ISMP Medication Error Report Analysis
U-500 Insulin Safety Concerns Mount
Improved Labeling Needed for Camphor Product
Cardizem–Cardene Mix-up
Initiative to Eliminate Tubing Misconnections
Michael R. Cohen, RPh, MS, ScD,* and Judy L. Smetzer, RN, BSN†
These medication errors have occurred in health care facilities at least once. They will happen again—perhaps where you work. Through education and alertness of personnel and procedural safeguards, they can be avoided. You should consider publishing accounts of errors in your newsletters and/or presenting them at your inservice training programs. Your assistance is required to continue this feature. The reports described here were received through the Institute for Safe Medication Practices (ISMP) Medication Errors Reporting Program. Any reports published by ISMP will be anonymous. Comments are also invited; the writers’ names will be published if desired. ISMP may be contacted at the address shown below. Errors, close calls, or hazardous conditions may be reported directly to ISMP through the ISMP Web site (www.ismp.org), by calling 800-FAIL-SAFE, or via e-mail at ismpinfo@ismp.org. ISMP guarantees the confidentiality and security of the information received and respects reporters’ wishes as to the level of detail included in publications.
Hosp Pharm 2014;49(2):117–120
2014 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj4902-117
U-500 Insulin Safety Concerns Mount
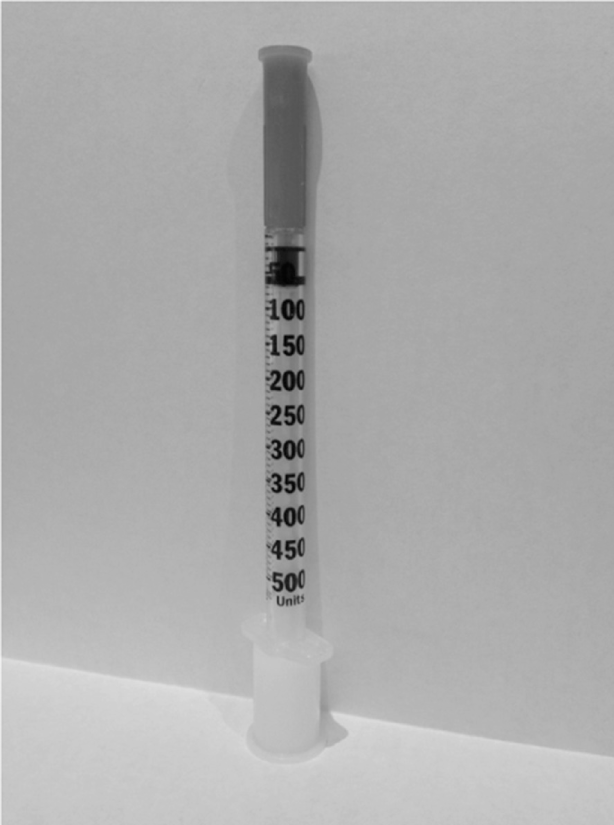
As the obesity epidemic continues and insulin resistance problems worsen, larger doses of insulin are more frequently required to meet glycemic goals. This has led to an increased use of U-500 insulin when dose requirements exceed 200 units per day. Along with the increased use of U-500 insulin, we have been receiving a growing number of U-500 insulin-related medication error reports and/or complaints from health professionals. Most of the reports are related to dosing confusion caused by not having a syringe with a U-500 scale. This usually requires practitioners to rely on measuring doses with a U-100 syringe and teaching the patient to communicate doses in “syringe units”—that is, measure 200 units of U-500 insulin by drawing up 40 “syringe units” on the U-100 syringe. Too often, patients do not understand the difference between U-100 and U-500, so they inaccurately state the actual dose. For example, they say that they take 40 units of insulin, which can lead to hyperglycemia if only 40 units are then prescribed. Confusion can also lead to overdoses. If people using a U-100 syringe misunderstand “syringe units,” a dose of 80 units of U-500 insulin might be prepared by measuring 80 units on the U-100 scale instead of 16 “syringe units,” resulting in a dose of 400 units.
It is difficult to understand how a high-alert drug like insulin was ever allowed to be available without a corresponding way to measure doses accurately (eg, a U-500 syringe). Instead, practitioners have been forced to improvise by using a U-100 syringe, which we believe is more error-prone than using a tuberculin syringe that measures U-500 doses by volume. Indeed, a U-500 syringe would seem to be a much better option by providing markings appropriate for U-500 insulin. Recently, the US Department of Veterans Affairs (VA) published research on a prototype U-500 syringe design (www.ismp.org/sc?id=257). One of the goals of the evaluation was to observe intuitive selection of the correct syringe for a corresponding dose. More than 100 subjects (clinicians and experienced and inexperienced patient groups) were asked to select the correct syringe between a U-500 syringe (see Figure 1) or a U-100 syringe given 3 different dosing situations. When the U-500 insulin doses were below 100 units, the majority of subjects chose a U-100 syringe instead of the U-500 syringe. In such a case, the patient could receive a 5-fold overdose when using U-500 insulin. Among clinicians alone, 47% chose the wrong syringe (U-100), probably thinking that it would be more accurate for doses under 100 units. The authors of this study noted a variety of other vulnerabilities with the U-500 syringe and offered helpful recommendations for improving its design.
What about an insulin pen for U-500? Given that far fewer patients receive U-500 insulin than U-100 insulin, and given the well-known confusion brought about by not having a corresponding U-500 syringe, we believe a U-500 insulin pen would be the best option, despite a recommendation we made in 2013 to consider transitioning away from insulin pen use in hospitals (www.ismp.org/sc?id=161). Unfortunately, Lilly and BD will not provide information about whether this is in the works, and the US Food and Drug Administration (FDA) is unable to comment on any products that may or may not be under development. However, there are additional strengths of insulin now under development that will only add to the confusion if a pen is not made available. Sanofi-Aventis is developing U-300 glargine (Lantus), Novo-Nordisk is developing U-200 degludec (brand name Tresiba outside the United States), and Lilly is developing a U-200 Humalog (insulin lispro). Tresiba is already on the market in the United Kingdom and is only available in a pen. Given the syringe selection issues raised in the U-500 insulin study, we strongly recommend that these new products be available in the United States only in a pen. If development of a U-200 syringe and/or a U-300 syringe is being considered, that will only add to inventory problems, selection issues, confusion, and medication errors. Indeed, mix-ups between syringe types were common when U-40 and U-80 insulins were available for a time, along with U-100 insulins (www.ismp.org/sc?id=262).
One final issue with U-500 insulin that perhaps can also lead to confusion is name similarity. Humulin R is the name for both the U-100 and the U-500 products, even though they have different concentrations and different pharmacokinetics. It is time to rethink this, as name similarity has contributed to product selection errors from the shelf during dispensing and from the computer screen while prescribing, as well as communication errors during medication reconciliation. The same issue could arise with the new higher strength insulins (eg, Lantus, Humalog). It is worthwhile studying whether a name change or the addition of a suffix to the more concentrated forms would help differentiate them.
Until U-500 syringes or pens are available for U-500 insulin, ISMP continues to believe that it would be far less confusing to all concerned if tuberculin syringes were used to measure doses by volume by using a dosing conversion chart (www.ismp.org/sc?id=260). Total doses should be expressed in terms of both units and volume (ie, 200 units [0.4 mL]). That way, the U-100 scale and associated confusion would not arise. One issue that has come up, however,
is reimbursement for patients using tuberculin syringes. Some insurers will not cover the cost. That is shortsighted given the financial cost of dosing errors. We can only hope that insurers will begin to cover the cost of using the most appropriate syringe with higher concentration insulin.
Improved Labeling Needed for Camphor Product
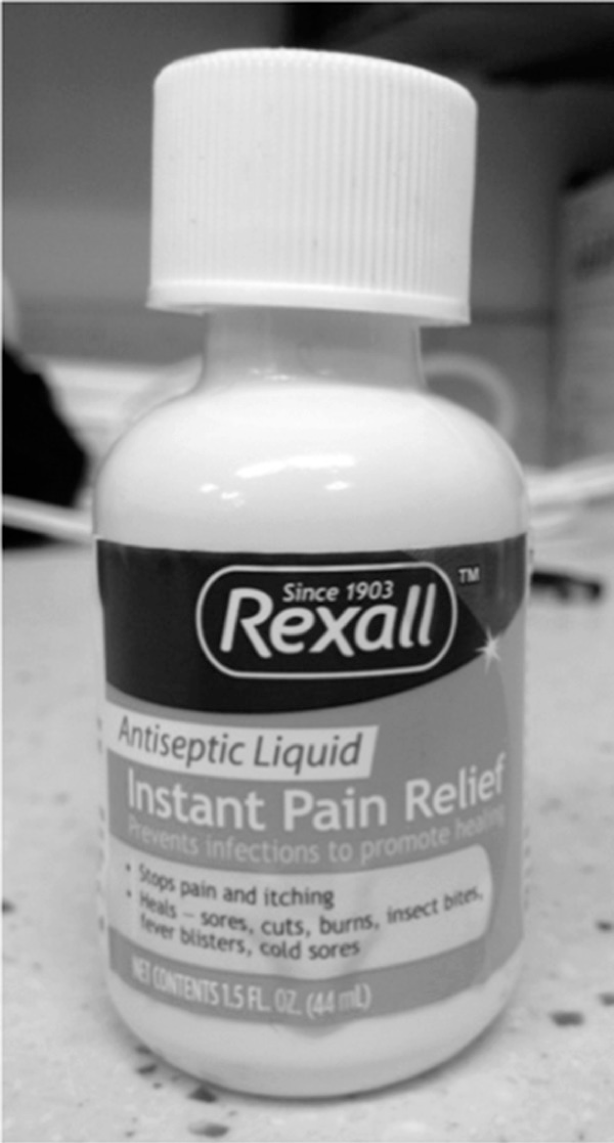
A 5-year-old child was accidentally given liquid camphorated phenol instead of acetaminophen. The child developed seizures and was taken to a hospital. In the emergency department (ED), the child’s mother produced a bottle of Rexall “Instant Pain Relief” antiseptic liquid from which she had given her child 10 mL (Figure 2). She said she mistook the bottle for acetaminophen liquid and had not noticed anything on the label about the product not being appropriate for oral use. The child was treated with phenytoin and a benzodiazepine and transferred to a critical care unit. He later recovered and was discharged.
Nationally, camphor ingestions occur in about 9,000 children under age 6 each year. Regulations keep camphor concentrations in liquid below 11%, so toxicity from unintentional ingestion is generally limited to children. In fact, ingestion of less than 10 mL (about 1 g of camphor) can result in a range of adverse neurological effects and death (www.ismp.org/?id=259).
Well-meaning parents might miss the “For external use only” warnings on some camphor product labels and give oral doses to children, not realizing that it can be harmful. The only place you can see “For external use only” is on a side panel (in Drug Facts). Some people may fail to read this warning, and some parents may not understand that terminology. Adding “Apply ONLY to the skin,” “Do NOT swallow,” or “Do NOT eat or drink” to the front label, along with a graphic that conveys the message, are much needed improvements.
We tried to contact Rexall, but we learned that the product is a store brand licensed to Dollar General Stores. The store put us in touch with the actual manufacturer, Lee Pharmaceuticals, and we asked them to consider label improvements. We also contacted the FDA, because other brands are similarly labeled. Meantime, outpatient pharmacies or stores that stock camphor products should separate oral and topical forms and alert customers that topical products are for topical use only.
Cardizem–Cardene Mix-Up
Several name mix-ups have been reported that involve Cardene (nicardipine) and Cardizem (diltiazem).
In one case, an ED nurse prepared and administered a Cardene infusion instead of the prescribed Cardizem infusion. The patient received the wrong medication for several hours before the mistake was discovered. The patient became hypotensive during the infusion, but he was quickly treated; there was no permanent harm, and no further medication management was required.
In another recently reported mix-up with these drugs, a nurse programmed a smart pump to infuse Cardizem instead of Cardene. The patient received the correct medication but at the wrong rate until the mistake was caught by a nurse on the next shift. Again, no harm was reported.
In an earlier case in which a mix-up occurred in the pharmacy at the time of dispensing, Cardizem was sent instead of Cardene. A nurse later assumed this was an intentional pharmacy substitution and did not question the change.
Recommendations for preventing drug name mix-ups like these can be found in the August 9, 2007 issue of the ISMP Medication Safety Alert! (www.ismp.org/sc?id=269). Although not a factor in the recent events, mix-ups are also likely if the mnemonic for the drugs start with C-A-R-D. ISMP will add these 2 drugs to the list of commonly confused drug names. An updated list of commonly confused names will be published early next year.
Initiative to Eliminate Tubing Misconnections
Catheter misconnections can occur when tubing from one type of delivery system is connected to another delivery system that serves a different function. These have been attributed in part to the universal design of Luer connector systems that are common to catheters, tubes, administration sets, syringes, and other connectors. An international effort is now underway to standardize the various types of connectors used in health care, making them incompatible with each other. Manufacturers of breathing systems and gases, enteral, limb cuff inflation, neuraxial, and urethral devices will need to redesign their products to accept the new connector standards as they are approved. Manufacturers are prepared to launch new connectors with minimal disruption to supply and clinical practice. There will be a phased approach to the launch of new connectors, starting with enteral devices in late summer or fall of 2014.
It is important for everyone to be aware of the forthcoming changes and plan for them. The Association for the Advancement of Medical Instrumentation, the International Organization for Standardization, and other key stakeholders from a joint working group have posted, Stay Connected: FAQs About Small-Bore Connectors and Tubing Misconnections, to answer questions about the project (www.ismp.org/sc?id=267). ![]()
*President, Institute for Safe Medication Practices, 200 Lakeside Drive, Suite 200, Horsham, PA 19044; phone: 215-947-7797; fax: 215-914-1492; e-mail: mcohen@ismp.org; Web site: www.ismp.org. †Vice President, Institute for Safe Medication Practices, Horsham, Pennsylvania.