Inhaled Nitric Oxide in Acute Pulmonary Embolism: A Systematic Review
Tariq Bhat, MD,1 Adi Neuman, MD,2 Mohmad Tantary, MD,2 Hilal Bhat, MD,3 Daniel Glass, MD,2 William Mannino, CCRN,1 Muhammad Akhtar, MD,1 Alina Bhat, MD,2 Sumaya Teli, MBChB,4 James Lafferty, MD1
1Division of Cardiology, Staten Island University Hospital, Staten Island, NY; 2Department of Medicine, Staten Island University Hospital, Staten Island, NY; 3Department of Medicine, Sher-i-Kashmir Institute of Medical Sciences, Soura, Srinagar, Jammu and Kashmir, India; 4University of Sheffield School of Medicine, Sheffield, South Yorkshire, United Kingdom
Acute pulmonary embolism (PE) is usually a complication secondary to migration of a deep venous clot or thrombi to lungs, but other significant etiologies include air, amniotic fluid, fat, and bone marrow. Regardless of the underlying etiology, little progress has been made in finding an effective pharmacologic intervention for this serious complication. Among the wide spectrum of PE, massive PE is associated with considerable morbidity and mortality, primarily due to severely elevated pulmonary vascular resistance leading to right ventricular failure, hypoxemia, and cardiogenic shock. We currently have limited therapeutic options at our disposal. Inhaled nitric oxide (iNO) has been proposed as a potential therapeutic agent in cases of acute PE in which hemodynamic compromise secondary to increased pulmonary vascular resistance is present, based on iNO's selective dilation of the pulmonary vasculature and antiplatelet activity. A systematic search of studies using the PubMed database was undertaken in order to assess the available literature. Although there are currently no published randomized controlled trials on the subject, except a recently publish phase I trial involving eight patients, several case reports and case series describe and document the use of iNO in acute PE. The majority of published reports have documented improvements in oxygenation and hemodynamic variables, often within minutes of administration of iNO. These reports, when taken together, raise the possibility that iNO may be a potential therapeutic agent in acute PE. However, based on the current literature, it is not possible to conclude definitively whether iNO is safe and effective. These case reports underscore the need for randomized controlled trials to establish the safety and efficacy of iNO in the treatment of massive acute PE. The purpose of this article is to review the current literature in the use of iNO in the setting of PE given how acute PE causes acute onset of pulmonary hypertension.
[Rev Cardiovasc Med. 2015;16(1):1-8 doi: 10.3909/ricm0718]
© 2015 MedReviews®, LLC
Inhaled Nitric Oxide in Acute Pulmonary Embolism: A Systematic Review
Tariq Bhat, MD,1 Adi Neuman, MD,2 Mohmad Tantary, MD,2 Hilal Bhat, MD,3 Daniel Glass, MD,2 William Mannino, CCRN,1 Muhammad Akhtar, MD,1 Alina Bhat, MD,2 Sumaya Teli, MBChB,4 James Lafferty, MD1
1Division of Cardiology, Staten Island University Hospital, Staten Island, NY; 2Department of Medicine, Staten Island University Hospital, Staten Island, NY; 3Department of Medicine, Sher-i-Kashmir Institute of Medical Sciences, Soura, Srinagar, Jammu and Kashmir, India; 4University of Sheffield School of Medicine, Sheffield, South Yorkshire, United Kingdom
Acute pulmonary embolism (PE) is usually a complication secondary to migration of a deep venous clot or thrombi to lungs, but other significant etiologies include air, amniotic fluid, fat, and bone marrow. Regardless of the underlying etiology, little progress has been made in finding an effective pharmacologic intervention for this serious complication. Among the wide spectrum of PE, massive PE is associated with considerable morbidity and mortality, primarily due to severely elevated pulmonary vascular resistance leading to right ventricular failure, hypoxemia, and cardiogenic shock. We currently have limited therapeutic options at our disposal. Inhaled nitric oxide (iNO) has been proposed as a potential therapeutic agent in cases of acute PE in which hemodynamic compromise secondary to increased pulmonary vascular resistance is present, based on iNO's selective dilation of the pulmonary vasculature and antiplatelet activity. A systematic search of studies using the PubMed database was undertaken in order to assess the available literature. Although there are currently no published randomized controlled trials on the subject, except a recently publish phase I trial involving eight patients, several case reports and case series describe and document the use of iNO in acute PE. The majority of published reports have documented improvements in oxygenation and hemodynamic variables, often within minutes of administration of iNO. These reports, when taken together, raise the possibility that iNO may be a potential therapeutic agent in acute PE. However, based on the current literature, it is not possible to conclude definitively whether iNO is safe and effective. These case reports underscore the need for randomized controlled trials to establish the safety and efficacy of iNO in the treatment of massive acute PE. The purpose of this article is to review the current literature in the use of iNO in the setting of PE given how acute PE causes acute onset of pulmonary hypertension.
[Rev Cardiovasc Med. 2015;16(1):1-8 doi: 10.3909/ricm0718]
© 2015 MedReviews®, LLC
Inhaled Nitric Oxide in Acute Pulmonary Embolism: A Systematic Review
Tariq Bhat, MD,1 Adi Neuman, MD,2 Mohmad Tantary, MD,2 Hilal Bhat, MD,3 Daniel Glass, MD,2 William Mannino, CCRN,1 Muhammad Akhtar, MD,1 Alina Bhat, MD,2 Sumaya Teli, MBChB,4 James Lafferty, MD1
1Division of Cardiology, Staten Island University Hospital, Staten Island, NY; 2Department of Medicine, Staten Island University Hospital, Staten Island, NY; 3Department of Medicine, Sher-i-Kashmir Institute of Medical Sciences, Soura, Srinagar, Jammu and Kashmir, India; 4University of Sheffield School of Medicine, Sheffield, South Yorkshire, United Kingdom
Acute pulmonary embolism (PE) is usually a complication secondary to migration of a deep venous clot or thrombi to lungs, but other significant etiologies include air, amniotic fluid, fat, and bone marrow. Regardless of the underlying etiology, little progress has been made in finding an effective pharmacologic intervention for this serious complication. Among the wide spectrum of PE, massive PE is associated with considerable morbidity and mortality, primarily due to severely elevated pulmonary vascular resistance leading to right ventricular failure, hypoxemia, and cardiogenic shock. We currently have limited therapeutic options at our disposal. Inhaled nitric oxide (iNO) has been proposed as a potential therapeutic agent in cases of acute PE in which hemodynamic compromise secondary to increased pulmonary vascular resistance is present, based on iNO's selective dilation of the pulmonary vasculature and antiplatelet activity. A systematic search of studies using the PubMed database was undertaken in order to assess the available literature. Although there are currently no published randomized controlled trials on the subject, except a recently publish phase I trial involving eight patients, several case reports and case series describe and document the use of iNO in acute PE. The majority of published reports have documented improvements in oxygenation and hemodynamic variables, often within minutes of administration of iNO. These reports, when taken together, raise the possibility that iNO may be a potential therapeutic agent in acute PE. However, based on the current literature, it is not possible to conclude definitively whether iNO is safe and effective. These case reports underscore the need for randomized controlled trials to establish the safety and efficacy of iNO in the treatment of massive acute PE. The purpose of this article is to review the current literature in the use of iNO in the setting of PE given how acute PE causes acute onset of pulmonary hypertension.
[Rev Cardiovasc Med. 2015;16(1):1-8 doi: 10.3909/ricm0718]
© 2015 MedReviews®, LLC
KEY WORDS
Nitric oxide • Pulmonary embolism • Pulmonary hypertension
KEY WORDS
Nitric oxide • Pulmonary embolism • Pulmonary hypertension
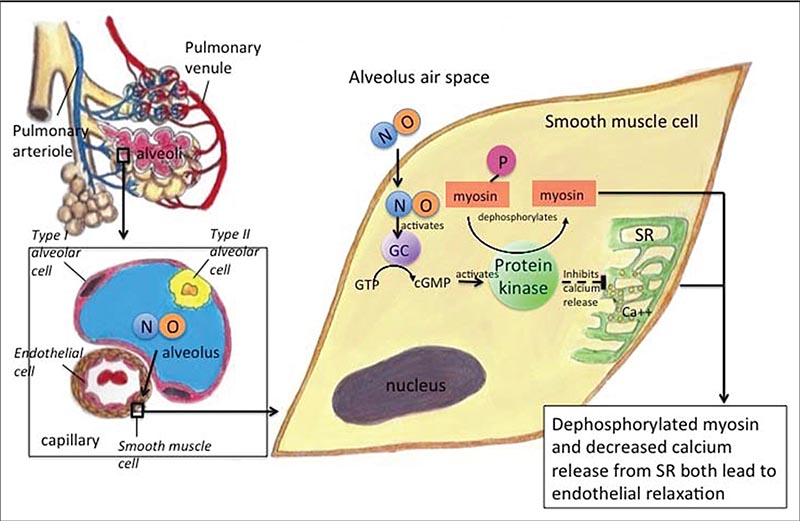
Figure 1. Schema of some of the vasodilatory effects of inhaled nitric oxide. Inhaled NO diffuses across the alveolus to the pulmonary arteriole in which, among other effects, it activates GC. This causes a cascade to eventually dephosphorylate myosin, as well as inhibit calcium release from the SR, both of which contribute to pulmonary arteriole endothelial relaxation and therefore decreased pulmonary pressure. cGMP, cyclic guanosine monophosphate; GC, guanylate cyclase; GTP, guanosine-5’-triphosphate; NO, nitric oxide; SR, sarcoplasmic reticulum.
... inhaled nitric oxide has been proposed as a potential therapeutic agent in cases of acute PE with associated increased pulmonary vascular resistance.

Acute PE is associated with high morbidity and mortality, and the mainstay of treatment is anticoagulation.
iNO has multiple effects that suggest a potential therapeutic role in the treatment of acute PE.
Main Points
• Acute pulmonary embolism (PE) is usually a complication secondary to migration of a deep venous clot or thrombi to the lungs; other significant etiologies include air, amniotic fluid, fat, and bone marrow. Massive PE is associated with considerable morbidity and mortality due to right ventricular failure, hypoxia, and cardiogenic shock, but little progress has been made in finding an effective pharmacologic intervention for this serious complication.
• Inhaled NO (iNO) has been reported to improve oxygenation and hemodynamics in conjunction with either chemical and/or mechanical thrombolysis in massive PE.
• iNO has multiple effects that suggest a potential therapeutic role in the treatment of acute PE. In addition, in both human and animal studies, NO has been shown to inhibit platelet adhesion and aggregation, prolong bleeding times, and reduce fibrinogen binding; these factors may prevent additional clot formation in acute PE.
• Large, randomized controlled trials need to be performed to determine if iNO provides a mortality benefit in acute PE. Whether NO should be administered to all patients with evidence of RV strain or limited to those with hemodynamic instability also requires further clarification.
Main Points
• Acute pulmonary embolism (PE) is usually a complication secondary to migration of a deep venous clot or thrombi to the lungs; other significant etiologies include air, amniotic fluid, fat, and bone marrow. Massive PE is associated with considerable morbidity and mortality due to right ventricular failure, hypoxia, and cardiogenic shock, but little progress has been made in finding an effective pharmacologic intervention for this serious complication.
• Inhaled NO (iNO) has been reported to improve oxygenation and hemodynamics in conjunction with either chemical and/or mechanical thrombolysis in massive PE.
• iNO has multiple effects that suggest a potential therapeutic role in the treatment of acute PE. In addition, in both human and animal studies, NO has been shown to inhibit platelet adhesion and aggregation, prolong bleeding times, and reduce fibrinogen binding; these factors may prevent additional clot formation in acute PE.
• Large, randomized controlled trials need to be performed to determine if iNO provides a mortality benefit in acute PE. Whether NO should be administered to all patients with evidence of RV strain or limited to those with hemodynamic instability also requires further clarification.
Acute pulmonary embolism (PE) is usually a complication secondary to migration of a deep venous clot or thrombi to the lungs, but other significant etiologies include air, amniotic fluid, fat, and bone marrow. Regardless of the underlying etiology, little progress has been made in finding an effective pharmacologic intervention for this serious complication. Massive PE is associated with considerable morbidity and mortality due to right ventricular (RV) failure, hypoxia, and cardiogenic shock. The incidence of acute PE in 2006 was reported to be 112.3 per 100,000 US adults, with a 7.8% mortality rate.1 Mortality is especially high in patients with hemodynamic instability or signs of RV dysfunction (RVD) and can range from 14% to 18% in patients with RVD.2 Acute PE increases pulmonary vascular resistance due to mechanical obstruction and arterial vasoconstriction, which is secondary to vasoactive mediators, such as thromboxane A2 and serotonin released by entrapped activated platelets.3 There are limited therapeutic options available for managing acute PE with severe RV failure and cardiogenic shock; these primarily include systemic anticoagulation, thrombolysis, and embolectomy, each of which has its own limitations. The elevated pulmonary resistance due to acute PE leads to an increase in RV afterload, which leads to RV failure and subsequent circulatory failure and death.4 Employing a therapeutic strategy to reduce RV afterload can prove pivotal in managing these patients. The possible therapeutic role of vasodilators in this situation is limited by their systemic vasodilatation and hypotension.5 In these situations, selective pulmonary vasodilators can have a significant role; thus, inhaled nitric oxide (iNO) has been proposed as a potential therapeutic agent in cases of acute PE with associated increased pulmonary vascular resistance.6
Background
Nitric oxide (NO) is a naturally occurring, colorless, odorless gas that is synthesized endogenously by NO synthase and is involved in the modulation of vascular tone. NO diffuses freely across cell membranes, thus diffusing rapidly from the alveoli to the highly resistant pulmonary arterioles.7 It activates guanylate cyclase, which activates cyclic guanosine monophosphate (GMP). Cyclic GMP, in turn, activates protein kinase, which dephosphorylates the myosin light chain, making it less sensitive to calcium, and inhibits release of calcium from the sarcoplasmic reticulum. These effects promote relaxation of the vascular smooth muscle (Figure 1).8
In an early study, iNO was shown to reverse pulmonary hypertension that was induced in healthy subjects breathing low concentrations of oxygen, without causing systemic vasodilation.9 However, the high cost of long-term iNO, along with concern for rebound hypoxemia and respiratory failure when use of the drug is abruptly discontinued, have been limiting factors in use of the agent in adult patients with pulmonary hypertension.
Because NO was shown to have limited effects on the peripheral vasculature, there was hope that the administration of NO could provide benefit in patients with acute respiratory distress syndrome (ARDS) and hypotension.10 One study in patients with acute lung injury (currently classified as mild ARDS) showed that iNO at a dose of 40 ppm induced pulmonary vasodilation and reduced the risk of pulmonary edema.11 Additionally, because iNO is administered by inhalation, it preferentially causes vasodilatation in areas of the lung that are well ventilated, improving ventilation/perfusion matching and decreasing pulmonary shunting.12 However, several large, randomized placebo-controlled trials failed to prove benefit with regard to duration of ventilation or mortality in ARDS patients administered NO, despite a short-lived increase in arterial partial pressures of oxygen.
Although iNO is available commercially, it is currently licensed only for persistent pulmonary hypertension and respiratory failure in the neonate. As a therapeutic agent, it can be administered to patients undergoing mechanical ventilation, as well as via face mask or nasal cannula.
Methods
A systematic search of studies concerning use of iNO in acute PE was conducted using the PubMed database from January 1990 to June 2013. The search terms used were acute pulmonary embolism, acute PE, acute thromboembolism, and acute VTE, together with inhaled NO, iNO, and NO. Articles that were found with the term NO were screened for whether or not the authors used iNO. Given the lack of large, randomized controlled trials involving iNO in acute PE, we included all case series and case reports. We limited studies to those published in 1990 or later. References of all included articles were reviewed in order to identify additional relevant studies. We excluded case reports describing patients who had underlying chronic pulmonary hypertension, or had a diagnosis of chronic pulmonary thromboembolic disease. The recently published small clinical trial did not include detailed information of the patients’ hemodynamic response; thus, although cited, it was excluded from analysis. In all relevant case series and reports, we extracted data including the dose and duration of iNO, imaging, and interventions that were performed, and treatment outcomes.
Results
To date, no large, randomized controlled studies have been performed that compare the use of iNO with placebo in acute PE. Recently, a small phase I clinical trial was published that concluded that iNO reduced dyspnea without adverse events in eight studied patients with severe sub-massive PE. In this study, eight patients with submassive PE diagnosed by computed tomography were administered NO using a commercial device and a nonre-breathing face mask. NO concentration was increased at 1 ppm/min until a maximum of 25 ppm and continued for 120 minutes and then weaned at 1 ppm/min. Dyspnea was assessed with the Borg score, oxygenation by pulse oximetry, and hemodynamic status by shock index (heart rate/systolic blood pressure). All patients tolerated the entire protocol without adverse events, and all had decreased numerical Borg score (a subjective measure of exertion) by > 50%. The changes from baseline to 155 minutes were as follows: Borg score 7.5 ± 2.5 to 2.3 ± 1.9 (P = .06; signed-ranktest), arterial oxygen saturation 93 ± 5 to 97 ± 3, and shock index 1.0 ± 0.11 to 0.86 ± 0.09. No patient showed signs of worsening during weaning.13
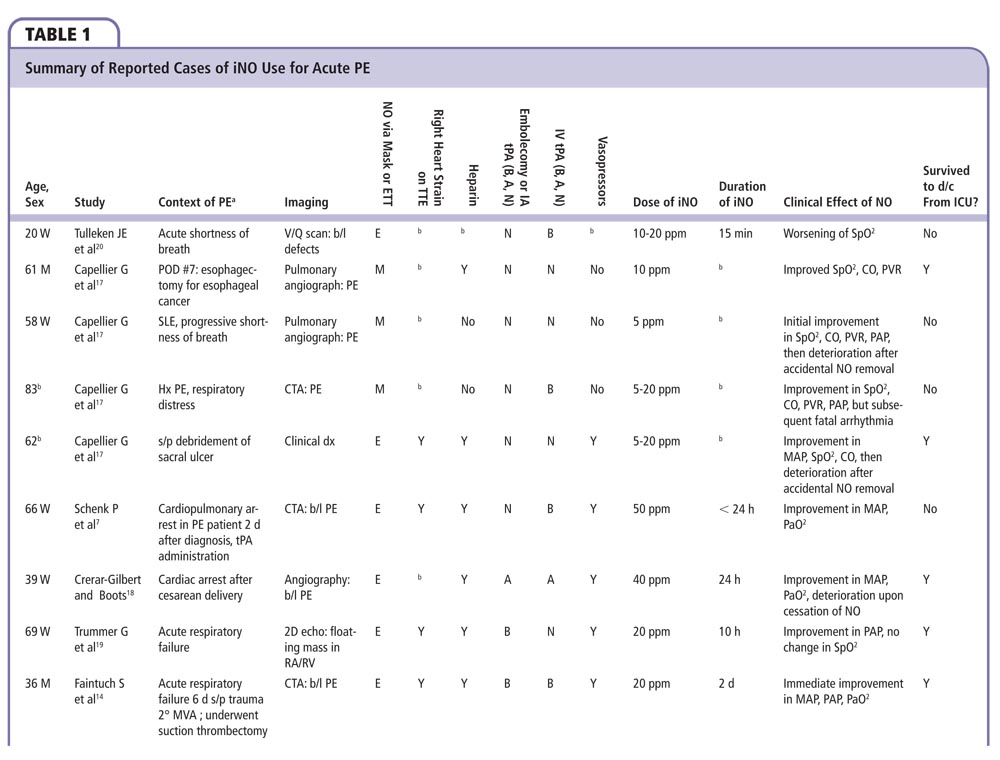
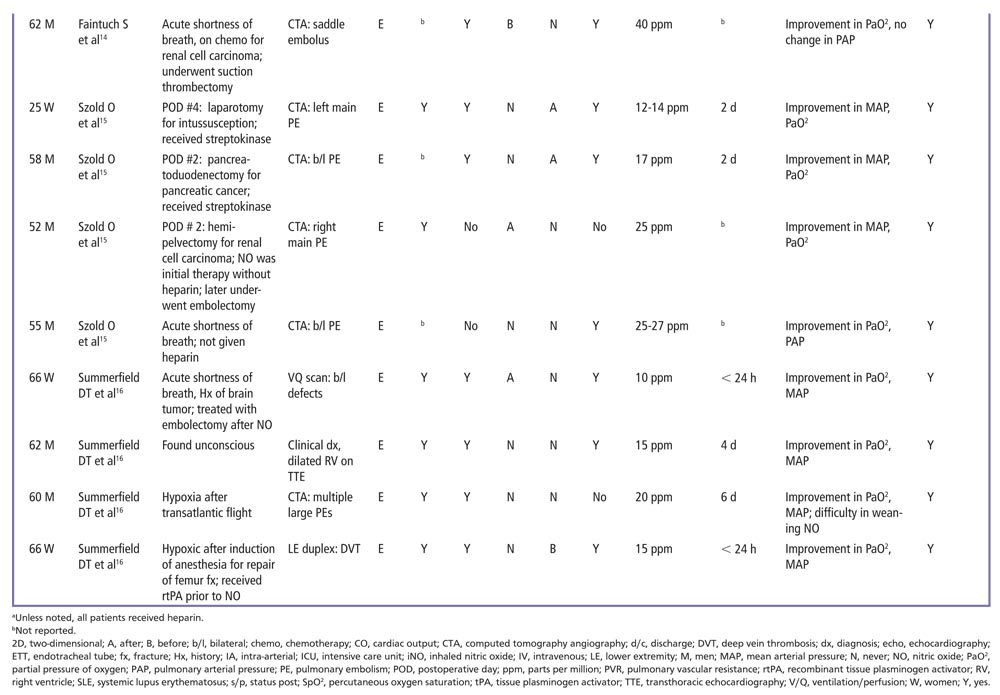
Additionally, 18 patients have been described either through case reports or case series who have been administered iNO for PE (Table 1). Many described improvement in hemodynamics and oxygenation following administration of iNO, often instantaneously upon administration.14,15
In the case reports, iNO was at times used to improve oxygenation and hemodynamics in conjunction with either chemical and/or mechanical thrombolysis. In three cases, iNO was delivered before thrombolysis, whereas in three others it was delivered after thrombolysis. Patients were between ages 25 and 83 years. Most patients had signs of RV strain on transthoracic echocardiography and received vasopressors in order to maintain systemic blood pressure. In the case reports, iNO was administered at a dose of 10 to 50 ppm for up to 6 days.
A total of 14 of 18 patients survived the intensive care unit, and there were no reported cases of clinical methemoglobinemia. In one case, the authors reported initial difficulty weaning patients from NO due to rebound hypoxemia.16 In three cases, accidental interruption of iNO resulted in an immediate drop in mean systemic arterial pressure, venous oxygen saturation, and cardiac output, which resolved after readministration.17,18 Another patient had recurrent pulmonary hypertension after being weaned off iNO, and the agent had to be used a second time.19
One early case report20 described rapid oxygen desaturation after iNO was administered to a patient with acute PE and shock. In this case, iNO was discontinued after 15 minutes, and partial pressures of oxygen only returned to preadministration levels after several hours. The patient underwent mechanical embolectomy but did not survive. The negative effect of iNO on oxygenation in this patient could not be readily explained, and was inconsistent with its effects in the other case reports.
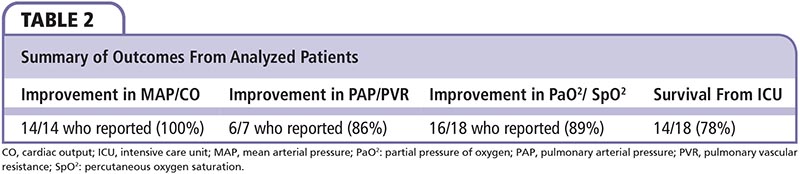
In all, 14 patients in whom systemic hemodynamics (mean arterial pressure or cardiac output) was documented experienced improvement, often immediately. Additionally, 86% of those documented experienced improvement in pulmonary pressures, and 89% in oxygenation. Only one patient had worsening of any of these parameters upon initiation of iNO (Table 2).20
Discussion
Acute PE is associated with high morbidity and mortality, and the mainstay of treatment is anticoagulation. When associated with hypotension, and particularly in the context of RV compromise, treatment options include thrombolytic therapy and mechanical embolectomy. However, thrombolysis is contraindicated in many patients, and mechanical embolectomy may be associated with unacceptable risk in patients with hemodynamic instability. In some cases, complicating factors, such as active gastrointestinal bleeding and recent surgery, may preclude or delay the implementation of these therapies. Therefore, there is a need for additional agents for those patients in whom thrombolysis and mechanical embolectomy is either delayed or contraindicated.
iNO has multiple effects that suggest a potential therapeutic role in the treatment of acute PE. Prognosis in acute PE has been linked to the degree of pulmonary resistance.21 It has been proposed that the dramatic increase in pulmonary resistance and resultant RV failure in massive PE may be due to pulmonary vasoconstriction secondary to the initial embolic event. This would suggest that the reduction of RV afterload may be critical to the treatment of PE. iNO, as noted above, has been established as a potent and selective dilator of pulmonary vasculature.21 In one study involving the use of micro-spheres to model massive PE in piglets, NO was shown to decrease pulmonary vascular resistance without affecting systemic blood pressure.22 Furthermore, it has been shown that—in patients with acute PE—free hemoglobin formed from hemolysis (either as a result of the embolism or the thrombolytic therapy) increases NO consumption and lowers the endogenous NO bioavailability.23
In addition, NO has been shown in both human and animal studies to inhibit platelet adhesion and aggregation, prolong bleeding times, and reduce fibrinogen binding.24 These factors may prevent additional clot formation in acute PE.
Concerns regarding treatment with iNO include rebound pulmonary hypertension and hypoxia upon withdrawal of the agent and methemoglobinemia. However, with regard to rebound effects, clinical experience has shown that they can usually be avoided with gradual as opposed to abrupt withdrawal of iNO. A small study in patients with ARDS demonstrated that doses of 1.25 to 40 ppm of iNO are generally well tolerated.25 Methemoglobinemia is also unlikely to occur at levels lower than doses of 40 ppm, except in patients with methemoglobin reductase deficiency (a relatively rare condition).
The available literature suggests that there is potential for iNO as a therapeutic agent in conjunction with available therapies, including anticoagulation and thrombolysis. This review has several limitations, including—most prominently—a lack of large, randomized controlled trials to assess the efficacy of iNO versus placebo. In addition, reporting bias most likely contributed to the relative lack of case reports documenting treatment failure or adverse effects. The mortality rate in patients with PE and hemodynamic compromise is high, and the fact that nearly all of the patients in the case reports survived suggests that many cases in which iNO is used are not being reported. On the other hand, iNO is likely to be given only to the patients with very poor prognoses, given the current lack of evidence regarding its role in PE. Additionally, iNO was given in conjunction with other established treatments and it is difficult to ascribe improvements to the iNO as an independent factor. However, in most patients, improvement was immediate upon iNO initiation; in some cases of accidental iNO interruption, the patient exhibited immediate clinical deterioration.17,18
Large, randomized controlled trials need to be performed to determine if iNO provides a mortality benefit in acute PE. Other issues that require clarification include the optimal dose and duration of iNO, whether NO should be administered to all patients with evidence of RV strain or limited to those with hemodynamic instability, and how best to monitor patients for methemoglobinemia.
Conclusions
In a review of 18 patients receiving iNO for acute PE, all but one showed improvement in either systemic or pulmonary pressures or oxygenation. Additionally, a recent phase I trial showed improvement in Borg score, pulse oximetry, and hemodynamics. Our hope is that this article will encourage investigators to conduct a large, randomized control trial to evaluate the efficacy and safety of iNO, as well as proper patient selection, timing, and dosage in those with acute PE. ![]()
The authors report no real or apparent conflicts of interest.
References
- Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171:831-837.
- ten Wolde M, Sohne M, Quak E, et al. Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med. 2004;164:1685-1689.
- Frostell C, Fratacci MD, Wain JC, et al. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation. 1991;83:2038-2047.
- Mclntyre KM, Sasahara AA. The hemodynamic response to pulmonary embolism in patients without prior cardiopulmonary disease. Am J Cardiol. 1971;28:288-294.
- Calvin JE Jr. Acute right heart failure: pathophysiology, recognition, and pharmacological management. J Cardiothorac Vasc Anesth. 1991;5:507-513.
- Pepke-Zaba J, Higenbottam TW, Dinh-Xuan AT, et al. Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet. 1991;338:1173-1174.
- Schenk P, Mittermayer C, Ratheiser K. Inhaled nitric oxide in a patient with severe pulmonary embolism. Ann Emerg Med. 1999;33:710-714.
- Griffiths MJ, Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med. 2005;353:2683-2695.
- Frostell CG, Blomqvist H, Hedenstierna G, et al. Inhaled nitric oxide selectively reverses human hypoxic pulmonary vasoconstriction without causing systemic vasodilation. Anesthesiology. 1993;78:427-435.
- Rossaint R, Falke KJ, López F, et al. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993;328:399-405.
- Benzing A, Bräutigam P, Geiger K, et al. Inhaled nitric oxide reduces pulmonary transvascular albumin flux in patients with acute lung injury. Anesthesiology. 1995;83:1153-1161.
- Zapol WM, Rimar S, Gillis N, et al. Nitric oxide and the lung. Am J Respir Crit Care Med. 1994;149:1375- 1380.
- Kline JA, Hernandez J, Garrett JS, Jones AE. Pilot study of a protocol to administer inhaled nitric oxide to treat severe acute submassive pulmonary embolism. Emerg Med J. 2014;31:459-462.
- Faintuch S, Lang EV, Cohen RI, Pinto DS. Inhaled nitric oxide as an adjunct to suction thrombectomy for pulmonary embolism. J Vasc Interv Radiol. 2004;15:1311-1315.
- Szold O, Khoury W, Biderman P, et al. Inhaled nitric oxide improves pulmonary functions following massive pulmonary embolism: a report of four patients and review of the literature. Lung. 2006;184:1-5.
- Summerfield DT, Desai H, Levitov A, et al. Inhaled nitric oxide as salvage therapy in massive pulmonary embolism: a case series. Respir Care. 2012;57: 444-448.
- Capellier G, Jacques T, Balvay P, et al. Inhaled nitric oxide in patients with pulmonary embolism. Intensive CareMed. 1997;23:1089-1092.
- Crerar-Gilbert A, Boots R. Use of inhaled nitric oxide in pulmonary embolism. Anaesth Intensive Care. 1999;27:412-414.
- Trummer G, Berchtold-Herz M, Martin J, Beyersdorf F. Successful treatment of pulmonary hypertension with inhaled nitric oxide after pulmonary embolectomy. Ann Thorac Surg. 2002;73:1299-1301.
- Tulleken JE, Zijlstra JG, Evers K, van der Werf TS. Oxygen desaturation after treatment with inhaled nitric oxide for obstructive shock due to massive pulmonary embolism. Chest. 1997;112:296-298.
- Dehring DJ, Arens JF. Pulmonary thromboembolism: disease recognition and patient management. Anesthesiology. 1990;73:146-164.
- Böttiger BW, Motsch J, Dörsam J, et al. Inhaled nitric oxide selectively decreases pulmonary artery pressure and pulmonary vascular resistance following acute massive pulmonary microembolism in piglets. Chest. 1996;110:1041-1047.
- Sertório JT, Neto-Neves EM, Dias-Junior CA, et al. Elevated plasma hemoglobin levels increase nitric oxide consumption in experimental and clinical acute pulmonary thromboembolism. Crit Care Med. 2013;41:ell8-el24.
- Gries A, Bode C, Peter K, et al. Inhaled nitric oxide inhibits human platelet aggregation, P-selectin expression, and fibrinogen binding in vitro and in vivo. Circulation. 1998;97:1481-1487.
- Dellinger RP, Zimmerman JL, Taylor RW, et al. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group. Crit CareMed. 1998;26:15-23.