Heart Failure With Preserved Ejection Fraction: An Insight Into Its Prevalence, Predictors, and Implications of Early Detection
Muhammad Asrar ul Haq, FRACP,1-3 Chiew Wong, PhD, FRACP,1,2,4 David L. Hare, FRACP2,3
1Department of Cardiology, The Northern Hospital, Melbourne, Australia; 2Department of Medicine, University of Melbourne, Melbourne, Australia; 3Department of Cardiology, Austin Health, Melbourne, Australia; 4Department of Cardiology, Western Health, Melbourne, Australia
Heart failure with preserved ejection fraction (HFPEF) is common, and at least half of patients presenting with signs and symptoms of heart failure are found to have preserved left ventricular systolic function. They have high mortality and morbidity and exert a substantial impact on health care costs worldwide. A range of conditions has been shown to predispose individuals to development of diastolic dysfunction and HFPEF. Chronic hypertension is the most common cause; it has been suggested that up to 60% of patients with HFPEF are hypertensive. Coronary artery disease, obesity, and diabetes are some of the other common contributory factors. Early detection of asymptomatic patients identified as at risk of developing this syndrome has the potential to reduce the risk of subsequent heart failure; this may be of benefit to focus our attention on prevention and intervention strategies in this population.
[Rev Cardiovasc Med. 2015;16(1):20-27 doi: 10.3909/ricm0725]
© 2015 MedReviews®, LLC
Heart Failure With Preserved Ejection Fraction: An Insight Into Its Prevalence, Predictors, and Implications of Early Detection
Muhammad Asrar ul Haq, FRACP,1-3 Chiew Wong, PhD, FRACP,1,2,4 David L. Hare, FRACP2,3
1Department of Cardiology, The Northern Hospital, Melbourne, Australia; 2Department of Medicine, University of Melbourne, Melbourne, Australia; 3Department of Cardiology, Austin Health, Melbourne, Australia; 4Department of Cardiology, Western Health, Melbourne, Australia
Heart failure with preserved ejection fraction (HFPEF) is common, and at least half of patients presenting with signs and symptoms of heart failure are found to have preserved left ventricular systolic function. They have high mortality and morbidity and exert a substantial impact on health care costs worldwide. A range of conditions has been shown to predispose individuals to development of diastolic dysfunction and HFPEF. Chronic hypertension is the most common cause; it has been suggested that up to 60% of patients with HFPEF are hypertensive. Coronary artery disease, obesity, and diabetes are some of the other common contributory factors. Early detection of asymptomatic patients identified as at risk of developing this syndrome has the potential to reduce the risk of subsequent heart failure; this may be of benefit to focus our attention on prevention and intervention strategies in this population.
[Rev Cardiovasc Med. 2015;16(1):20-27 doi: 10.3909/ricm0725]
© 2015 MedReviews®, LLC
Heart Failure With Preserved Ejection Fraction: An Insight Into Its Prevalence, Predictors, and Implications of Early Detection
Muhammad Asrar ul Haq, FRACP,1-3 Chiew Wong, PhD, FRACP,1,2,4 David L. Hare, FRACP2,3
1Department of Cardiology, The Northern Hospital, Melbourne, Australia; 2Department of Medicine, University of Melbourne, Melbourne, Australia; 3Department of Cardiology, Austin Health, Melbourne, Australia; 4Department of Cardiology, Western Health, Melbourne, Australia
Heart failure with preserved ejection fraction (HFPEF) is common, and at least half of patients presenting with signs and symptoms of heart failure are found to have preserved left ventricular systolic function. They have high mortality and morbidity and exert a substantial impact on health care costs worldwide. A range of conditions has been shown to predispose individuals to development of diastolic dysfunction and HFPEF. Chronic hypertension is the most common cause; it has been suggested that up to 60% of patients with HFPEF are hypertensive. Coronary artery disease, obesity, and diabetes are some of the other common contributory factors. Early detection of asymptomatic patients identified as at risk of developing this syndrome has the potential to reduce the risk of subsequent heart failure; this may be of benefit to focus our attention on prevention and intervention strategies in this population.
[Rev Cardiovasc Med. 2015;16(1):20-27 doi: 10.3909/ricm0725]
© 2015 MedReviews®, LLC
KEY WORDS
Heart failure • Diastolic dysfunction • Heart failure with preserved ejection fraction • Heart failure with normal ejection fraction
KEY WORDS
Heart failure • Diastolic dysfunction • Heart failure with preserved ejection fraction • Heart failure with normal ejection fraction
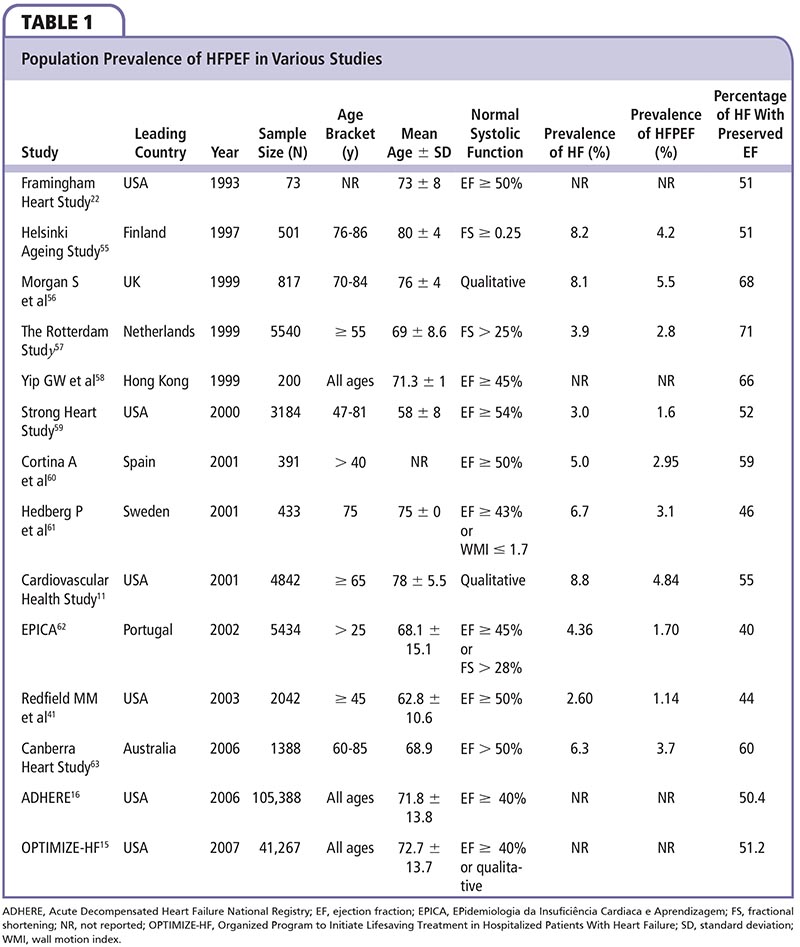
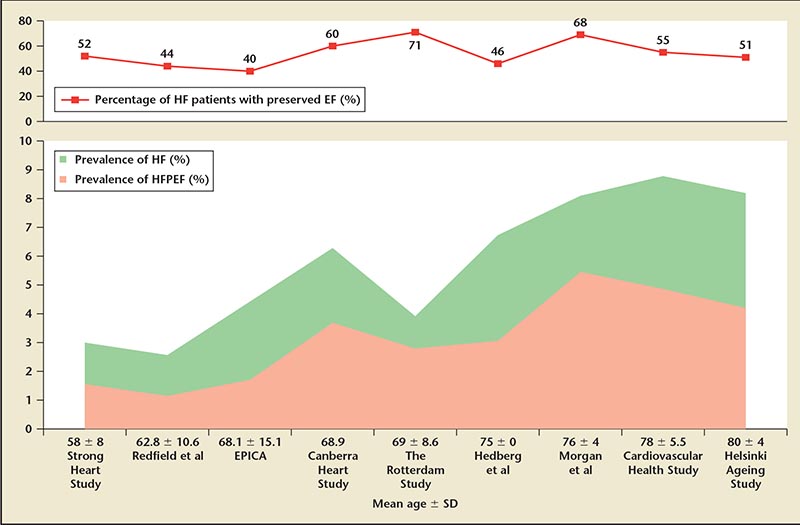
Figure 1. Reported prevalence of HF and HFPEF by age group. EF, ejection fraction; EPICA, EPidemiologia da Insuficiência Cardiaca e Aprendizagem; HF, heart failure; HFPEF, heart failure with preserved ejection fraction.
In patients with DHF, cardiovascular diseases (60%) are the leading cause of death…
Age is by far the strongest predictor of mortality in HFPEF.
Main Points
• Heart failure with preserved ejection fraction (HFPEF) is common; at least 50% of patients presenting with signs and symptoms of heart failure have preserved left ventricular systolic function. This segment of the heart failure population consists predominantly of women, the elderly, and people with hypertension and other cardiovascular risk factors.
• A range of conditions predisposes individuals to development of diastolic dysfunction and HFPEF. These include—but are not limited to—chronic hypertension, coronary artery disease, obesity, diabetes, hypertrophic or restrictive cardiomyopathies, and valvular heart disease.
• Early detection of asymptomatic patients identified as at risk of developing this syndrome has the potential to reduce the risk of subsequent heart failure.
Main Points
• Heart failure with preserved ejection fraction (HFPEF) is common; at least 50% of patients presenting with signs and symptoms of heart failure have preserved left ventricular systolic function. This segment of the heart failure population consists predominantly of women, the elderly, and people with hypertension and other cardiovascular risk factors.
• A range of conditions predisposes individuals to development of diastolic dysfunction and HFPEF. These include—but are not limited to—chronic hypertension, coronary artery disease, obesity, diabetes, hypertrophic or restrictive cardiomyopathies, and valvular heart disease.
• Early detection of asymptomatic patients identified as at risk of developing this syndrome has the potential to reduce the risk of subsequent heart failure.
Although the possibility of heart failure with normal systolic function has been recognized, it is only in the past 2 to 3 decades that a more detailed understanding of diastolic heart failure (DHF) syndrome has emerged, leading to insights into the mechanics of diastology and limitations of ejection fraction (EF). After large epidemiologic studies provided evidence for the existence of this type of heart failure, clinicians began to recognize presentations of pulmonary edema and other heart failure signs and symptoms without impaired systolic function. It is now well established that at least half of patients with heart failure have preserved EF.
The term diastolic heart failure, as opposed to systolic heart failure (SHF), was initially used to denote this syndrome. This term provides a clean distinction between the two phases of heart function, suggesting an impairment of either will lead to its respective type of heart failure. This provides a good initial understanding of the difference between pathophysiology and mechanics of SHF and DHF; however, in practice it is often not possible to differentiate between the two. Both SHF and DHF often coexist and overlap. This led to the use of the term heart failure with normal ejection fraction (HFNEF), which has been adopted in the most recent literature. This term encompasses a more complex process of the disease beyond diastolic and contractile functions of the heart, and classifies the disease between clinical rather than pathophysiologic entities. However, the definition of normal EF is variable, and different cutoffs have been used in different studies and various populations (Table 1); hence, the term heart failure with preserved ejection fraction (HFPEF) is now being promoted. Three obligatory conditions need to be satisfied to diagnose HFPEF: clinical signs and symptoms of heart failure, normal or mildly abnormal systolic function, and diastolic dysfunction.1
Interestingly, despite growing evidence of its significance and high prevalence, most existing management recommendations are based on information obtained from patients with reduced (HFREF) rather than preserved EF (HFPEF).2 To date, the results from various drug trials have not been very successful,3 highlighting the difference in pathophysiology and possibly the therapeutic targets to achieve similar clinical benefits as SHF.
How Common Is HFPEF?
Diastolic dysfunction has been increasingly recognized as a major factor in reduced exercise tolerance and symptoms of dyspnea,4-7 both in HFREF and HFPEF.8-10 Prevalence of diastolic dysfunction and HFPEF varies markedly depending on age, sex, and diagnostic criteria; however, there is enough evidence indicating that despite overlap between the two, and their interrelation in context of heart failure syndrome, DHF and SHF are equally distributed in the population, and they differ with regard to the pathologic mechanisms, sex, and age of those affected.11-13
Numerous studies have confirmed that at least half of patients with heart failure have preserved EF, and this segment of the heart failure population consists predominantly of women, the elderly, and people with hypertension and other cardiovascular risk factors (ie, people considered at risk of developing cardiovascular disease).11,13-16 The prevalence of HFPEF within the population varies from 1.14% to 5.5%, depending on age (Figure 1) and other variables, such as diagnostic criteria and methods; when younger age groups are excluded and studies are confined to a population ≥ 65 years, the prevalence rises to between 3.1% and 5.5% (Table 1).
Clinical Predictors and Risk Factors for HFPEF
A range of conditions predisposes individuals to development of diastolic dysfunction and HFPEF.1 Chronic hypertension is the most common cause; up to 60% of patients with HFPEF may be hypertensive.17 Coronary artery disease is another common cause of this condition. Obesity and diabetes also contribute independently to the development of diastolic dysfunction; other conditions associated with diastolic dysfunction are hypertrophic or restrictive cardiomyopathies, valvular heart disease (most commonly aortic stenosis and mitral regurgitation), and— rarely—restrictive cardiomyopathy.
Age
Age remains one of the most predictive factors of diastolic dysfunction. Age-related physiologic and pathologic changes likely contribute to the development of diastolic dysfunction.18 Left ventricular (LV) wall thickness increases, due to an increase in the size of cardiac myocytes and an increased deposition of collagen,19 leading to greater LV stiffness and reduced compliance. Left atrial size also increases with age in otherwise healthy people. Evidence suggests that the number of cardiac myocytes, which are in the postmitotic phase, are also reduced with aging.20 The remaining myocytes exhibit an alteration in both phenotype and function. Resulting hemodynamic changes involve a decline in maximal heart rate, cardiac output, and maximal oxygen consumption (VO2P). Systemic vascular resistance is increased with increased blood pressure and afterload.5,18,21
Hypertension
There is sufficient evidence suggesting that hypertension is one of the most important predictors of diastolic dysfunction, in addition to age and sex, and is one of the most commonly associated cardiovascular risk factors. Through a variety of mechanisms, including increased afterload, LV hypertrophy, myocardial fibrosis, and impaired diastolic filling, hypertension may lead to subsequent heart failure. Epidemiologic studies have consistently shown up to a threefold higher risk of developing HFPEF in people with hypertension.11,22 Diastolic dysfunction can occur in hypertensive patients with or without LV hypertrophy.23 This suggests that LV hypertrophy may be a part of the process or an aggravating factor but not necessarily the mechanism of hypertensive etiology of diastolic dysfunction.
Diabetes Mellitus
Diastolic dysfunction may be one of the early signs of diabetic cardiomyopathy.24 Zabalgoitia and colleagues25 studied the prevalence of diastolic dysfunction in 86 normotensive patients with well-controlled type 2 diabetes who were asymptomatic for ischemic heart disease or heart failure and reported that 47% of subjects had diastolic dysfunction, and all had preserved EF. This important study demonstrated the high prevalence of diastolic abnormalities in people with diabetes at a young age despite satisfactory apparent control of the disease. Similar results were reported by the Strong Heart Study.26
Changes in myocardial metabolism of glucose and fatty acids may be responsible for structural changes in myocardium, including myocyte hypertrophy, increased collagen deposition, interstitial fibrosis, and intramyocardial microangiopathy.26 All these changes lead to diastolic dysfunction and HFPEF.
Obesity
Increased body mass index (BMI) is associated with diastolic abnormalities.27,28 Powell and colleagues29 reported that obesity was associated with LV remodeling and increased LV end-diastolic pressure but not with EF. On echocardiography, a higher BMI was associated with increased LV mass, LV wall thickness, and LV end-diastolic diameter. These changes may be in response to the increased stroke volume required for higher metabolic demands and indicate an association of obesity with HFPEF.
Pulmonary Disease
Chronic pulmonary diseases with chronic hypoxia, such as obstructive sleep apnea, obesity hypoventilation syndrome, or chronic obstructive pulmonary disease, are not only associated with pulmonary hypertension and right ventricular dysfunction secondary to increased right ventricular afterload,30,31 but can also lead to diastolic dysfunction. There are studies suggesting that intrinsic mechanisms are responsible for diastolic dysfunction associated with chronic hypoxia, in addition to the mechanical effect of interventricular septal bulge secondary to increased right ventricular pressure.32,33 The exact mechanisms leading to DHF in people with obstructive sleep apnea are not clear. It is possible that increased nocturnal blood pressure and sympathetic activity contribute to the underlying mechanism.34,35
Why Is Early Detection Important?
It is now well established that the morbidity and mortality associated with HFPEF is much higher than in the healthy population.36 Several studies have reported an annual mortality rate ranging from 5% to 8%,4,5,37 which is much higher than that of age-matched control subjects.38-40 Some studies have reported a 1-year hospital readmission rate approaching 50%.4,5,41 In patients with DHF, cardiovascular diseases (60%) are the leading cause of death, including sudden cardiac death (26%), heart failure (15%), myocardial infarction (5%), and stroke (9%), followed by noncardiovascular (30%) and unknown causes (10%).42
The etiology of HFPEF plays a major role in its prognosis and associated mortality. Commonly associated comorbidities, such as hypertension, diabetes, and obesity, and their management, have the potential to affect clinical outcomes. Given the accumulated data of various studies, it appears that all-cause mortality of heart failure patients in the community is similar whether or not their EF is above or below 50%. Likewise, all-cause annual mortality for HFPEF or HFREF patients admitted with acute decompensated heart failure is similar, and very high. It is likely that older age and related multiple noncardiovascular comorbidities in people with HFPEF contribute disproportionately to their high all-cause mortality, although this remains unconfirmed.
Development of Symptoms in Asymptomatic Diastolic Dysfunction
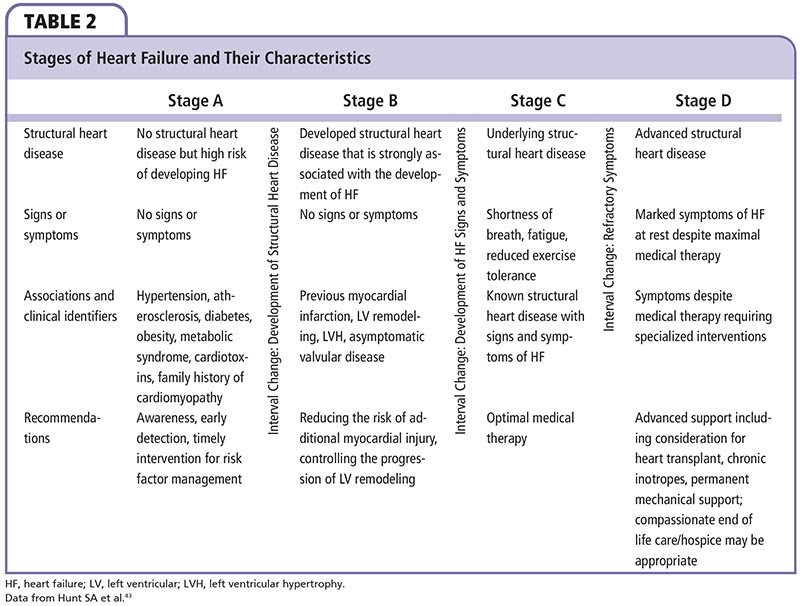
The American Heart Association/ American College of Cardiology (AHA/ACC) classification of heart failure defines stage B as patients with abnormal heart structure and/ or function without clinical symptoms.43 This stage of heart failure is also termed asymptomatic or preclinical diastolic dysfunction (PDD) that may progress to symptomatic heart failure. The evidence of abnormal heart structure or function can be confirmed using complementary methods such as echocardiography, and is associated with increased risk of adverse cardiovascular events, including atrial fibrillation (AF).44,45
Correa de Sa and associates46 have analyzed the echocardiographic data of 82 patients with moderate or severe diastolic dysfunction and preserved EF by Doppler criteria using the echocardiographic database, and have suggested a moderate degree of progression to development of symptoms and cardiac hospitalizations over 2 years in this patient population. The beginning of follow-up was defined as first echocardiogram with EF > 50% and moderate or severe diastolic dysfunction. The primary endpoint was the time to the development of (1) heart failure according to the Framingham criteria or (2) any symptoms of dyspnea, edema, or fatigue that was a chief complaint and not explained by another medical condition. The secondary end-point was cardiac hospitalization. Mean follow-up was 721 days. The authors found a cumulative probability of development of heart failure according to the Framingham criteria was 1.9%; however, a cumulative probability of development of any symptoms was 31.1%. The cumulative probability for cardiac hospitalization was 21.2%. It was also noted that peripheral vascular disease and hypertension were independently associated with increased likelihood for the development of symptoms.
Clinical Predictors of Poor Prognosis
Age is by far the strongest predictor of mortality in HFPEF.5,12,47 In patients over age 70, mortality rates for DHF approach those of SHF5; the approximate 5-year mortality rate in patients who were < 50 years was reported to be 15%, for those age 50 to 70 years was 33%, and for those > 70 years was 50%.
O'Connor and associates12 prospectively evaluated 2498 consecutive patients with HFPEF and found the independent predictors of mortality using a multivariable Cox proportional hazard model were as follows: (1) age, (2) New York Heart Association class IV symptoms, (3) EF, (4) coronary artery disease index, (5) diabetes, (6) peripheral vascular disease, and (7) minority ethnic group.
In another study, the Digitalis Investigation Group47 employed survival analyses to identify the predictors of mortality in 988 patients with HFPEF (EF > 45%) and recognized older age and male sex as important predictors of death. The overall 3-year mortality rate in this primarily ambulatory population was 23%. Among the 18 possible clinical predictors, the strongest independent predictors of death in this study included (1) glomerular filtration rate (hazard ratio [HR] 1.50; 95% confidence interval [CI], 1.35-1.67; P < .0001), (2) New York Heart Association functional class III or IV (HR 1.64; 95% CI, 1.20-2.18; P = .0011), (3) male sex (HR 1.71; 95% CI, 1.26-2.32; P = .0005), and (4) older age (HR 1.28; 95% CI, 1.08-1.50; P = .0019).
Atrial Fibrillation
AF is common in HFPEF patients, with a prevalence of up to 41%.48 Presence and severity of diastolic dysfunction are independently predictive of first documented non-valvular AF in the elderly.49 The prognostic implications of loss of atrial-kick effect in people with HFPEF and chronic AF remain controversial.
A meta-analysis of 16 studies for the prognostic significance of AF in heart failure involving 53,969 patients suggested that the presence of AF was associated with an adverse prognosis in heart failure irrespective of LV systolic function.50
Anemia
Anemia is an independent predictor of survival in patients with DHF.51-53 The exact underlying mechanisms causing anemia in patients with heart failure are not well understood. Some of the proposed etiologic mechanisms include hemodilution causing pseudoanemia, defective iron utilization, renal dysfunction, insufficient erythropoietin production, and anemia of chronic disease.54 Future studies are warranted in order to evaluate the potential benefit of aggressively managing treatable anemia in patients who have DHF. Management options include etiology and a symptom-based approach, such as iron supplementation for iron deficiency, transfusions for myelofibrosis, and recombinant erythropoietin therapy for patients who have refractory anemia and DHF.
Implications of Early Detection
The recent AHA/ACC guidelines43 classify heart failure into four stages based on structural abnormality and damage to the heart muscle (Table 2):
Stage A: At high risk for developing heart failure. No identified structural or functional abnormality; no signs or symptoms.
Stage B: Developed structural heart disease that is strongly associated with the development of heart failure, but without signs or symptoms.
Stage C: Symptomatic heart failure associated with underlying structural heart disease.
Stage D: Advanced structural heart disease and marked symptoms of heart failure at rest despite maximal medical therapy.
The above-mentioned conditions associated with an increased risk of structural heart disease can be identified before patients show any evidence of structural abnormalities. These patients with risk factors are labeled as high-risk for HFPEF. Early detection and modification of many of these factors have the potential to reduce the risk of heart failure. Appropriate medical interventions to these patients (Stage A) can be the first step to reduce the impact of heart failure on the community. Asymptomatic patients with ventricular dilatation and reduced EF carry a substantially higher risk for subsequent morbidity and mortality than the general population; however, there are no data available looking specifically at people with high risk of diastolic dysfunction. It would be beneficial to evaluate the risk of their subsequent development of diastolic dysfunction and form strategies to identify such patients early by aggressive screening for appropriate and timely intervention. However, a routine periodic echocardiographic assessment of these patients has not been recommended at this stage.
Similarly, Stage B (PDD) patients who don't have heart failure symptoms but have evidence of diastolic dysfunction are at considerable risk for developing HFPEF. In patients with no symptoms but with evidence of structural heart disease, the incidence of heart failure can be decreased by reducing the risk of additional injury and by controlling the progression of LV remodeling.3
Conclusions
HFPEF is common and at least half of patients with heart failure have preserved EF. They also have high mortality and morbidity. Asymptomatic patients identified as at risk of developing this syndrome may be a population on whom to target our prevention and intervention strategies. ![]()
The authors report no real or apparent conflicts of interest.
References
- Asrar ul Haq M, Wong C. Heart failure with preserved myocardial contractility: understanding the pathophysiology. Eur J Health. 2013(2013):Article ID 6.
- Asrar Ul Haq M, Wong C, Levinger I, et al. Effect of exercise training on left ventricular remodeling in diabetic patients with diastolic dysfunction: rationale and design. Clin Med Insights Cardiol. 2014;8:23-28.
- Asrar ul Haq M, Wong C, Mutha V, et al. Therapeutic interventions for heart failure with preserved ejection fraction: a summary of current evidence. World J Cardiol. 2014;6:67-76.
- Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part II: causal mechanisms and treatment. Circulation. 2002;105:1503-1508.
- Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387-1393.
- Becker M, Bilke E, Kühl H, et al. Analysis of myocardial deformation based on pixel tracking in two dimensional echocardiographic images enables quantitative assessment of regional left ventricular function. Heart. 2006;92:1102-1108.
- Notomi Y, Martin-Miklovic MG, Oryszak SJ, et al. Enhanced ventricular untwisting during exercise: a mechanistic manifestation of elastic recoil described by Doppler tissue imaging. Circulation. 2006;113:2524-2533.
- Skaluba SJ, Litwin SE. Mechanisms of exercise intolerance: insights from tissue Doppler imaging. Circulation. 2004; 109:972-977.
- Hadano Y, Murata K, Yamamoto T, et al. Usefulness of mitral annular velocity in predicting exercise tolerance in patients with impaired left ventricular systolic function. Am J Cardiol. 2006;97:1025-1028.
- Asrar ul Haq M, Mutha V, Lin T, et al. Left ventricular torsional dynamics post exercise for LV diastolic function assessment. Cardiovasc Ultrasound. 2014;12:8.
- Kitzman DW, Gardin JM, Gottdiener JS, et al. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413-419.
- O’Connor CM, Gattis WA, Shaw L, et al. Clinical characteristics and long-term outcomes of patients with heart failure and preserved systolic function. Am J Cardiol. 2000;86:863-867.
- Smith GL, Masoudi FA, Vaccarino V, et al. Outcomes in heart failure patients with preserved ejection fraction: mortality, readmission, and functional decline. J Am Coll Cardiol. 2003;41:1510-1518.
- Fischer M, Baessler A, Hense HW, et al. Prevalence of left ventricular diastolic dysfunction in the community. Results from a Doppler echocardiographic-based survey of a population sample. Eur Heart J. 2003;24:320-328.
- Fonarow GC, Stough WG, Abraham WT, et al; OPTIMIZE- HF Investigators and Hospitals. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768-777.
- Yancy CW, Lopatin M, Stevenson LW, et al; ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76-84.
- Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470-479.
- Lakatta EG. Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev. 2002;7:29-49.
- Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560-1568.
- Anversa P, Palackal T, Sonnenblick EH, et al. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res. 1990;67: 871-885.
- Kitzman DW, Daniel KR. Diastolic heart failure in the elderly. Clin Geriatr Med. 2007;23:83-106.
- Vasan RS, Larson MG, Benjamin EJ, et al. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948-1955.
- Phillips RA, Goldman ME, Ardeljan M, et al. Determinants of abnormal left ventricular filling in early hypertension. J Am Coll Cardiol. 1989;14:979-985.
- Asrar ul Haq M, Mutha V, Rudd N, Wong C. Diabetic cardiomyopathy—what do we know about it? World J Cardiovasc Dis. 2013;3:26-32.
- Zabalgoitia M, Ismaeil MF, Anderson L, Maklady FA. Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled type 2 diabetes mellitus. Am J Cardiol. 2001;87: 320-323.
- Liu JE, Robbins DC, Palmieri V, et al. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol. 2003;41:2022-2028.
- Iribarren C, Karter AJ, Go AS, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001;103:2668-2673.
- Hardin NJ. The myocardial and vascular pathology of diabetic cardiomyopathy. Coron Artery Dis. 1996;7:99-108.
- Powell BD, Redfield MM, Bybee KA, et al. Association of obesity with left ventricular remodeling and diastolic dysfunction in patients without coronary artery disease. Am J Cardiol. 2006;98:116-120.
- Barberà JA, Peinado VI, Santos S. Pulmonary hypertension in chronic obstructive pulmonary disease. Eur Respir J. 2003;21:892-905.
- Baum GL, Schwartz A, Llamas R, Castillo C. Left ventricular function in chronic obstructive lung disease. N Engl J Med. 1971;285:361-365.
- Schena M, Clini E, Errera D, Quadri A. Echo-Doppler evaluation of left ventricular impairment in chronic corpulmonale. Chest. 1996;109:1446-1451.
- Gomez A, Mink S. Increased left ventricular stiffness impairs filling in dogs with pulmonary emphysema in respiratory failure. J Clin Invest. 1986;78: 228-240.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897-1904.
- Bradley TD, Hall MJ, Ando S, Floras JS. Hemodynamic effects of simulated obstructive apneas in humans with and without heart failure. Chest. 2001;119: 1827-1835.
- Ryan TJ, Antman EM, Brooks NH, et al. 1999 update: ACC/AHA guidelines for the management of patients with acute myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction). J Am Coll Cardiol. 1999;34:890-911.
- Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282-2289.
- Cohn JN, Johnson G. Heart failure with normal ejection fraction. The V-HeFT Study. Veterans Administration Cooperative Study Group. Circulation. 1990;81(2 suppl):III48-III53.
- Judge KW, Pawitan Y, Caldwell J, et al. Congestive heart failure symptoms in patients with preserved left ventricular systolic function: analysis of the CASS registry. J Am Coll Cardiol. 1991;18:377-382.
- McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285: 1441-1446.
- Redfield MM, Jacobsen SJ, Burnett JC Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194-202.
- Zile MR, Gaasch WH, Anand IS, et al; I-Preserve Investigators. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. 2010;121:1393-1405.
- Hunt SA; American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2005;46:e1-e82.
- Takemoto Y1, Barnes ME, Seward JB, et al. Usefulness of left atrial volume in predicting first congestive heart failure in patients > or = 65 years of age with well-preserved left ventricular systolic function. Am J Cardiol. 2005;96:832-836.
- Tsang TS, Barnes ME, Gersh BJ, et al. Prediction of risk for first age-related cardiovascular events in an elderly population: the incremental value of echocardiography. J Am Coll Cardiol. 2003;42:1199-1205.
- Correa de Sa DD, Hodge DO, Slusser JP, et al. Progression of preclinical diastolic dysfunction to the development of symptoms. Heart. 2010;96: 528-532.
- Jones RC, Francis GS, Lauer MS. Predictors of mortality in patients with heart failure and preserved systolic function in the Digitalis Investigation Group trial. J Am Coll Cardiol. 2004;44:1025-1029.
- Olsson LG, Swedberg K, Ducharme A, et al; CHARM Investigators. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol. 2006;47:1997-2004.
- Tsang TS, Gersh BJ, Appleton CP, et al. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol. 2002;40:1636-1644.
- Mamas MA, Caldwell JC, Chacko S, et al. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail. 2009;11:676-683.
- O’Meara E, Clayton T, McEntegart MB, et al; CHARM Committees and Investigators. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation. 2006;113:986-994.
- Felker GM, Shaw LK, Stough WG, O’Connor CM. Anemia in patients with heart failure and preserved systolic function. Am Heart J. 2006;151:457-462.
- Latado AL, Passos LC, Darzé ES, Lopes AA. Comparison of the effect of anemia on in-hospital mortality in patients with versus without preserved left ventricular ejection fraction. Am J Cardiol. 2006;98:1631-1634.
- Anand IS. Anemia and chronic heart failure implications and treatment options. J Am Coll Cardiol. 2008;52:501-511.
- Kupari M, Lindroos M, Iivanainen AM, et al. Congestive heart failure in old age: prevalence, mechanisms and 4-year prognosis in the Helsinki Ageing Study. J Intern Med. 1997;241:387-394.
- Morgan S, Smith H, Simpson I, et al. Prevalence and clinical characteristics of left ventricular dysfunction among elderly patients in general practice setting: cross sectional survey. BMJ. 1999;318:368-372.
- Mosterd A, Hoes AW, de Bruyne MC, et al. Prevalence of heart failure and left ventricular dysfunction in the general population; The Rotterdam Study. Eur Heart J. 1999;20:447-455.
- Yip GW, Ho PP, Woo KS, Sanderson JE. Comparison of frequencies of left ventricular systolic and diastolic heart failure in Chinese living in Hong Kong. Am J Cardiol. 1999;84:563-567.
- Devereux RB, Roman MJ, Liu JE, et al. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the Strong Heart Study. Am J Cardiol. 2000;86:1090-1096.
- Cortina A, Reguero J, Segovia E, et al. Prevalence of heart failure in Asturias (a region in the north of Spain). Am J Cardiol. 2001;87:1417-1419.
- Hedberg P, Lönnberg I, Jonason T, et al. Left ventricular systolic dysfunction in 75-year-old men and women; a population-based study. Eur Heart J. 2001;22:676-683.
- Ceia F, Fonseca C, Mota T, et al. Prevalence of chronic heart failure in Southwestern Europe: the EPICA study. Eur J Heart Fail. 2002;4:531-539.
- Abhayaratna WP, Smith WT, Becker NG, et al. Prevalence of heart failure and systolic ventricular dysfunction in older Australians: the Canberra Heart Study. Med J Aust. 2006;184:151-154.