Effect of Polypropylene Contamination on Weld Strength of Recycled Polyamide 6
Previous Article Next Article
By Hesam Ghasemi¹, Philip J. Bates¹, Amin Mirzadeh², Ying Zhang¹, and Musa R. Kamal²
¹Department of Chemistry and Chemical Engineering, Royal Military College, Kingston, Ontario, Canada ²Chemical Engineering Department, McGill University, Montreal, Québec, Canada
Effect of Polypropylene Contamination on Weld Strength of Recycled Polyamide 6
Previous Article Next Article
By Hesam Ghasemi¹, Philip J. Bates¹, Amin Mirzadeh², Ying Zhang¹, and Musa R. Kamal²
¹Department of Chemistry and Chemical Engineering, Royal Military College, Kingston, Ontario, Canada ²Chemical Engineering Department, McGill University, Montreal, Québec, Canada
Effect of Polypropylene Contamination on Weld Strength of Recycled Polyamide 6
Previous Article Next Article
By Hesam Ghasemi¹, Philip J. Bates¹, Amin Mirzadeh², Ying Zhang¹, and Musa R. Kamal²
¹Department of Chemistry and Chemical Engineering, Royal Military College, Kingston, Ontario, Canada ²Chemical Engineering Department, McGill University, Montreal, Québec, Canada
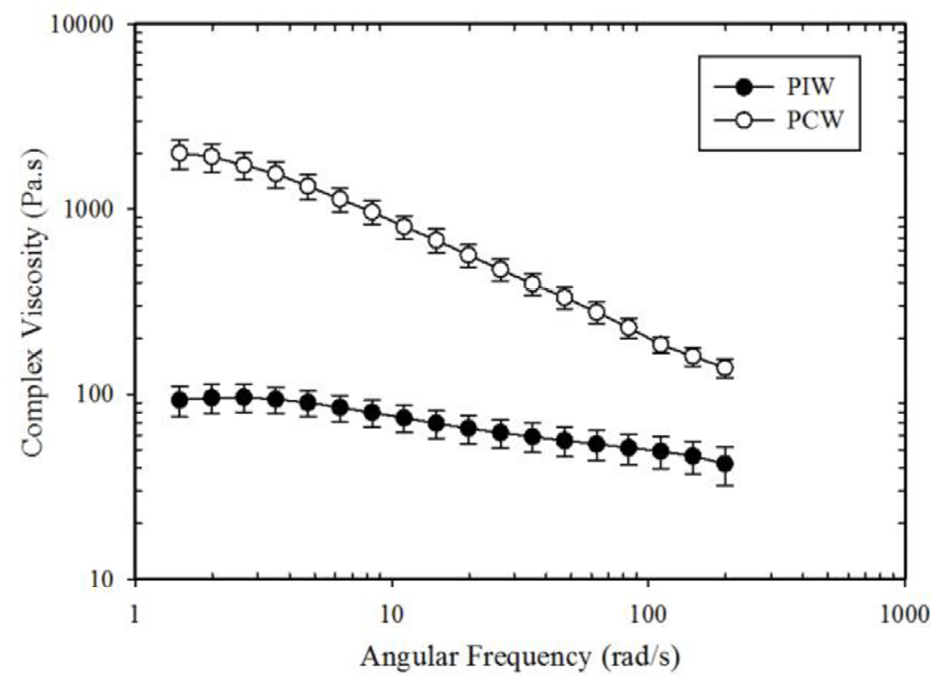
Figure 1: Melt viscosity of PIW and PCW.

Figure 1: Melt viscosity of PIW and PCW.
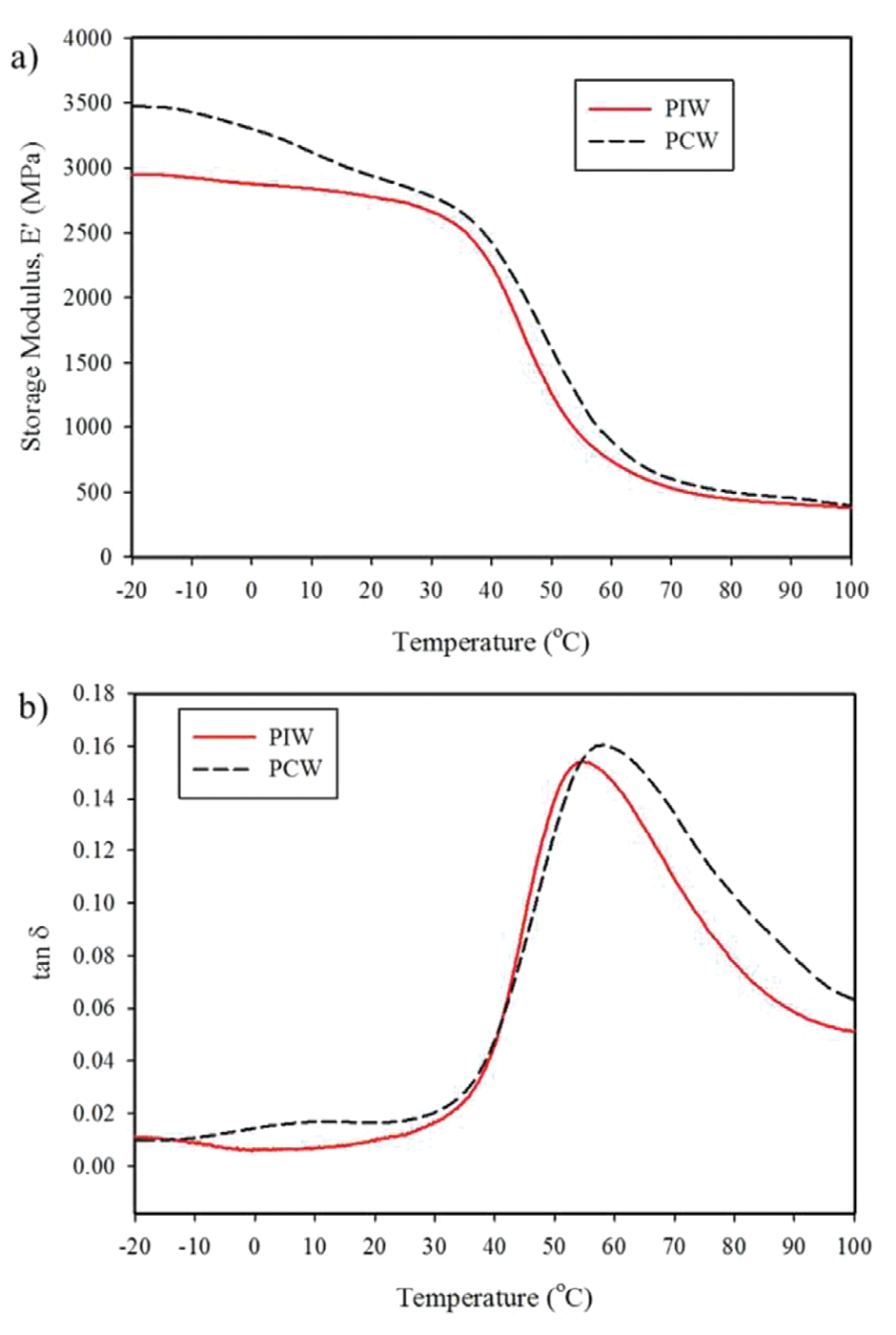
Figure 2: (a) Storage modulus vs. temperature and (b) tan δ of PIW and PCW polyamide 6.

Figure 2: (a) Storage modulus vs. temperature and (b) tan δ of PIW and PCW polyamide 6.
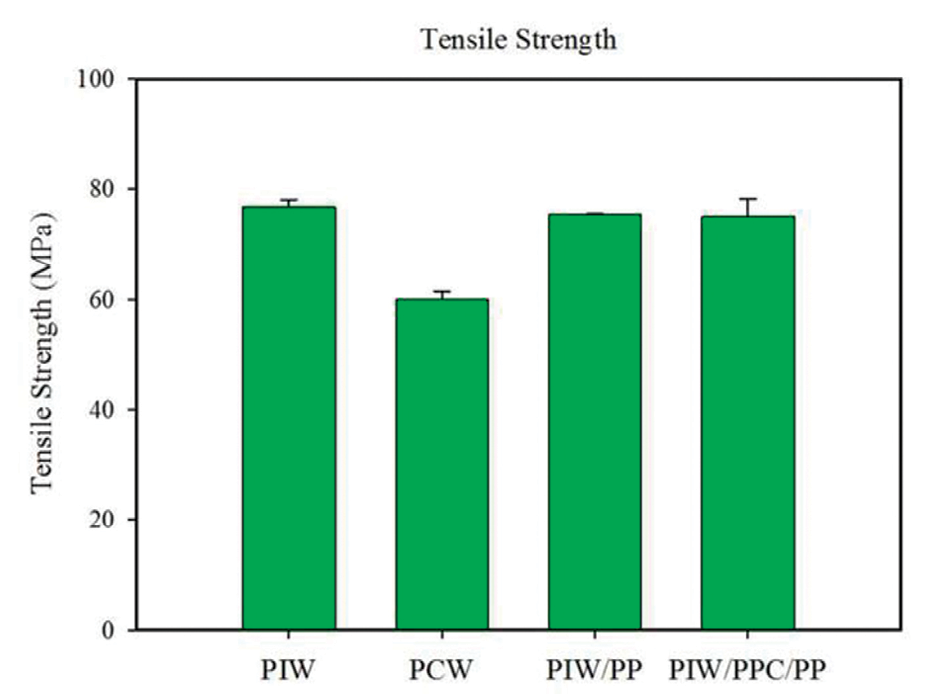
Figure 3: Tensile strength of PIW and PCW and model compounds.
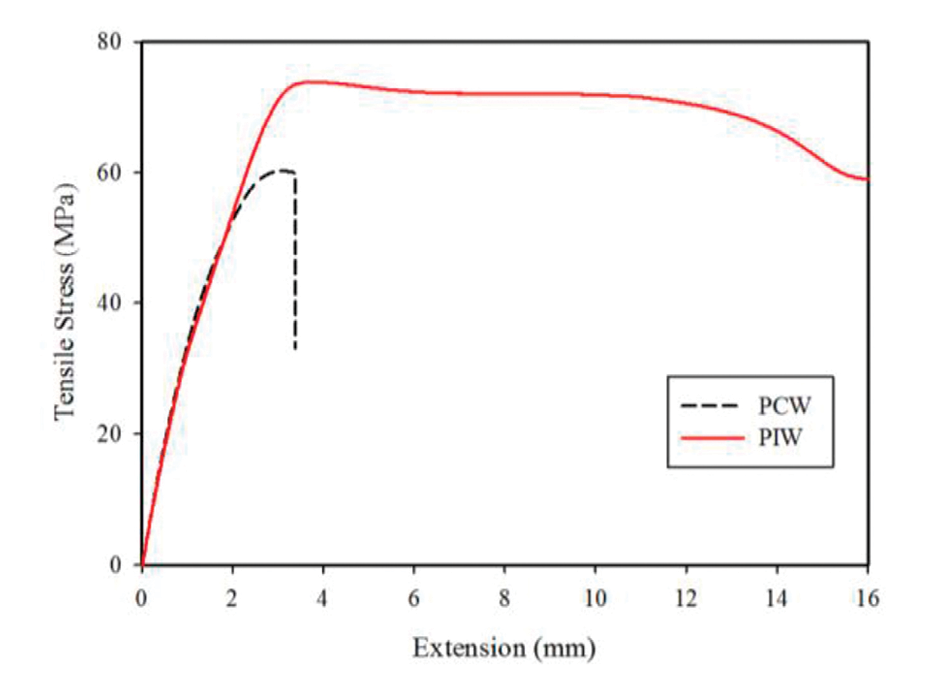
Figure 4: Tensile stress vs. extension plot of PIW and PCW.
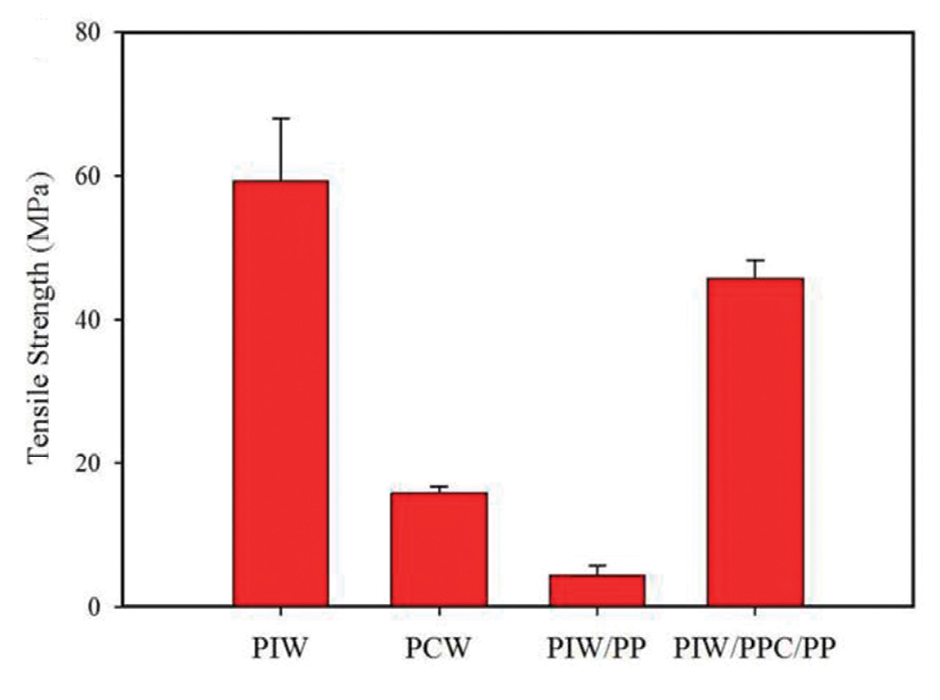
Figure 5: Weld strength of PIW and PCW and model compounds.
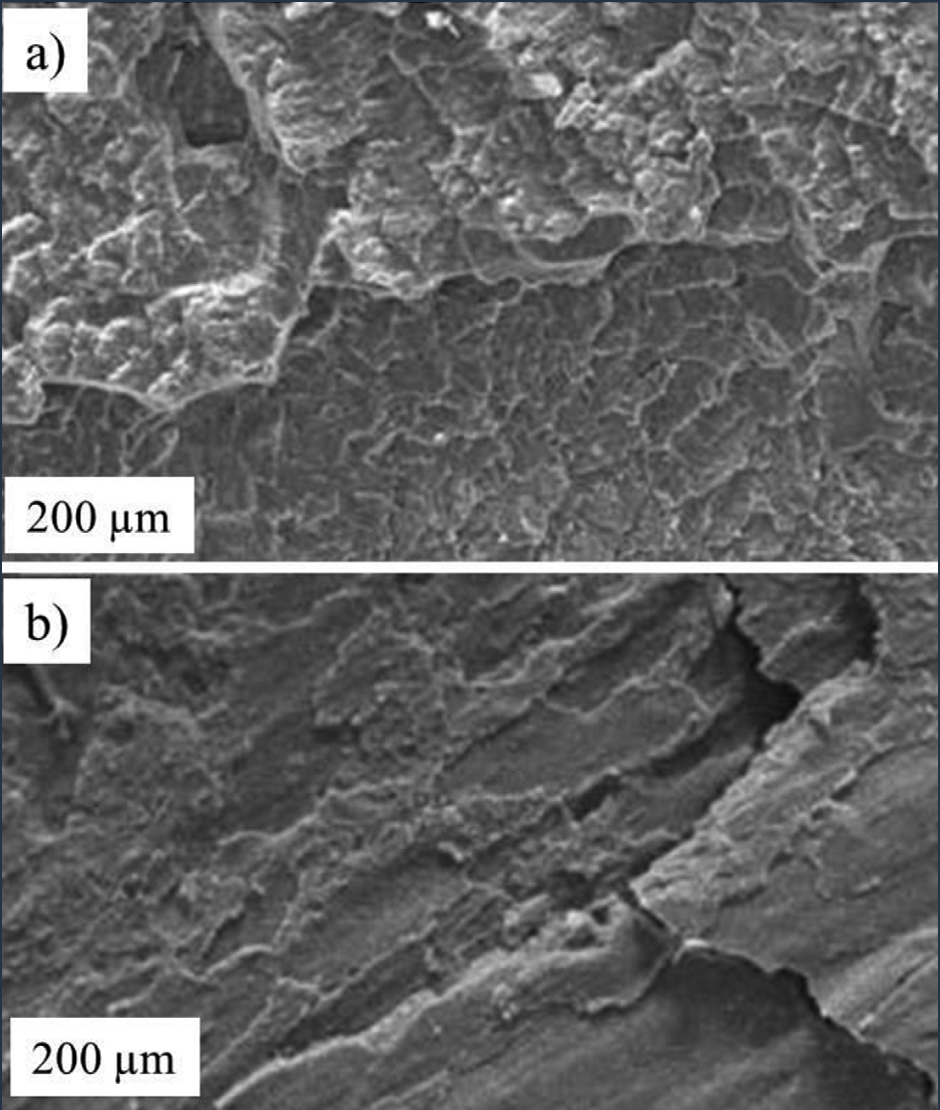
Figure 6: SEM images of weld fracture surface of (a) PIW and (b) PCW.
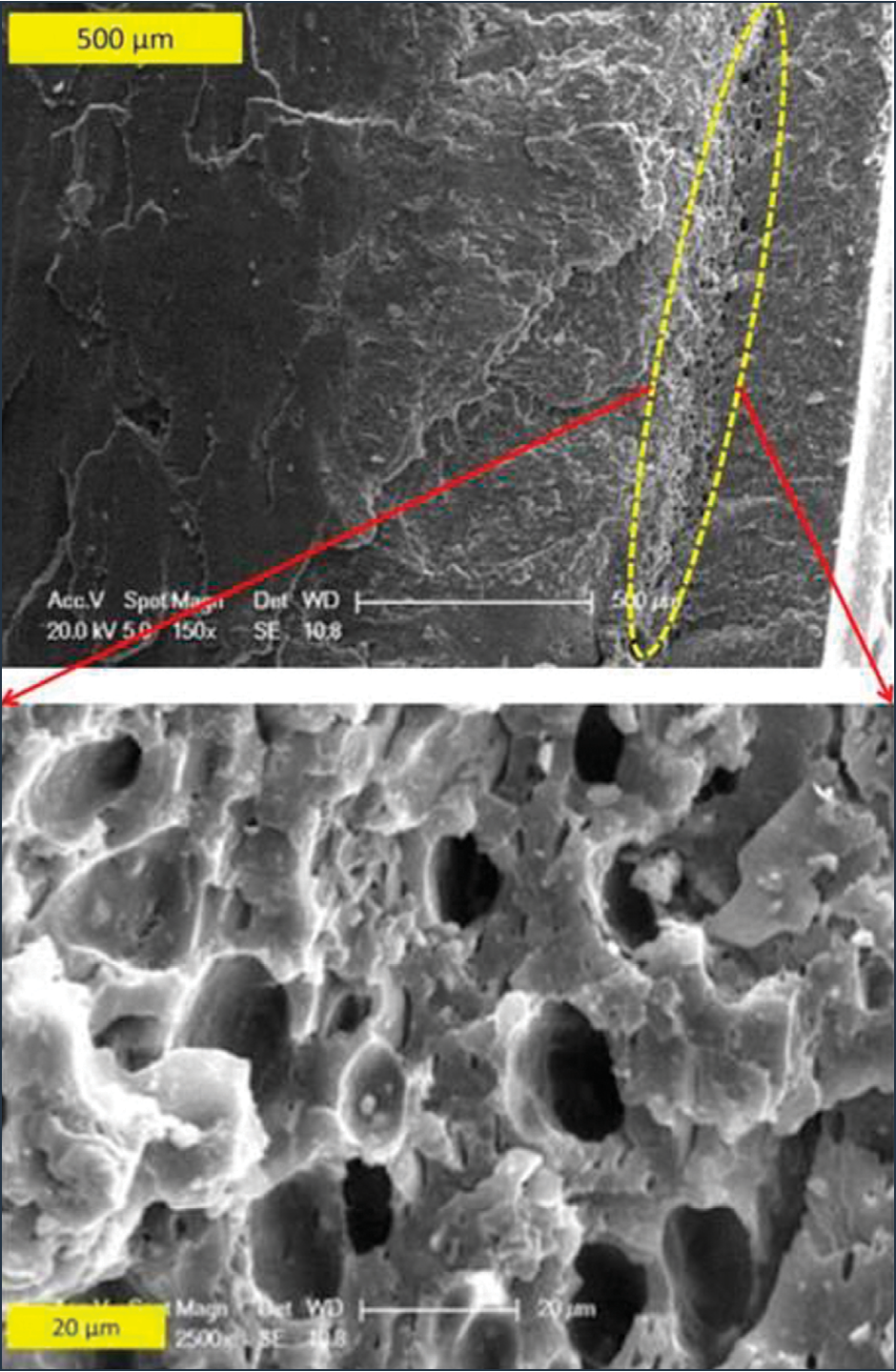
Figure 7: SEM images of the PCW weld fracture surface after etching in boiling xylenes..
Ed. Note: This paper won the SPE Joining of Plastics and Composites Special Interest Group’s best paper award, sponsored by Branson Ultrasonics Corp., at ANTEC® Orlando 2015.
Abstract: Two different recycled polyamide 6 (PA6) resins were used in this study: post-industrial waste (PIW) PA6 obtained from a fiber manufacturer and post-consumer waste (PCW) PA6 recycled from used carpets. Differential scanning calorimetry (DSC) and dynamic mechanical analysis (DMA) proved the presence of polypropylene (PP) in the PCW. Moreover, thermal gravimetric analysis (TGA) showed that the PCW contained approximately ten times more ash content than the PIW. The PP and inorganic contaminations of PCW come from the PP carpet backing and calcium carbonate-filled latex binder, respectively.1 Due to higher inorganic filler content, PCW exhibited higher melt viscosity and also higher storage modulus than PIW. Tensile tests were performed on dog-bone specimens cut from injection molded plaques: PIW displayed approximately 20% higher tensile strength than PCW. A 70% drop in PCW vibration weld strength was observed; this is attributed to its PP contamination.
Introduction
A significant amount of waste is collected each year during the manufacturing of PA6 fibers and also from used carpets. Disposal of these waste streams into landfills has negative environmental impacts. Public awareness and legislation, and also economic and environmental incentives, have encouraged the use of waste PA6 fiber streams as possible resources to produce new products.2,3 PIW and PCW PA6 are usually obtained by mechanical recycling, which involves separation, grinding, re-melting, and processing of waste streams.4 It results in a homogenous waste product for further downstream processes such as compounding and injection molding.
One of the processes that recycled material can be used in is vibration welding. Vibration welding is a practical and useful method to join injection molded articles and form complex hollow assemblies such as air intake manifolds.5 The process does not require an external heating source, adhesives, or fastening. Two parts are brought together under pressure. One component is oscillated laterally at frequencies of 100-300 Hz over amplitudes of 1-2 mm. The interface heats up to the melting/softening temperature by Coulomb friction. Further heating occurs by viscous dissipation of polymer in a melt film between the two parts. Molten polymer is squeezed out of the film by the applied pressure, causing meltdown to occur. The vibration is stopped once a desired meltdown is reached.6 Meltdown depth, frequency, and amplitude have negligible effects on weld strength, while weld pressure plays the key role on the microstructure and mechanical performance of the welded joints.7
Previously, the thermal properties8 and the effect of polyamide 66 on mechanical properties9 of recycled PA6 have been studied. In this research, the mechanical properties of PIW and PCW are compared, and the effect of PP contamination on vibration weld strength of PCW is investigated.
Experimental
PIW and PCW compounds were generously provided by Mahle Filter Systems Canada, ULC. A carpet backing grade of PP (Pinnacle PP 1703, melt flow rate 3.5 g/10 min) was kindly supplied by Pinnacle Polymers. A maleic anhydride-grafted PP (Bondyram® 1101 from Polyram USA) was used as a compatibilizer and is henceforth referred to as PPC.
Two model compounds (PIW/PP and PIW/PPC/PP) were made in a co-rotating twin screw extruder (ZE25 from KraussMaffei Berstorff). The extruder had a 25-mm screw diameter and L/D of 30. The extrusion was carried out at 100 RPM and a temperature profile of 240, 265, 280, 285, 285, and 285°C (from feeder to die). PP concentration in both model compounds was kept at 3 wt%, while 2 wt% PPC was added into the second model compound.
All compounds were injection molded into a series of 100 x 100 x 3.2 mm (length x width x thickness) plaques using an Engel 55-ton press. Molding was conducted under a nozzle-to-feeder temperature profile of 280, 280, 285, and 275°C. The mold temperature was 35°C, and the injection, hold, and cooling times were 1, 10, and 20 sec, respectively. Injection speed and hold pressure were 50 mm/sec and 8 MPa, respectively.
Injection molded plaques were cut parallel to the flow direction during filling, in order to obtain a series of 60 x 100 x 3.2 mm long specimens. These specimens were then butt welded along the 100 x 3.2 mm face, using a Branson Mini II linear vibration welder. The target meltdown, weld pressure, frequency, and peak-to-peak amplitude were 1.5 mm, 2 MPa, 211.8 Hz, and 1.78 mm, respectively.
A series of analyses was performed on the unwelded and welded materials:
- The ash content of PCW and PIW was determined using a furnace at 800°C.
- Rheological measurements were carried out at 280°C using a stress-controlled Bohlin Gemini HR rheometer (Malvern Instruments) with 25-mm diameter parallel plate geometry under nitrogen atmosphere.
- Three-point bending dynamic mechanical analysis (DMA-Q800, TA Instruments) was performed using a 58 x 14 x 3.2 mm specimen cut from the middle of an injection-molded plaque. Temperature sweep tests were performed at 1 Hz frequency and 20 μm amplitude from -20 to 100°C.
- A Perkin Elmer CHNS analyzer (series II, 2400) was used for elemental analysis of the PCW.
- The tensile and vibration weld strengths were measured at a 5 mm/min extension rate at room temperature, using an Instron universal testing machine. Dog-bone pieces were cut from an injection-molded plaque along the flow direction to measure tensile strength, and from a welded specimen to obtain weld strength. The gauge distance was 25 mm. The dog-bone specimens had a gauge cross-section of 16 x 3.2 mm.
- The weld fracture surfaces of the PIW and PCW were coated with gold and observed using a scanning electron microscope (SEM; a Philips-XL30).
Results and Discussion
Ash content measurements revealed that the PCW and PIW had approximately 6 wt% and 0.5 wt% inorganic content respectively. The higher ash content of PCW is believed to be from the calcium carbonate-filled latex binder between PA6 fibers and PP backing.
Figure 1 shows the melt viscosity of the PIW and PCW compounds. The viscosity of PCW at low shear rates is observed to be over an order of magnitude higher than that of PIW. Previous studies have confirmed that the molecular weights of these two compounds are similar.8 This difference is therefore likely due to the higher inorganic contamination of PCW.
Figure 2(a) shows the storage modulus (E’) for both compounds as a function of temperature. PCW had higher storage modulus than PIW. Again this is attributed to the calcium carbonate (CaCO3) content of PCW which comes from carpet backing during the recycling process.
The tan δ data are presented in Figure 2(b). The small peak in the region of -10 to 10°C is likely related to the PP contamination of PCW. The presence of PP in PCW was also confirmed in DSC results for PCW (data not shown). As seen in Figure 2(b), PCW had a slightly higher Tg than PIW. This is also attributed to the higher inorganic contamination of PCW.10
To evaluate an approximate PP content of PCW, CHNO elemental analysis was run on a small quantity of PCW. It was found that PCW contained 58.7 and 8.9 wt% carbon (C) and hydrogen (H), respectively. Knowing the amount of C and H in PA6 and PP monomer (C: 63.7 wt% and H: 9.7 wt% in PA6 monomer, and C: 85.7 wt% and H: 14.3 wt% in PP monomer) and also the amount of C in the ash of PCW (C: 12 wt% in CaCO3), one can calculate the approximate amount of PP in PCW, which was circa 4 wt%.
Given the PP content of PCW, two model compounds based on PIW were prepared. Both model compounds had small quantities of PP (3 wt%), while the second model compound contained 2 wt% PPC (compatibilizer).
The unwelded tensile strengths of all compounds are presented in Figure 3. PIW demonstrated 20% higher tensile strength than PCW. Model compounds based on PIW exhibited tensile strengths very close to PIW. It would appear that PP contamination had no significant effect on tensile properties of recycled PA6. The drop in PCW static properties is likely due to the CaCO3 content.11
Figure 4 shows the stress-strain behavior of the PIW and PCW compounds. PCW exhibited a brittle behavior during the tensile test, while PIW showed ductile behavior and more elongation in the cold draw region (plateau after yielding point). The model compounds exhibit behavior more closely resembling that of PIW. This drastic reduction in PCW properties could therefore be associated with the higher inorganic content in PCW.
The vibration weld strength results are shown in Figure 5. All compounds containing PP displayed a noticeable drop in weld strength relative to PIW. The PIW/PPC/PP model compound showed less reduction in weld strength than PIW. It seems that the presence of the compatibilizer attenuated the loss in weld strength.
Figure 6 illustrates the weld fracture surface (WFS) of PIW and PCW. The texture and structure of the WFS of PCW was very different from PIW’s. The very low-weld strength PCW had a more planar and smoother WFS, compared to the rougher and coarser WFS of PIW.
The WFS of PCW and PIW were etched in boiling xylenes for approximately 10 min. This allowed the xylene to dissolve the PP content of PCW. Etched samples were then coated with gold and observed under a SEM. As shown in Figure 7, after etching, a series of holes appeared close to the edge of the WFS of PCW. A closer look at this area confirmed the presence of 20-μm diameter holes (formerly PP droplets) near the edges of weld fracture surface. These droplets are similar to those seen in the PCW bulk material. Interestingly, no holes were observed in the center of the weld fracture surface. This morphology was not observed for the PIW etched samples (not shown).
It is clear that PP is significantly interfering with the joining process. The mechanism is, however, not clear. One possible explanation is the creation of a PP film at the interface as the polyolefin melts, coalesces, and is then sheared by both the oscillatory motion and the lateral flow of melt from the interface. Once the vibratory motion stops, the planar orientation of the film may be frozen in place, creating stress concentrations or planes of weakness in the weld zone. The droplets present at the edge of the weld fracture surface may be material that was able to coalesce during solidification, or it may be visible bulk material. More work on the microstructure of the heat-affected zone is required.
Conclusions
Recycled PA6 from used carpets (referred to as PCW in this article) is contaminated with PP and calcium carbonate. These contaminants come from the PP carpet backing and latex binder. Hence, the higher inorganic content of PCW led to a higher melt viscosity and storage modulus below the glass transition temperature. Higher ash content of PCW also changed the ductile behavior of PA6 to brittle.
The presence of PP in recycled polyamide compounds had a negative impact on vibration weld strength. The drop in weld strength can be attenuated somewhat through the use of compatibilizer.
Acknowledgements
The authors would like to express their gratitude to Jim Vanderveen and Bobbye Baylis at Mahle for providing the resins and valuable discussions. The authors would also like to thank the AUTO21 Network of Centres of Excellence for their funding.
References
- Muzzy, J., Wang, Y., Satcher, M., Shaw, B., McNamara, A., Jin, K., and Norton, J., 8th Annual Conference on Recycling of Fibrous Textile and Carpet Waste (2003).
- Mancosh, D., Murdock, D.E., and Przybylinski, J., U.S. Patent 7,875,655 B2 (2011).
- Weiser, A., Streltses, B., Afek, U., and Yermolaev, A., U.S. Patent 8,366,977 B2 (2013).
- Vilaplana, F. and Karlsson, S., Macromol. Mater. Eng., 293, 274 (2008).
- Kagan, V.A., Journal of Injection Molding Technology, 4, 8 (2000).
- Stokes, V.K., Polym. Eng. Sci., 28, 718 (1988).
- Bates, P.J., Mah, J.C., Zou, X.P., Wang, C.Y., and Baylis, B., Composites: Part A, 35, 1107 (2004).
- Mirzadeh, A., Ghasemi, H., Bates, P.J., and Kamal, M.R., Intern. Polymer Processing, 29, 4 (2014).
- Ghasemi, H., Mirzadeh, A., Bates, P.J., and Kamal, M.R., Polymer-Plastics Technology and Engineering, (2014).
- Droste, D.H. and Dibenedetto, A.T., Journal of Applied Polymer Science, 13, 2149 (1969).
- Zuiderduin, W.C.J., Westzaan, C., Huetink, J., and Gaymans, R.J., Polymer, 44, 261 (2003).
