Best of the 2014 TCT Annual Meeting
Highlights From the Transcatheter Cardiovascular Therapeutics Conference, September 13-17, 2014, Washington DC
[Rev Cardiovasc Med. 2015;16(1):74-80 doi: 10.3909/ricm0787]
© 2015 MedReviews®, LLC
Reviewed by Sandeep K. Krishnan, MD, Cedars-Sinai, Los Angeles, CA.
Best of the 2014 TCT Annual Meeting
Highlights From the Transcatheter Cardiovascular Therapeutics Conference, September 13-17, 2014, Washington DC
[Rev Cardiovasc Med. 2015;16(1):74-80 doi: 10.3909/ricm0787]
© 2015 MedReviews®, LLC
Reviewed by Sandeep K. Krishnan, MD, Cedars-Sinai, Los Angeles, CA.
Best of the 2014 TCT Annual Meeting
Highlights From the Transcatheter Cardiovascular Therapeutics Conference, September 13-17, 2014, Washington DC
[Rev Cardiovasc Med. 2015;16(1):74-80 doi: 10.3909/ricm0787]
© 2015 MedReviews®, LLC
Reviewed by Sandeep K. Krishnan, MD, Cedars-Sinai, Los Angeles, CA.
KEY WORDS
Dual antiplatelet therapy • Transcatheter aortic valve replacement • Percutaneous coronary intervention • Percutaneous transluminal angioplasty
KEY WORDS
Dual antiplatelet therapy • Transcatheter aortic valve replacement • Percutaneous coronary intervention • Percutaneous transluminal angioplasty
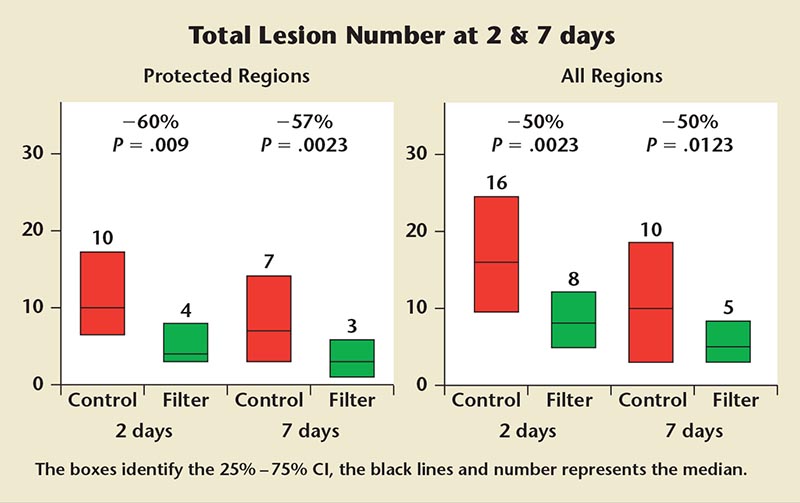
Figure 1. Claret Embolic Protection and Transcatheter Aortic Valve Implantation (CLEAN-TAVI) trial magnetic resonance imaging data.
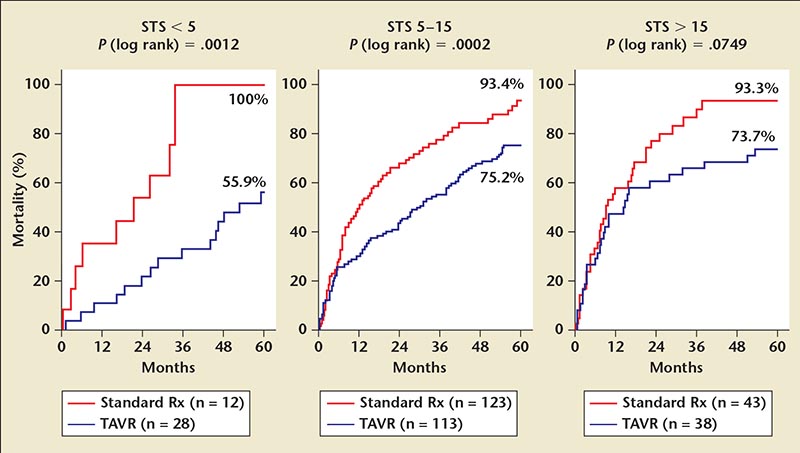
Figure 2. All-cause mortality stratified by STS score in standard versus TAVR groups. ITT, intention-to-treat; Rx, therapy; STS, Society of Thoracic Surgeons Predicted Risk of Mortality; TAVR, transcatheter aortic valve replacement.
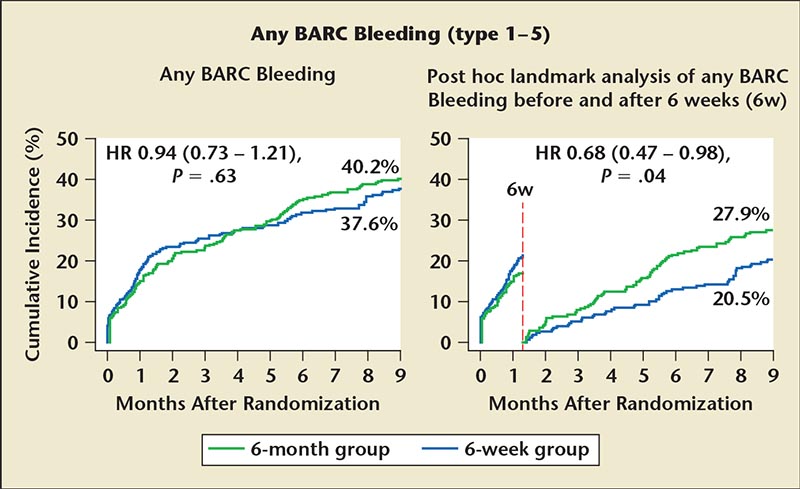
Figure 3. BARC bleeding differences between 6-week and 6-month group including post hoc analysis data. BARC, Bleeding Academic Research Consortium; HR, hazard ratio.
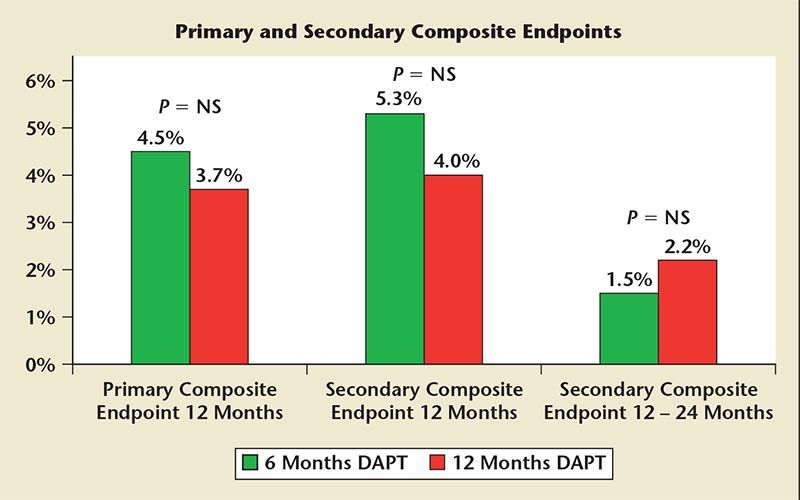
Figure 4. Six versus 12 months of DAPT in the SECURITY trial. DAPT, dual antiplatelet therapy; NS, not significant; SECURITY, Second Generation Drug-Eluting Stent Implantation Followed by Six- Versus Twelve-Month Dual Antiplatelet Therapy.
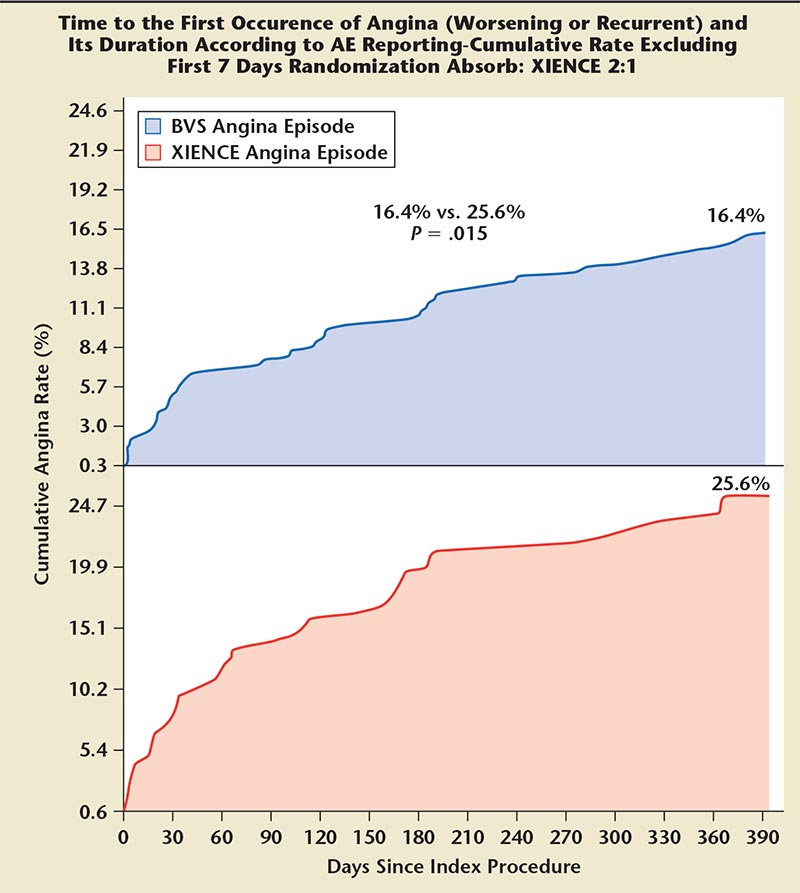
Figure 5. Time to first occurrence of angina: Absorb versus XIENCE stents (Abbott Vascular; Abbott Park, IL). AE, adverse event; BVS, bioresorbable vascular scaffold.
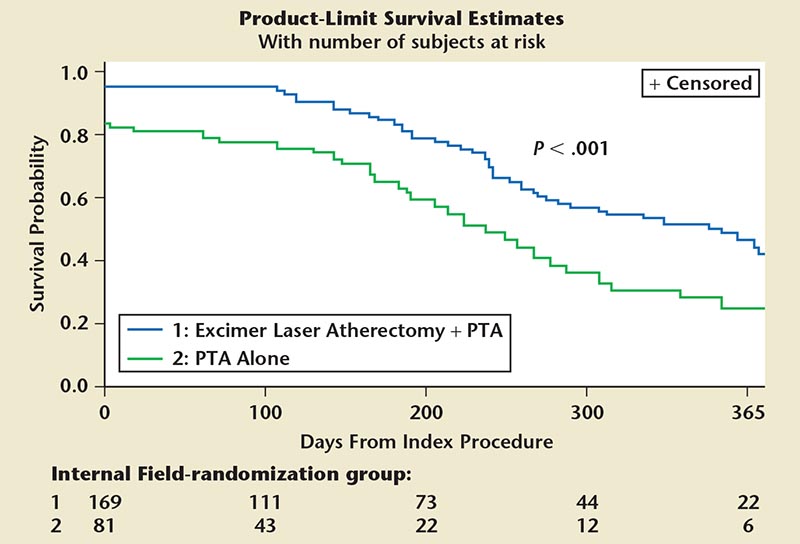
Figure 6. Freedom from MAE up to 1 year out. ELA 1 PTA cohort is significantly superior in this regard compared with PTA alone. ELA, excimer laser atherectomy; MAE, major adverse events; PTA, percutaneous transluminal angioplasty.
The 26th annual Transcatheter Cardiovascular Therapeutics (TCT) conference was held in Washington, DC, September 13-17, 2014. Several thousand interventional cardiologists, cardiothoracic surgeons, and cardiovascular team members gathered from across the globe to focus on catheter-based cardiac and vascular therapies. Sponsored by the Cardiovascular Research Foundation (New York, NY), this is the largest interventional cardiovascular conference in the world, with customized programs for interventional cardiologists, vascular surgeons, cardiothoracic surgeons, and interventional radiologists. Herein we examine important late-breaking clinical research studies and first reports of new data presented at TCT 2014 on the subject of pioneering developments in the realm of interventional cardiology—from valvular heart disease, to the duration of dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI), to the use of bioresorbable vascular scaffolds to treat coronary artery disease.
Transcatheter Aortic Valve Replacement
Claret Embolic Protection and Transcatheter Aortic Valve Implantation (CLEAN-TAVI): Cerebral Embolic Protection in High-risk Patients With Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement
Stroke remains a major complication in many patients who undergo transcatheter aortic valve replacement (TAVR), particularly those with high surgical risk. Ischemic brain lesions on post-TAVR imaging are found in more than two thirds of patients.1 Moreover, the presence of silent brain infarcts increased the risk for major stroke more than threefold; silent infarcts are well recognized to be associated with several adverse neurologic and cognitive consequences.2
Unfortunately, the occurrence of stroke increases 30-day mortality by as much as threefold in some studies.3 Thus, the Claret Medical dual-filter cerebral protection system (Claret Medical, Inc., Santa Rosa, CA) was developed to protect the brain from injury caused by embolic debris. It is an embolic protection device delivered percutaneously via the radial artery and is used during TAVR in high-risk patients with aortic stenosis; it significantly reduced the number of cerebral lesions and lesion volume when compared with TAVR procedures performed without the filter.
Axel Linke, MD, of the University of Leipzig Heart Center (Leipzig, Germany), presented the study, which randomized 100 patients in a 1:1 fashion to the control group (TAVR without a filter) and the filter group (TAVR with filter). Patients in both groups underwent magnetic resonance imaging (MRI) at three points: 2 days postprocedure, 7 days postprocedure, and 30 days postprocedure. All patients in the study received a CoreValve® (Medtronic; Minneapolis, MN). Key exclusion criteria included patients with > 70% stenosis of the carotid artery or relevant stenosis of the brachiocephalic trunk or subclavian artery. The primary efficacy endpoint was numeric reduction in positive post-procedure diffusion-weighted MRI (DW-MRI) perfused brain lesions relative to baseline at 2 days in protected territories. Secondary end-points included serial volumetric and numeric reduction in positive postprocedure DW-MRI perfused brain lesions at 2, 7, 30, and 360 days; serial neurologic assessment by National Institutes of Health Stroke Scale-trained specialist; serial neurocognitive assessment; and periprocedural transcranial Doppler assessment.
At postoperative day 2, there were 10 lesions detected in those undergoing TAVI without the filter versus four lesions in those who received embolic protection— a significant 60% reduction. At postoperative day 7, there were seven and three lesions in the control and filter arms, respectively. This difference was also statistically significant. When the entire region of brain was analyzed (not just the protected region), the total number of lesions and the lesion volume at 2 and 7 days was significantly reduced in the filter-treated patients (Figure 1). In their neurocognitive assessment of patients, Linke said they observed lower rates of ataxia in the patients treated with the embolic protection device, and “this supports the notion that the filter has the potential to improve neurological outcomes.”4 Interestingly, the study showed a reduction in the total number of lesions and lesion volume from day 2 to day 7, suggesting that some lesions may disappear over time. The fluoroscopy time was slightly longer and statistically significant by approximately 3 minutes in the filter group, but the contrast used for both groups did not vary significantly. With regard to safety, the mortality rate at 30 days was 1% in the entire cohort.
Panelists overall agreed that the Sentinel™ cerebral protection device (Claret Medical) showed promise in reducing particulate debris to the brain. However, some interventionalists noted that neurologists remain concerned about lesions detected at 48 hours, even if they resolve, as the damage may already be done.
The multicenter Cerebral Protection in Transcatheter Aortic Valve Replacement (SENTINEL) trial is currently ongoing in the United States and Europe; it randomizes 360 TAVI patients to treatment with the embolic filter or to TAVR alone. The primary end-point is an assessment of cerebral lesions by MRI at 4 to 7 days post-procedure. The Sentinel™ cerebral protection system has Conformité Européenne (CE) mark approval in Europe for use during TAVI procedures but is awaiting approval in the United States.
5-Year Placement of Aortic Transcatheter Valve (PARTNER) Trial Data
Although the vast majority of patients with inoperable aortic valve disease who were treated in the original PARTNER protocol are no longer alive after their treatment with the SAPIEN transcatheter valve (Edwards Lifesciences, Irvine, CA) or usual care, investigators saw a significant difference in survival and repeat hospitalizations, suggesting that patients are both living longer and better lives following transcatheter valve replacement according to data presented by Samir Kapadia, MD.
The PARTNER trial randomized 358 patients with inoperable aortic stenosis in a 1:1 fashion to standard therapy versus transfemoral transcatheter aortic valve replacement with the Edwards Sapien device. The key endpoints for the 5-year analysis included all-cause mortality; cardiac mortality; rehospitalization; stroke; New York Heart Association (NYHA) functional class; echocardiography-derived valve areas, transvalvular gradients, and paravalvular leak; and mortality outcomes stratified by Society of Thoracic Surgeons Predicted Risk of Mortality (STS) score, paravalvular leak, and age.
For the mortality endpoint at 5 years the majority of patients in both groups had died and only 6 of 179 remained alive in the standard therapy group (6.4% survival), whereas 51 of 179 were alive in the TAVR group (28.2% survival). All six of the patients in the former group had an aortic valve procedure—balloon valvuloplasty or aortic valve replacement. The cause of death in the standard care group was cardiovascular in 48% of patients, whereas in the TAVR group the cause of death was evenly distributed between cardiovascular, other, and unknown. Furthermore, the survival advantage for TAVR was seen across all three tertiles of STS scores, including STS > 15 (Figure 2).
With regard to the other end-points, 87% in the standard care group were rehospitalized versus 48% in the TAVR-treated group; 40% of the standard therapy group were in NYHA class 3 or 4 heart failure by 5 years versus 14.3% of the TAVR-treated patients (although this was not statistically significant). And there was no significant increase in transvalvular gradient or attrition of valve area, which suggests that there is good durability for at least 5 years.
These promising data prompted many panelists to comment on the use of TAVR in the very elderly population. The majority of panelists felt that the 5-year data underscore the fact that age alone should not be a reason to deny patients this life-saving procedure. As long as the patient is living and functioning independently without dementia, there is no need to withhold this therapy.
PCI
Intracoronary Stenting and Antithrombotic Regimen-Testing of a Six-Week Versus A Six-Month Clopidogrel Treatment Regimen In Patients With Concomitant Aspirin And Oral Anticoagulant Therapy Following Drug-eluting Stenting (ISAR-TRIPLE): 6-Week Versus 6-Month Clopidogrel in Drug-eluting Stent and AtriaI Fibrillation
Because the optimal duration of triple therapy with oral anticoagulation and DAPT after drug-eluting stent (DES) implantation has not been defined, Nikolaus Sarafoff, MD, and coauthors decided to investigate this in the ISAR-TRIPLE study.
Their objective was to evaluate clinical outcomes after 6 weeks versus 6 months of clopidogrel therapy in patients after DES placement who are on concomitant aspirin and warfarin therapy. In total, 614 patients at three European centers were randomized (in a 1:1 fashion) into the short (6-week) and the long (6-month) therapy groups.5 Patients were approximately 74 years old, and one-third presented with an acute coronary syndrome. Over 80% had atrial fibrillation as the reason for oral anticoagulation, and the majority of patients received new-generation DES (second-generation permanent polymer DES). However, equal numbers of patients in both groups received therapies not yet available for commercial use in the United States, including biodegradable polymer DES, polymer-free DES, bioresorbable vascular scaffolds (BVS), and drug-eluting balloons. The hypothesis was that 6 weeks of triple therapy would be superior to 6 months of triple therapy. Clopidogrel was the drug that was stopped at either 6 weeks or 6 months.
The primary endpoint included death, myocardial infarction (MI), definite stent thrombosis, stroke, or Thrombolysis in Myocardial Infarction (TIMI) major bleeding at 9 months. Secondary endpoints included ischemic and bleeding complications. Ischemic complications included cardiac death, MI, definite stent thrombosis, and ischemic stroke; bleeding complications only measured TIMI major bleeding.
There was no difference in primary endpoints between the two groups. There was a statistically significant increase in MI in the 6-week group; however, five of the six Mis occurred within 6 weeks (when all patients were still on triple therapy) and the other event occurred after 6 months (when all patients were on only warfarin and aspirin), suggesting that this was more random chance. Although there was no significant decrease in major bleeding events, when Bleeding Academic Research Consortium (BARC) bleeding was examined, a post hoc analysis demonstrated that, after 6, weeks there was a statistically significant decrease in bleeding in the 6-week group compared with the 6-month group (Figure 3).
Thus, the authors concluded that 6 weeks of triple therapy is not superior to 6 months of triple therapy with regard to net clinical outcomes. Shortening the duration of triple therapy neither reduced the incidence of major bleeding nor increased the incidence of ischemic events. The results of ISAR-TRIPLE suggest that physicians should weigh the tradeoff between ischemic and bleeding risk when choosing the shorter or longer duration of triple therapy.
Second-generation DES Implantation Followed by 6- Versus 12-Month DAPT (SECURITY)
Six months of DAPT is noninferior to the 12 months or more recommended in US guidelines for the treatment of low-risk patients with stable or unstable angina and documented ischemia undergoing PCI, at least when newer-generation DES are used.6 Antonio Colombo, MD, of the San Raffaele Scientific Institute in Milan, Italy, presented the results of the SECURITY trial, which was a multicenter, randomized controlled trial conducted in Spain, the Netherlands, and Italy using second-generation stents. Stents implanted in the trial included Endeavor Resolute (Medtronic), XIENCE (Abbott Vascular; Abbott Park, IL), PROMUS (Boston Scientific; Marlborough, MA), Nobori® (Terumo Corporation; Tokyo, Japan), and the Biomatrix™ (Biosensors International; Singapore).
A total of 1399 low-risk patients were randomized just prior to their PCI to either 6 or 12 months of DAPT with aspirin and clopidogrel and were followed for 12 months. Inclusion criteria included any type or number of lesions, no other DES in the patients’ coronary trees, no bare metal stents implanted within 3 months of enrollment, and a de novo diagnosis of angina pectoris, unstable angina, or silent angina. Exclusion criteria included stents placed in bypass grafts, unprotected left main lesions, ST-elevation MI patients, patients with left ventricular ejection fraction ≤ 30%, or patients with non-ST-elevation MI in the 6 months preceding randomization. The primary endpoint was a composite of cardiac death, MI, stroke, stent thrombosis, and bleeding. Secondary endpoints included following the patients for the primary endpoints out to 24 months, as well as composite of urgent target revascularization, MI, any bleeding, or all-cause mortality.
There were no differences in any of the endpoints at any time period; the rate of stent thrombosis did not differ significantly between the two groups (0.3 vs 0.4% in the 6- and 12-month groups, respectively). Bleeding was slightly higher among patients taking DAPT for 12 months but did not reach statistical significance. Of note, however, a full 33% of patients randomized to the 6-month group actually continued taking DAPT for 12 months but by 2 years 97% of patients in both groups were only on aspirin (Figure 4).
Panelists were surprised about the lack of statistically significant increase in bleeding but felt this may be due to the one-third of patients in the 6-month group who were still on DAPT at 12 months. Colombo noted that event rates are so low with these newer stents that the contribution of these smaller randomized trials individually is quite small. More definitive answers will come from two much larger, randomized trials of DAPT that will be unveiled at the upcoming American Heart Association meeting: the Dual Antiplatelet Therapy and the Randomized, Double-blind Trial of 6 Versus 12 Months of Dual Antiplatelet Therapy After DES Implantation (ISAR-SAFE) trials.
ABSORB II: Everolimus-Eluting Bioresorbable Scaffold Versus an Everolimus-eluting Metallic Stent in Patients with Coronary Artery Disease
Patrick Serruys, MD, of the Imperial College in London, United Kingdom, presented the interim 1-year preliminary results of this prospective randomized trial given the widespread adoption of this novel therapy. The interim results suggest that, although the acute lumen gain was significantly smaller among patients receiving the BVS compared with those receiving an everolimus-eluting metallic stent (XIENCE), the clinical outcomes and angina were equivalent between the Absorb (Abbott Vascular) and XIENCE stents.7
A total of 501 patients were randomized in an intention-to-treat analysis in a 2:1 fashion to the Absorb BVS group (n = 335) and the XIENCE group (n = 166). Baseline characteristics were evenly matched between the groups. The coprimary endpoints of the ABSORB II study are vasomotion (assessed by the change in mean lumen diameter before and after nitrate administration at 3 years) and minimum lumen diameter at 3 years after nitrate administration minus the minimum lumen diameter after nitrates postprocedure. The secondary endpoints were exercise performance and angina status assessed by the Seattle Angina Questionnaire.
Procedural characteristics were almost identical with the exception of the nominal diameter of the last balloon used for postdeployment balloon dilation; the Absorb group used slightly smaller balloons compared with the XIENCE group (3.08 vs 3.16 mm), inflated to lower pressures (both variables reached statistical significance). Postprocedure residual vessel diameter was 2.64 versus 2.80 (P < .001).
The device success rates in this population with B1/B2 lesions were comparable between the two arms (device success: Absorb = 99% vs XIENCE = 100%). There was a reduction in nitrate use at 6 months (P = .02) and a trend toward lower nitrate use at 1 year with Absorb (P = .09). A post hoc analysis showed cumulative rates of angina were lower in the Absorb arm at 1 year. Also, if angina episodes occurring in the index admission or in the 7 days after the stent implantation were excluded, angina rates were lower in the Absorb BVS arm (Figure 5). Regarding the primary endpoints for the study, the rates of MI were 4% and 1% in the Absorb and XIENCE arms, respectively (P = .06), and this difference was primarily the result of periprocedural non-Q-wave Mis. There were two reported cases of stent thrombosis, one acute with 24 hours of stent implantation and the second on day 2, in the Absorb-treated patients. The rate of definite scaffold thrombosis was 0.6% in the Absorb arm and 0% in the XIENCE arm (P = 1.0).
Panelists agreed that the findings were in line with what was expected—a trade-off in performance and a slight increase in MI, but the trial was underpowered to draw any definitive conclusions. Furthermore, many noted that angina was a soft endpoint and difficult to reproduce (depending on how and when the patient was asked about his or her symptoms). The main question was the safety of the device. The recently published data from the Gauging Coronary Healing With Bioresorbable Scaffolding Platforms in Europe (GHOST-EU) registry of 1189 patients treated with the Absorb BVS reveals the cumulative incidence of probable/definite stent thrombosis was 1.5% at 30 days and 2.1% at 6 months.8
However, other panelists did not seem too concerned about the rates of stent thrombosis in the GHOST-EU registry as those clinical events were not adjudicated and it's difficult to know what events were being captured in the registry. They were excited about the potential for the BVS to help patients’ angina symptoms given that stent patients who return to the hospital are both difficult and expensive to treat. Regardless, the world awaits the ongoing data collected in ABSORB II and is looking forward to seeing data from ABSORB III (enrollment completed) and ABSORB IV (enrolling now).
Peripheral Arterial Disease
Excimer Laser Randomized Controlled Study for Treatment of Femoropopliteal In-stent Restenosis (EXCITE-ISR)
Eric J. Dippel, MD, of the Genesis Heart Institute in Davenport, IA, presented the findings from the EXCITE-ISR trial proving Spectranetics (Colorado Springs, CO) laser atherectomy devices are clinically superior to percutaneous transluminal angioplasty (PTA) alone in both safety and efficacy in the treatment of femoropopliteal in-stent restenosis. This prospective, randomized, multicenter clinical trial to evaluate the safety and efficacy of Spectranetics excimer laser atherectomy (ELA) system enrolled 250 patients in a 2:1 fashion to ELA plus PTA and PTA alone in 40 clinical sites in the United States. Endpoints were examined at 37 and 212 days. Follow-up points included discharge, 30 days postprocedure, 6 months postprocedure, and 1 year postprocedure.
The primary endpoints were divided into the primary safety and primary efficacy endpoints. The primary safety endpoint was major adverse events at 30 days: death, unplanned major amputation, and target lesion revascularization (TLR). The primary efficacy endpoint was freedom from clinically driven TLR at 6 months—duplex ultrasound binary restenosis, return of clinical symptoms, and deteriorated ankle brachial index or Rutherford Classification. The trial did not place a limit on lesion length and included multiple stents including popliteal stents. Key exclusion criteria included target lesions that extended > 3 cm beyond the stent margin, patients with untreated inflow lesions, and grade 4 or 5 stent fractures. Baseline characteristics between the two groups were similar, including mean lesion length (in both groups 20% of lesions were > 30 cm). Key findings included the following:
- Primary safety endpoint: major adverse event rates at 30 days 5.8% ELA + PTA group versus 20.5% with PTA alone (P < .001) (Figure 6)
- Primary efficacy endpoint, 73.5% freedom from TLR in ELA + PTA group at 6 months vs 51.8% with PTA alone (P < .005)
- ELA + PTA was associated with a 52% reduction in TLR (hazard ratio 0.48; 95% confidence interval, 0.31-0.74)
- Dissection rate of 7.7% in ELA + PTA group vs 17.2% with PTA alone (P = .03)
- Bailout stenting 4.1% in ELA + PTA group vs 11.1% with PTA alone (P = .05)
- There was no procedurally related stent damage observed in either cohort
- 93.5% procedural success rate using Turbo-Tandem (Spectranetics) with PTA vs 82.7% with PTA alone (P = .01)
ELA with adjunctive PTA treatment of in-stent restenosis is superior to PTA alone for treatment of femoropopliteal in-stent restenosis both in terms of safety (freedom from major adverse events) and efficacy (higher procedural success rate and significantly lower TLR rate). This is the first US Food and Drug Administration-approved investigational device exempted randomized controlled study demonstrating the benefits of laser atherectomy in the lower extremities. ![]()
References
- Daneault B, Kirtane AJ, Kodali SK, et al. Stroke associated with surgical and transcatheter treatment of aortic stenosis: a comprehensive review. J Am Coll Cardiol. 2011;58:2143-2150.
- Vermeer SE, Hollander M, van Dijk EJ, et al; Rotterdam Scan Study. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126-1129.
- Eggebrecht H, Schmermund A, Voigtländer T, et al. Risk of stroke after transcatheter aortic valve implantation (TAVI): a meta-analysis of 10,037 published patients. EuroIntervention. 2012;8:129-138.
- O’Riordan M. Embolic filter reduces cerebral lesions during TAVI. Medscape website. http://www.medscape.com/viewarticle/831673. Updated September 13, 2014. Accessed March 2, 2015.
- Fiedler KA, Byrne RA, Schulz S, et al. Rationale and design of The Intracoronary Stenting and Antithrombotic Regimen-Testing of a six-week versus a six-month clopidogrel treatment Regimen In Patients with concomitant aspirin and oraL anticoagulant therapy following drug-Eluting stenting (ISAR-TRIPLE) study. Am Heart J. 2014;167:459-465.e1.
- Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol. 2014;64:2086-2097.
- Serruys PW, Ormiston JA, Onuma Y, et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373:897-910.
- Capodanno D, Gori T, Nef H, et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention. 2015;10:1144-1153.