Original Article
Effects of Etomidate on Adrenal Suppression: A Review of Intubated Septic Patients
Melissa L. Thompson Bastin, PharmD, BCPS*; Stephanie N. Baker, PharmD, BCPS*,†; and Kyle A. Weant, PharmD, BCPS*,†
Abstract
Background: Etomidate is a commonly used sedative during rapid sequence intubation (RSI). Septic patients are at an increased risk of independently developing adrenal suppression, which has been associated with increased mortality in some studies. Since etomidate affects cortisol production, its use in septic patients is controversial. However, data are still lacking to prove that etomidate should be avoided in this patient population.
Objectives:The objective was to review patients diagnosed with sepsis who received etomidate during RSI. Our hypothesis is that patients who receive etomidate will experience clinically significant hypotension within the first 24 hours of intubation.
Methods:A retrospective cohort study was conducted on patients intubated in the emergency department (ED) and medical/surgical floors at our institution from 2004 to 2010. Once patients with a diagnosis of sepsis were identified, it was determined whether the patients received etomidate or a different sedative during intubation. The primary endpoint was clinically significant hypotension: systolic blood pressure
Results: One hundred fifty-seven patients, 110 etomidate and 47 non-etomidate, were included in the final analysis. Hypotension was seen in 79 (71.8%) patients who received etomidate and in 14 (29.8%) patients who received another sedative (P ≤ .001). There were no statistically significant differences in secondary objectives.
Conclusion: Etomidate use for induction of anesthesia during RSI was associated with clinically significant hypotension when compared to other sedatives. The hypotension was transient and did not translate into statistically significant differences in the secondary clinical endpoints.
Key Words—adrenal suppression, etomidate, intubation, rapid sequence intubation, sepsis
Hosp Pharm—2014;49(2):177–183
Hosp Pharm 2014;49(2):177–183
2014 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj4902-177
Etomidate is a short-acting, sedative hypnotic that is commonly used for inducing short-term anesthesia during rapid sequence intubation (RSI) for patients requiring mechanical ventilation. This agent induces amnesia within 5 to 15 seconds after a single bolus dose and may shorten the onset of neuromuscular blocking agents when used during RSI. It is touted for exhibiting fewer detrimental side effects, mainly hemodynamic compromise, when compared to other sedatives commonly used for induction such as the benzodiazepines.1 Etomidate has neutral effects on blood pressure, a predictable reduction in intracranial pressure comparable to thiopental and propofol, and minimal effects on lung function and cardiac function. The favorable side effect profile and predictable pharmacokinetic and pharmacodynamic parameters make etomidate an attractive choice for induction of anesthesia in critically ill patients experiencing hemodynamic instability.2
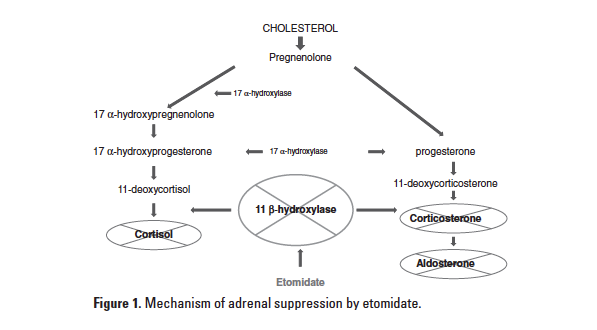
A well-documented side effect of etomidate is suppression of the adrenal synthesis of cortisol. In a dose-dependent fashion, it inhibits adrenal mitochondrial
11-β hydroxylase, the enzyme responsible for the final conversion of 11-deoxycortisol to cortisol (Figure 1).
This side effect, shown to be a risk factor for increased mortality, can be detrimental in septic patients who may have a baseline adrenal insufficiency due to critical illness.3 Previous studies have evaluated the effect of etomidate on adrenocortical suppression in septic patients; however none have been sufficiently powered to make direct claims on this agent’s effects on mortality. It has been shown, however, that mortality increases in septic patients with each incremental decrease in adrenal function.3
Although etomidate can cause relative adrenal insufficiency, the question of how this effect translates into clinical outcomes has not fully been answered by the primary literature currently available. We designed this retrospective study to evaluate whether etomidate is associated with adverse hemodynamic effects in the treatment of septic patients. Monitoring cortisol levels during treatment of septic shock is no longer in line with contemporary practice, so we chose to look at hypotension as a surrogate outcome for reduced cortisol production. Because the inhibition of cortisol by etomidate is a temporal phenomenon, we screened our patients for prolonged hypotension within the first 24 hours of receiving etomidate. Working under the assumption that etomidate will inhibit adrenal production of cortisol, we conducted the study to determine whether etomidate given to patients diagnosed with sepsis, severe sepsis, or septic shock would result in clinically significant hypotension despite maximal resuscitation efforts within the first 24 hours. A systolic blood pressure (SBP)
Materials and Methods
Setting
A retrospective cohort study was performed on patients who were intubated in the emergency department (ED) or medical floors of a 489-bed tertiary care academic teaching hospital. Study approval was granted by the university’s institutional review board. During the study period, the ED was comprised of 45 acute care beds and provided services to 45,000 patients yearly. Our institution discharges 22,000 patients each year and is a referral center for a significant part of our state. The emergency resident and attending physicians perform intubations using a rapid sequence technique with an intubation box, readily available in the ED, that holds the medications and supplies. Medications available during the study included etomidate, lidocaine, vecuronium, and rocuronium. Midazolam and fentanyl were also available in automated dispensing cabinets as options for sedation during the intubation. When a patient requires intubation on the medicine floor, a code is called and a pharmacist responds to facilitate the delivery of medications with an RSI kit that holds the same medications. The responding physician to an intubation code is usually an emergency medicine resident. There is not an algorithm or protocol for selection of induction agents during RSI, instead the agent is selected on an individual patient basis by the physician performing the procedure. We did not collect information on which prescribers used certain induction agents over another.
Patients
Adult patients aged 18 years and older were included if they were intubated during the study period (January 1, 2004 to March 30, 2010) and had an ICD-9 discharge code for sepsis. Due to low patient yield during our first search based on ICD-9 codes alone, additional patients were identified by reviewing those who received a neuromuscular blocker during their stay. These patients were then screened for a diagnosis of sepsis. Patients were considered septic if they had received a diagnosis of sepsis in the medical chart at the time of intubation and met criteria according to the Surviving Sepsis Campaign guidelines.4 Patients who were immunosuppressed, receiving steroids before admission, or being treated with an antifungal medication within the past 30 days of admission (if known) were excluded from the analysis to rule out potential confounding causes of hypotension.
Variables
A sequential organ failure assessment (SOFA) score was calculated for each patient at the time of intubation to assess for severity of illness. Baseline hemodynamic measurements including blood pressure (BP), MAP, and heart rate (HR) were recorded right before intubation and 24 hours after intubation. Information on the use, dose, time to initiation, and duration of vasopressors and corticosteroids was also collected. Intravenous fluids administered were recorded for the first 24 hours on the day of intubation and the 24 hours following intubation to assess for appropriateness of fluid resuscitation. The 24-hour time frame for collecting hemodynamic measurements was based on previous studies which revealed that etomidate’s effect on adrenal synthesis of cortisol and its clinical manifestations are temporary after a single bolus dose is administered and the effects tend to resolve after 24 hours.2,5 Any cortisol level or adrenocorticotropin stimulation test result was recorded if the test was conducted after the patient was intubated. A positive response to an adrenocorticotropin stimulation test was defined as a change in the serum cortisol level ≥9 mcg/mL inside a time period of 90 minutes. Alternatively, a patient was considered a nonresponder to the adrenocorticotropin stimulation test if the serum cortisol failed to reach a ≥9 mcg/dL increase. Nonresponders to an adrenocorticotropin stimulation test meet diagnostic criteria for adrenal insufficiency in the current literature and for the sake of this study.3 The number of days on mechanical ventilation, number of days in the intensive care unit (ICU), and total length of stay for the hospitalization were also recorded.
Statistical Analysis
We calculated that 94 patients in each group would be needed to determine a 20% absolute difference between groups with 80% power. Significance was set at a P value of t test and chi-square test where appropriate.
Results
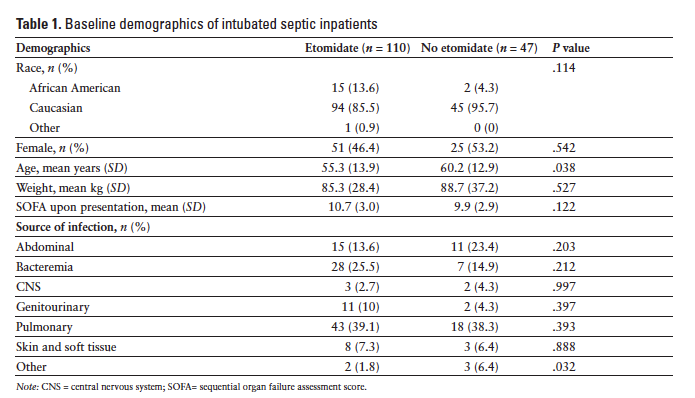
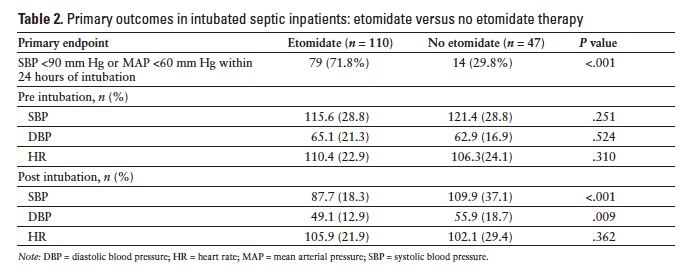
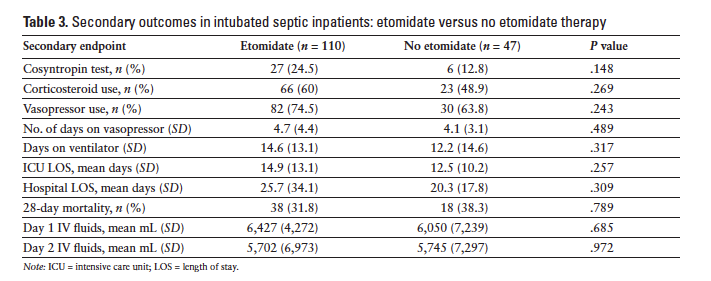
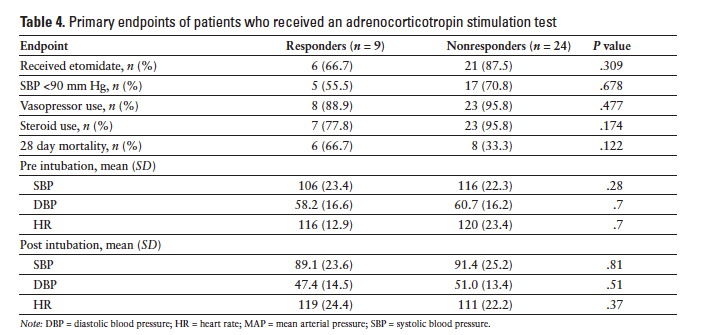
Nine hundred and seventy-one patients were identified to be critically ill and requiring intubation during the defined time period. One hundred and fifty-seven met the inclusion criteria. The 814 patients who were excluded from the analysis were either intubated before arrival to the ED (at another hospital or by emergency medical services), were not septic at the time of intubation, or met exclusion criteria previously listed. Of the 157 patients who met inclusion criteria, 110 (70%) received etomidate as the induction agent and 47 (30%) received another induction agent during RSI. The most common agent used in lieu of etomidate was midazolam, followed by fentanyl or no agent. Baseline demographic data of the study groups are summarized in Table 1. The primary endpoint was met in 79 (71.8%) patients who received etomidate and in 14 (29.8%) patients who received another agent (P < .001). Changes in BP and HR are summarized in Table 2. Patients who received etomidate were more likely to receive steroids (66; 60%) versus those who did not (23; 48.9%), however this difference did not reach statistical significance (P = .269). There was not a significant difference in vasopressor use between the 2 groups (etomidate, n = 82, 74.5%; no etomidate, n = 30, 63.8%) (P = .243). Days on the ventilator, ICU length of stay, hospital length of stay, and 28-day mortality were not found to be statistically different (Table 3). Patients who received etomidate were more likely to have an adrenocorticotropin stimulation test ordered, but there were no differences in the outcomes of these 2 groups (Table 4).
Discussion
Etomidate use in the septic patient population is controversial among emergency medicine physicians and critical care practitioners. Some experts have recommended that etomidate should be avoided in the septic patient population.6 Previous studies of etomidate use in this population have produced discordant results.
Etomidate has been associated with an increased risk of mortality when used as a continuous infusion for sedation in the ICU.7 In a study of trauma patients receiving etomidate continuous infusion for sedation compared to sedation with a benzodiazepine, mortality was significantly increased (77% vs 28%). Patients who were switched from etomidate to a benzodiazepine saw a 25% reduction in overall mortality. Based on this study, etomidate is currently only used as a one-time bolus injection. The clinical implications of adrenal suppression from 1 or 2 doses of etomidate are not well defined. Mohammad et al found that etomidate blunted the response to an adrenocorticotropin stimulation test in 152 patients with septic shock.8 However, the study was underpowered to evaluate mortality between groups. Jaber and colleagues conducted a noninferiority trial of ketamine to etomidate on patients intubated in the ED.9 They found higher rates of adrenal suppression in the etomidate group, yet similar rates of organ dysfunction and mortality. It is important to note that their study was not powered to evaluate the septic patients in their population, so the results cannot be generalized to this population.
The largest sepsis trial to date included an a priori analysis of the intubated patients who received etomidate (N = 96). It found that patients who received etomidate were more likely to be nonresponders to an adrenocorticotropin stimulation test, and 28-day mortality was higher when etomidate use was added to the logistical regression analysis.10 The most recent review by Dmello and colleagues of septic patients who received etomidate found that etomidate patients received statistically significant more corticosteroids during their stay, but mortality, vasopressor use, and length of hospital stay were not affected by the use of etomidate.11
The effect of etomidate on the results of the adrenocorticotropin stimulation test demonstrates a blunted response to this stressful stimulus.12 However, the routine use of this stimulation test is no longer recommended in the treatment of sepsis due to the lack of correlation with clinical outcomes and the benefit of steroid use during the resuscitation period regardless of result.3 This current practice recommendation is reflected in the low percentage of patients in our study who received an adrenocorticotropin stimulation test. Therefore, SBP
Limitations
Limitations of the previously reported literature have been a small sample size, heterogeneous patient populations, and inconsistent primary outcomes for comparison. Limitations of our study include the retrospective study design and a small sample size; 157 patients met inclusion criteria. We found that a disproportionate number of our patients received etomidate, reflecting the current practice and experience level of intubating physicians at our institution.
It would be difficult to surmise why some physicians chose not to prescribe etomidate during the RSI in this retrospective review. Etomidate is available in the RSI kits that are brought to codes on the floors and is inside the intubation box available in the ED, therefore it seems that it would be easy to choose etomidate when in emergent situations as compared to a drug from the automated cabinet. Other potential causes of hypotension in this medical critically ill patient population could not be controlled for, other than standardizing baseline demographic information including organ dynfunction at the time of intubation. Home medication data were not collected outside of immunosuppressant medications, steroids, or antifungal medications, as those patients were excluded from the analysis. The most significant limitation of the current study was the identification of patients who were septic during the study period. Patient identification relied upon the appropriate coding of sepsis or septic shock and the time at which the individual charts were reviewed; additional patients were identified who were not coded at discharge. The results of the current study cannot be generalized to other patient populations, such as trauma patients. We were able to identify 157 patients who were septic as the primary diagnosis; this limits these conclusions to this patient population. We cannot comment on the effects on adrenal suppression by etomidate in other patient populations. Although we did not meet our original power calculation, we identified enough septic patients who received etomidate to find a statistically significant difference between the 2 groups.
Conclusion
In our study of 157 intubated septic patients, etomidate was associated with development of clinically significant hypotension during the first 24 hours following administration of a bolus dose. As in the study by Dmello and colleagues, our etomidate population received more corticosteroids than the comparator group, although our results did not reach statistical significance. 11 The increased use of corticosteroids in the etomidate group is likely linked to the transient hypotension seen in this group. We did not have enough patients who received an adrenocorticotropin stimulation test to find a difference between groups. The response rate to the test was similar between groups, although the etomidate group was more likely to receive one of these tests. Current clinical practice does not recommend adrenocorticotropin stimulation testing in septic patients, because of the lack of correlation of a positive response to overall morbidity and mortality.10 The recommendations correlate with the results of our study, because our patient populations had similar response rates to the stimulation test. The effect of etomidate on adrenal synthesis of cortisol was short-lived in our septic patient population, as evidenced by similar mortality rates at 28 days between groups. Usual interventions for hypotension, including intravenous fluids, vasopressor agents, and days on mechanical ventilation, were not different between the 2 groups. This implies that stabilization of these patients takes 24 hours or more, and the amount of resources required to achieve stability is not affected by whether or not they experience additive hypotension from a drug like etomidate. We did, however, see a significant decrease in blood pressure in the etomidate group, implying that etomidate has an effect on cortisol production but that the adverse effect of the drug is transient in nature and not significant enough to cause long-term consequences when used as a one-time bolus.
Acknowledgments
The authors have no conflicts of interest to report regarding the content of this article.
References
- Bergen JM, Smith DC. A review of etomidate for rapid sequence intubation in the emergency department. J Emerg Med 1997;15(2):221-230.
- Wells RM, Murphy MF. Clinical controversies: Etomidate as an induction agent for endotracheal intubation in patients with sepsis. Ann Emerg Med. 2008;52(1):13-14.
- Annane D, Sebille V, Troche G, et al. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000;283:1038-1045.
- Dellinger RP, Levy MM, Carlet JM, et al Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(4):1394-1396.
- Vinclair M, Broux C, Faure P, et al. Duration of adrenal inhibition following a single dose of etomidate in critically ill patient. Intens Care Med. 2008;34:714-719.
- Annane D. ICU Physicians should abandon the use of etomidate! Intens Care Med. 2005;31:235-326.
- Ledingham IM, Watt I. Influence of sedation on mortality in critically ill multiple trauma patients. Lancet.1983;1:1270.
- Mohammad Z, Afessa B, Finkielman JD. The incidence of relative adrenal insufficiency in patients with septic shock after the administration of etomidate. Crit Care. 2006;10:R105.
- Jaber P, Combes X, Lapostolle F, et al. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: A multicentre randomized controlled trial. Lancet. 2009:374:293-300.
- Cuthbertson BH, Sprung CL, Annane D, et al. The effects of etomidate on adrenal responsiveness and mortality in patients with septic shock. Intens Care Med. 2009;35:1868-1876.
- Dmello D, Taylor S, O’Brien J, Matuschak, GM. Outcomed of etomidate in severe sepsis and septic shock. Chest. 2010;138(6):1327-1332.
- Malerba G, Romano-Girard F, Cravoisy A, et al. Risk factors of relative adrenocortical deficiency in intensive care patients needing mechanical ventilation. Intens Care Med. 2005;31:388-392.

*Department of Pharmacy, †Department of Emergency Services, University of Kentucky HealthCare, Lexington, Kentucky. Corresponding author: Melissa L. Thompson Bastin, PharmD, BCPS, 800 Rose Street, Room H110, Lexington, KY 40536; e-mail: mth226@uky.edu