Formulary Drug Reviews
Dolutegravir
Dennis J. Cada, PharmD, FASHP, FASCP (Editor)*; Terri L. Levien, PharmD†; and Danial E. Baker, PharmD, FASHP, FASCP‡
Each month, subscribers to The Formulary Monograph Service receive 5 to 6 well-documented monographs on drugs that are newly released or are in late phase 3 trials. The monographs are targeted to Pharmacy & Therapeutics Committees. Subscribers also receive monthly 1-page summary monographs on agents that are useful for agendas and pharmacy/nursing in-services. A comprehensive target drug utilization evaluation/medication use evaluation (DUE/MUE) is also provided each month. With a subscription, the monographs are sent in print and are also available on-line. Monographs can be customized to meet the needs of a facility. A drug class review is now published monthly with The Formulary Monograph Service. Through the cooperation of The Formulary, Hospital Pharmacy publishes selected reviews in this column. For more information about The Formulary Monograph Service, call The Formulary at 800-322-4349. The February 2014 monograph topics are sofosbuvir, simeprevir capsules, ibrutinib, hydrocodone extended-release capsules, and eslicarbazepine. The DUE/MUE is on sofosbuvir.
Hosp Pharm 2014;49(2):184–195
2014 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj4902-184
Indications
Dolutegravir is indicated for use in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and adolescents 12 years and older weighing at least 40 kg. Genotype testing may be valuable for patients experiencing virologic failure during antiretroviral therapy. Poor virologic response was observed in patients with an integrase strand transfer inhibitor (INSTI)–resistance Q148 substitution plus 2 or more additional INSTI-resistance substitutions, including L741/M, E138A/D/K/T, G140A/S, Y143H/R, E157Q, G163E/K/Q/R/S, or G193E/R.1
Raltegravir, another oral integrase inhibitor, is indicated for use in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and children 2 years and older.2 The other available integrase inhibitor, elvitegravir, is only available in a combination formulation indicated as a complete regimen for the treatment of HIV-1 infection in adults who are antiretroviral treatment naive.3
Clinical Pharmacology
Dolutegravir is an HIV-1 INSTI.1,4 Dolutegravir has exhibited binding differences that enable it to form strengthened interactions with viral DNA compared with raltegravir, as well as an ability to adjust its position and conformation in response to structural changes in the active sites of raltegravir-resistant integrase.4 In addition, it has displayed slower dissociation from wild-type and integrase inhibitor–resistant integrase DNA complexes than raltegravir and elvitegravir.5
Integrase mutations potentially conferring resistance to dolutegravir are at positions 92, 101, 118, 124, 148, 153, 193, and 263.1,6,7 T124A and L1011/T124A mutations have been observed in raltegravir-treated patients and in treatment-naive patients. These mutations may induce some degree of cross-resistance, but they have not been observed to induce resistance when present in clinical isolates from integrase inhibitor–naive patients.6,8-10 The S153F/Y mutations have not been detected clinically.6,8 Reduced response rates have been observed in patients with Q148 substitutions present at baseline, as well as in patients with 3 or more of the following INSTI-resistance substitutions: L74/M, E138A/D/K/T, G140A/S, Y143H/R, Q148H/R, E157Q, G163E/K/Q/R/S, or G193E/R.1
HIV-1 clinical isolates susceptible to raltegravir have also demonstrated susceptibility to dolutegravir.5,11 Dolutegravir retained in vitro activity against clinical isolates resistant to raltegravir at levels similar to that against wild-type isolates for variants containing the Y143 and N155 resistance mutations, but dolutegravir exhibited reduced susceptibility to variants containing the Q148 mutation in conjunction with other integrase mutations.5,7,11
In a 10-day study assessing dolutegravir monotherapy in 35 antiretroviral therapy–naive and antiretroviral therapy–experienced (integrase inhibitor–naive) patients, dolutegravir reduced plasma HIV RNA by 1.51 log10 copies/mL with a 2 mg once daily dose, 2.03 log10 copies/mL with a 10 mg once daily dose, and 2.46 log10 copies/mL with a 50 mg once daily dose, compared with a 0.05 log10 copies/mL increase in a placebo group (P < .001 for all dolutegravir doses vs placebo). A reduction in viral load to less than 50 copies/mL was achieved in 7 of 10 patients in the 50 mg group.12
Pharmacokinetics
Following oral administration, peak plasma levels of dolutegravir were achieved within approximately 0.5 to 1 hour with a suspension formulation and within 1.5 to 2.5 hours with the tablet and granule formulations.12-15 Administration with food slowed the rate and increased the extent of absorption. The time to peak increased to 3 to 5 hours when administered with food. The area under the concentration-time curve (AUC) increased 33%, 41%, and 66% when administered with low-, moderate-, and high-fat meals, respectively, and the peak concentration (Cmax) increased similarly (46%, 52%, and 67%, respectively).1,16 At least 34% of the oral dose was absorbed.14 Dolutegravir plasma concentrations increased in a less than dose-proportional manner at doses greater than 50 mg.1 Dolutegravir is highly plasma protein bound (greater than 98.9%).1 Dolutegravir is distributed into the cerebrospinal fluid.17 Its apparent volume of distribution is 17.4 L.1
The mean half-life is 11 to 16 hours.12-14,16 Dolutegravir is metabolized primarily via uridine diphosphate–glucuronosyltransferase 1A1 (UGT1A1) and cytochrome P450 3A4 (CYP3A4), with lesser metabolism via UGT1A3, UGT1A9, human breast cancer resistance protein (BCRP), and P-glycoprotein (P-gp).1,18 Patients with UGT1A1 genotypes that result in poor drug metabolism may lower dolutegravir clearance by 32% and increase its AUC by 46%.1 The primary metabolite is inactive.14 Because of its high membrane permeability, the efflux transporters P-gp and BCRP have minimal impact on its intestinal absorption.18 Dolutegravir excretion is primarily in the feces (approximately 64% of the dose) and to a lesser extent in the urine (approximately 32% of the dose). Less than 1% of the dose is excreted unchanged in the urine.14
Dolutegravir reversibly increases serum creatinine by inhibiting the organic cation transporter 2, which is responsible for the tubular secretion of creatinine.19 Dolutegravir pharmacokinetics were not altered in subjects with mild to moderate renal impairment.1,20 In patients with severe renal impairment, dolutegravir exposure was reduced 40% and Cmax was reduced 23%, although the cause of these reductions is not known.1
Dolutegravir elimination was not significantly altered in subjects with moderate hepatic impairment; no dosage adjustment is necessary in patients with mild to moderate hepatic impairment (Child-Pugh score A or B). The effect of severe hepatic impairment (Child-Pugh score C) on dolutegravir pharmacokinetics has not been studied.1
Comparative Efficacy
Indication: HIV Infection in Treatment-Naive Patients
Guidelines
Guideline: Guidelines for the use of antiretroviral agents in HIV-1–infected adults and adolescents
Reference: US Department of Health and Human Services, 201321
Comments: Current guidelines recommend the following preferred regimens for antiretroviral-naive patients initiating antiretroviral therapy:
- Efavirenz/tenofovir disoproxil fumarate/emtricitabine
- Ritonavir-boosted atazanavir + tenofovir disoproxil fumarate/emtricitabine
- Ritonavir-boosted darunavir + tenofovir disoproxil fumarate/emtricitabine
- Raltegravir + tenofovir disoproxil fumarate/emtricitabine
Selection of a regimen should be individualized on the basis of virologic efficacy, adverse effects, pill burden, dosing frequency, drug interaction potential, resistance testing results, and comorbid conditions. Alternative regimens may be preferred based on individual patient characteristics.
Studies
Drug: Dolutegravir vs Efavirenz
Reference: van Lunzen J, et al, 2012 (SPRING-1)22,23
Study Design: Randomized, multicenter, phase 2b, dose-finding study
Study Funding: Shionogi-GlaxoSmithKline Pharmaceuticals (now Shionogi-ViiV Healthcare)
Patients: 205 antiretroviral-naive adults with HIV infection and plasma HIV-1 RNA viral loads of at least 1,000 copies/mL (mean, 4.5 log10 copies/mL; 21% with viral load exceeding 100,000 copies/mL) and CD4 counts of at least 200 cells/mcL (mean, 324 cells/mcL). Median age was 37 years; 80% of patients were White and 86% were male.
Intervention: Dolutegravir 10, 25, or 50 mg once daily without regard to food or efavirenz 600 mg once daily, in conjunction with either tenofovir plus emtricitabine or abacavir plus lamivudine at the investigator’s discretion; patients and investigators were blind to the dolutegravir dose but not to drug allocation. Therapy was continued for 96 weeks.
Results:
Primary Endpoint(s):
- Proportion of patients with viral load of less than 50 copies/mL at week 16 was 93% (144 of 155 patients) receiving dolutegravir versus 60% (30 of 50 patients) receiving efavirenz.
Secondary Endpoint(s):
- Proportion of patients with viral load of less than 50 copies/mL at week 48 was 90% (139 of 155 patients) receiving dolutegravir versus 82% (41 of 50 patients) receiving efavirenz.
- Proportion of patients with viral load of less than 50 copies/mL at week 96 was 82% with dolutegravir (79% with 10 mg, 78% with 25 mg, and 88% with 50 mg) and 72% with efavirenz.
Endpoint(s):
- Median increase in CD4 count at week 48 was 231 cells/mcL with dolutegravir and 174 cells/mcL with efavirenz (P = .076).
- Median increase in CD4 count at week 96 was 338 cells/mcL with dolutegravir and 301 cells/mcL with efavirenz (P = .155).
Comments: Response rates (viral load less than 50 copies/mL) for dolutegravir exceeded those of efavirenz by week 4; the response rates at week 4 were 66% with dolutegravir compared with 18% with efavirenz. Sustained efficacy and tolerability was seen through 96 weeks of therapy.
Limitations: Limited enrollment of non-White and female patients. The levels of the HIV-1 RNA viral load and CD4 counts are reflective of patients with fairly nonadvanced disease.
Reference: Walmsley S, et al, 2012 (SINGLE)1,24
Study Design: Randomized, double-blind, double-dummy, multicenter, noninferiority study
Study Funding: Not provided
Patients: 833 treatment-naive adults with HIV-1 infection and plasma HIV-1 RNA viral loads of at least 1,000 copies/mL (median, 4.7 log10
copies/mL; 32% with viral load exceeding 100,000 copies/mL); 68% of patients were White and 84% were male, median age was 35 years, and 7% had hepatitis C virus coinfection (hepatitis B coinfection was excluded).
Intervention: Dolutegravir 50 mg once daily plus abacavir/lamivudine once daily or efavirenz/tenofovir/emtricitabine once daily for 48 weeks.
Results:
Primary Endpoint(s):
- Proportion of patients with HIV-1 RNA less than 50 copies/mL at week 48 was 88% receiving the dolutegravir regimen versus 81% receiving the efavirenz regimen (difference, 7.4%; 95% confidence interval [CI], 2.5 to 12.3; P = .003 for superiority).
Secondary Endpoint(s):
- Median time to HIV-1 RNA less than 50 copies/mL was 28 days with the dolutegravir regimen and 84 days with the efavirenz regimen (hazard ratio, 2.3; 95% CI, 2 to 2.7; P < .001).
- Change from baseline in CD4 count increased by 267 cells/mcL with the dolutegravir regimen and 208 cells/mcL with the efavirenz regimen (difference, 59 cells/mcL; 95% CI, 33 to 84; P < .001).
Comments: Response rates were consistently greater in the dolutegravir group when analyzed by baseline viral load, CD4+ cell count, age, gender, and race. The dolutegravir-containing regimen was associated with fewer psychiatric and nervous system adverse events than the efavirenz-containing regimen.
Limitations: Limited enrollment of non-White and female patients. Results only reported in a meeting abstract and the product labeling.
Drug: Dolutegravir vs Raltegravir
Reference: Raffi F, et al, 2013 (SPRING-2)1,25,26
Study Design: Randomized, double-blind, double-dummy, multicenter, noninferiority study
Study Funding: ViiV Healthcare
Patients: 822 treatment-naive adult patients with HIV infection, a plasma HIV-1 RNA of at least 1,000 copies/mL (median, 4.52 log10 copies/mL in the dolutegravir group and 4.58 log10 copies/mL in the raltegravir group; 28% with viral load exceeding 100,000 copies/mL in both groups), and no primary resistance in reverse transcriptase or protease enzymes. Median age was 37 years in the dolutegravir group and 35 years in the raltegravir group, 85% of patients were White and 86% were male, and 11% of patients had hepatitis B and/or C virus coinfection.
Intervention: Dolutegravir 50 mg once daily or raltegravir 400 mg twice daily in conjunction with a regimen of tenofovir/emtricitabine (59% of patients) or abacavir/lamivudine (41% of patients) at the discretion of the investigator.
Results:
Primary Endpoint(s):
- Proportion of patients with HIV-1 RNA less than 50 copies/mL at week 48 was 88% with dolutegravir and 85% with raltegravir (difference, 2.5%; 95% CI, −2.2 to 7.1; met noninferiority criteria).
Secondary Endpoint(s):
- Proportion of patients with HIV-1 RNA less than 50 copies/mL at week 96 was 81% with dolutegravir and 76% with raltegravir (difference, 4.5%; 95% CI, −1.1 to 10; met noninferiority criteria).
- Change from baseline to week 48 in CD4 cell counts: increased by a median of 230 cells/mcL in both groups.
- Change from baseline to week 96 in CD4 cell counts: increased by 276 cells/mcL with dolutegravir and by 264 cells/mcL with raltegravir.
- Incidence and severity of adverse events did not differ between groups.
- Changes in laboratory parameters did not differ between groups.
- Genotypic or phenotypic evidence of resistance was not observed in the dolutegravir group. Resistance mutations to the integrase inhibitor developed in 1 patient on raltegravir, and resistance mutations to the background therapy developed in 4 patients on raltegravir.
- Health outcomes showed similar improvements from baseline occurring in both groups (data not provided).
Comments: Response rates were similar when analyzed by baseline viral load, CD4+ cell count, age, gender, race, and background nucleoside reverse transcriptase inhibitor (NRTI) regimen. The values reported in the product labeling are a little different than those reported in the study results but generally are similar in number and range.
Limitations: Limited enrollment of non-White and female patients.
Indication: HIV Infection in Treatment-Experienced Patients
Guidelines
Guideline: Guidelines for the use of antiretroviral agents in HIV-1–infected adults and adolescents
Reference: USDepartment of Health and Human Services, 201321
Comments: For the management of treatment-experienced patients, current guidelines recommend assessment of the severity of the patient’s HIV disease, antiretroviral therapy history, drug interaction potential, HIV RNA and CD4 count trends over time, and prior drug-resistance testing results. When selecting a new regimen, the patient’s treatment history and past and current resistance test results should be used to identify at least 2, and preferably 3, fully active agents to combine with an optimized background antiretroviral regimen.
Studies
Drug: Dolutegravir
Reference: Eron JJ, et al, 2013 (VIKING)27
Study Design: Single-arm, open-label, multicenter study
Study Funding: ViiV Healthcare
Patients: 107 antiretroviral therapy–experienced patients with HIV-1 infection, HIV-1 RNA levels of at least 1,000 copies/mL, and current or historic raltegravir treatment failure and evidence of raltegravir resistance at screening, as well as genotypic or phenotypic resistance to at least 1 compound in each of 2 other approved antiretroviral classes.
Intervention: Cohort 1 (27 subjects) received dolutegravir 50 mg once daily; cohort 2 (24 subjects) received dolutegravir 50 mg twice daily. Dolutegravir was initially administered for 10 days with the failing background regimen, then dolutegravir was continued at the assigned dose for up to 24 weeks with optimized background therapy.
Results:
Primary Endpoint(s):
- Proportion of subjects on day 11 with a plasma HIV-1 RNA less than 400 copies/mL or at least 0.7 log10 copies/mL below baseline was 78% in cohort 1 and 96% in cohort 2.
Secondary Endpoint(s):
- Mean change from baseline in plasma HIV-1 RNA on day 11 was −1.45 log10 copies/mL in cohort 1 and −1.76 log10 copies/mL in cohort 2 (difference, −0.32 log10 copies/mL; P = .017).
- Mean change from baseline in plasma HIV-1 RNA at week 24 was −1.3 log10 copies/mL in cohort 1 and −2.3 log10 copies/mL in cohort 2.
- Proportion of subjects with plasma HIV-1 RNA less than 400 copies/mL on day 11 was 41% in cohort 1 and 54% in cohort 2.
- Proportion of subjects with plasma HIV-1 RNA less than 400 copies/mL at week 24 was 52% in cohort 1 and 83% in cohort 2.
- Proportion of subjects with plasma HIV-1 RNA less than 50 copies/mL on day 11 was 11% in cohort 1 and 17% in cohort 2.
- Proportion of subjects with plasma HIV-1 RNA less than 50 copies/mL at week 24 was 41% in cohort 1 and 75% in cohort 2.
- Change from baseline CD4 count at week 24 showed an increase of 54 cells/mcL in cohort 1 and 60 cells/mcL in cohort 2.
Comments: The initial group of patients was treated with dolutegravir 50 mg once daily, but the protocol was amended because of the inadequate viral response in some of these patients. The dosage of dolutegravir was increased to 50 mg twice daily in the second cohort of patients. Mutations that emerged during dolutegravir treatment were previously described raltegravir-associated mutations. No novel mutations emerged that resulted in a clinically important drop in dolutegravir susceptibility.
Limitations: Small study, no comparator group.
Reference: Nichols G, et al, 2012 (VIKING-3)1,28
Study Design: Single-arm, open-label, multicenter study
Study Funding: ViiV Healthcare
Patients: 183 antiretroviral therapy–experienced patients with HIV-1 infection, HIV-1 RNA levels of at least 500 copies/mL, and current or historic raltegravir and/or elvitegravir treatment failure and evidence of raltegravir and/or elvitegravir resistance at screening. Median age was 48 years, 77% were male, 29% were non-White, and 20% had hepatitis B and/or C virus coinfection. The median duration of prior antiretroviral treatment was 13 years; 79% of patients had resistance to 2 or more NRTIs, 75% had resistance to 1 or more nonnucleoside reverse transcriptase inhibitors (NNRTIs), 71% had 2 or more protease inhibitor resistance–associated mutations, and 62% had non–R5 virus.
Intervention: Dolutegravir 50 mg twice daily initially administered for 7 days with the failing background regimen, then with optimized background therapy.
Results:
Primary Endpoint(s):
- Mean change from baseline in plasma HIV-1 RNA at day 8 was −1.4 log10 copies/mL (95% CI, 1.3 log10 to 1.5 log10).
- Proportion of subjects with a plasma HIV-1 RNA level less than 50 copies/mL at week 24 was 63%.
Secondary Endpoint(s):
- Median change from baseline CD4+ cell count at week 24 was 65 cells/mcL.
Comments: Response rates ranged from 80% in subjects with the N155H genotype without a Q148 substitution, 56% in subjects with the Y143C/H/R genotype without a Q148 substitution, 56% in subjects with the Q148H/R + G140A/S genotype without additional INSTI-resistance substitutions, and 18% in subjects with the Q148H/R genotype plus 2 or more INSTI-resistance substitutions. Emergent INSTI-resistance substitutions included T97A, E138K or A, G140S or A, or Q148H or R or K. Emergence of resistance to 1 or more background drugs occurred in 30% of subjects.
Limitations: Small study, no comparator group. Results only published in abstract form and in the product labeling.
Drug: Dolutegravir vs Raltegravir
Reference: Cahn P, et al, 2013 (SAILING)1,29
Study Design: Randomized, double-blind, double-dummy, multicenter noninferiority study
Study Funding: ViiV Healthcare
Patients: 715 antiretroviral-experienced patients with HIV-1 infection who had not previously received integrase inhibitor therapy and who had a baseline HIV-1 RNA of at least 400 copies/mL, resistance to 2 or more classes of antiretroviral drugs, and 1 to 2 fully active drugs for background therapy. The median HIV-1 RNA was 4.18 log10 copies/mL (20% with HIV-1 RNA greater than 100,000 copies/mL); 42% of patients were of African descent and 68% were male; the median age was 43 years and 16% had hepatitis B and/or C virus coinfection.
Intervention: Dolutegravir 50 mg once daily or raltegravir 400 mg twice daily for 48 weeks, plus investigator-selected background therapy of no more than 2 agents.
Results:
Primary Endpoint(s):
- Proportion of subjects with HIV-1 RNA less than 50 copies/mL at week 48 was 71% of patients in the dolutegravir group and 64% in the raltegravir group (difference, 7.4%; 95% CI, 0.7 to 14.2); noninferiority was prespecified with a lower bound of a 2-sided 95% CI less than −12%. Noninferior criteria was met for dolutegravir, so a specified test for superiority was conducted (P = .03).
Secondary Endpoint(s):
- Mean change in CD4 count from baseline to week 48 increased by 162 cells/mcL with dolutegravir and 153 cells/mcL with raltegravir at week 24.
- Proportion of patients with virologic failure and treatment-emergent integrase inhibitor resistance was 4 patients (1%) with dolutegravir and 17 patients (5%) with raltegravir (difference, −3.7%; 95% CI, −6.1 to −1.2; P = .003).
Comments: Higher response rates with dolutegravir were maintained across baseline characteristics, including CD4+ cell count and age. The values reported in the product labeling are a little different than those reported in the study results but are generally similar in number and range.
Contraindications, Warnings, and Precautions
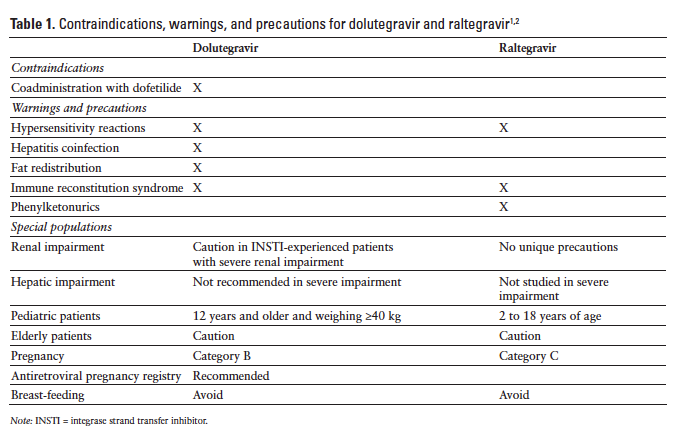
The contraindications, warnings, and precautions for dolutegravir and raltegravir are compared in Table 1.
Contraindications
Coadministration of dofetilide with dolutegravir is contraindicated.1
Warnings and Precautions
Hypersensitivity reactions, characterized by rash, constitutional findings, and organ dysfunction in some patients, have been reported in 1% or fewer patients treated with dolutegravir. Therapy should be immediately discontinued if signs or symptoms of hypersensitivity occur. Clinical status, including liver aminotransferases, should be monitored and appropriate therapy initiated. A delay in the discontinuation of dolutegravir therapy after the onset of hypersensitivity may result in a life-threatening reaction. Dolutegravir therapy should not be used in patients who have experienced a previous hypersensitivity reaction to dolutegravir.1
Patients with underlying hepatitis B or C infection may have an increased risk of worsening or development of transaminase elevations with the use of dolutegravir. Patients with underlying hepatic disease should undergo laboratory testing prior to initiating therapy and monitoring for hepatotoxicity during therapy.1
Body fat redistribution/accumulation has been observed in patients receiving antiretroviral therapy.1
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including dolutegravir. Patients may develop an immune response to indolent or residual opportunistic infections (eg, Mycobacterium avium, cytomegalovirus, Pneumocystis jiroveci pneumonia, tuberculosis) during the early phase of therapy. Autoimmune disorders (eg, Graves disease, polymyositis, Guillain-Barré syndrome) have also been reported in the setting of immune reconstitution but may occur many months after initiation of treatment.1
No dosage adjustment is necessary in patients with mild to moderate hepatic impairment (Child-Pugh score A or B). The pharmacokinetics of dolutegravir has not been evaluated in patients with severe hepatic impairment; therefore, use is not recommended in patients with severe hepatic impairment. Patients with underlying hepatic disease should also be closely monitored for hepatotoxicity during dolutegravir therapy.1
No dosage adjustment is necessary for treatment-naive or treatment-experienced INSTI-naive patients with mild, moderate, or severe renal impairment or for INSTI-experienced patients with potential resistance and mild or moderate impairment. Dolutegravir levels are reduced in patients with severe renal impairment, so caution is advised for INSTI- experienced patients with potential resistance and severe renal impairment because the decrease in dolutegravir concentrations may result in loss of therapeutic effect or development of resistance.1
Dolutegravir is not recommended in pediatric patients younger than 12 years or weighing less than 40 kg.1
Dolutegravir is in Pregnancy Category B. No evidence of impaired fertility or harm to the fetus was observed in animal studies; however, dolutegravir was shown to cross the placenta in animal studies. There are no adequate studies in pregnant women. An antiretroviral pregnancy registry has been established to monitor maternal-fetal outcomes of pregnant women with HIV who are exposed to dolutegravir.1 The Centers for Disease Control and Prevention recommends that mothers should not breast-feed, because of the potential for HIV transmission and the potential for adverse reactions in breast-feeding infants.1
Adverse Reactions
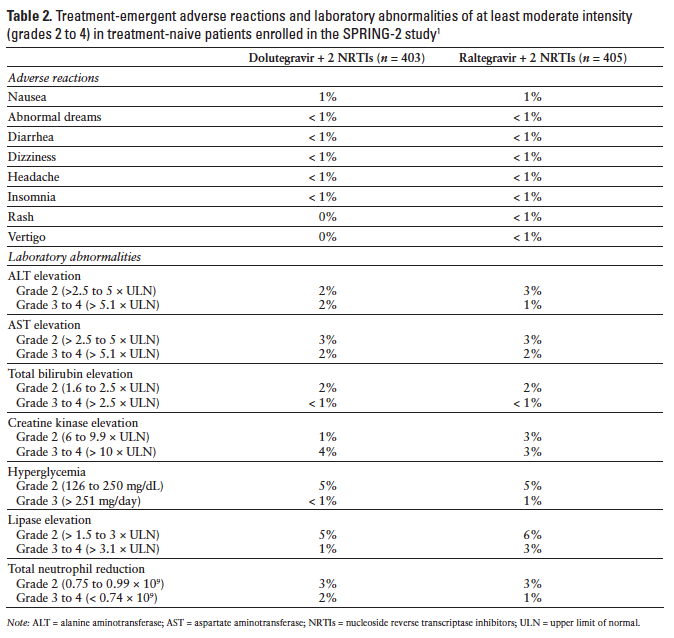
Adverse events reported most frequently in clinical trials, including dolutegravir 50 mg once daily in the treatment regimen, were nausea, diarrhea, headache, fatigue, asthenia, nasopharyngitis, insomnia, dizziness, abnormal dreams, pyrexia, and depression.22,30 Common laboratory abnormalities included elevated alanine aminotransferase (ALT), aspartate aminotransferase (AST), cholesterol and lipase, and hyperglycemia.1 Adverse events have occurred with similar frequency in dolutegravir and raltegravir treatment groups (Table 2).1,31 Adverse events observed in dolutegravir-treated children have been similar to those reported in adults.1
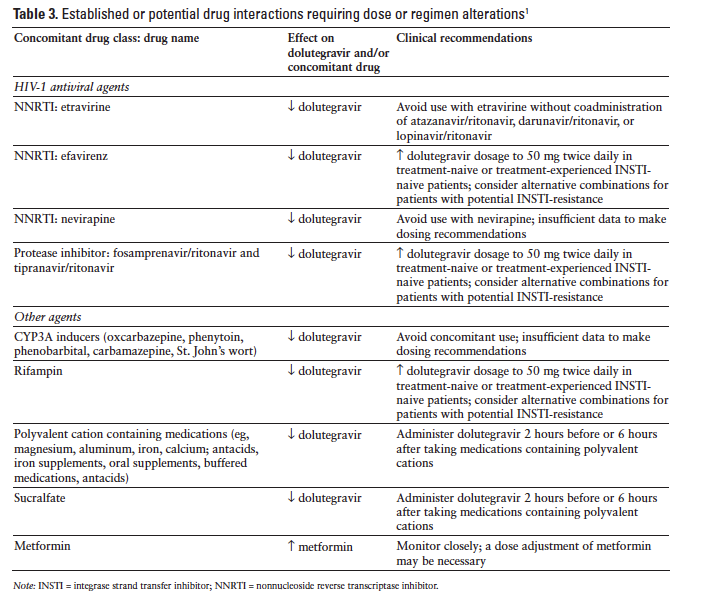
Drug Interactions
Dolutegravir inhibits the renal organic cation transporter 2, potentially resulting in increased plasma concentrations of drugs eliminated via organic cation transporter 2 (eg, dofetilide and metformin). Table 3 summarizes potential dolutegravir drug interactions requiring dose or regimen alterations.1 Dolutegravir is not expected to affect the elimination of agents that are substrates of other enzymes or transporters. Dolutegravir does not inhibit P-gp, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A, UGT1A1, UGT2B7, BCRP, multidrug resistance protein 2, organic anion-transporting polypeptide (OATP) 1B1, OATP 1B3, or organic cation transporter 1.1,13,18 Dolutegravir does not induce CYP1A2, CYP2B6, or CYP3A4.13,18 Dolutegravir did not affect the pharmacokinetics of methadone, tenofovir, midazolam, rilpivirine, or an ethinyl estradiol/norgestimate oral contraceptive.1,32
Coadministration of dolutegravir with a proton pump inhibitor (omeprazole) or a multivitamin (One-A-Day Maximum) did not have any important effect on dolutegravir absorption; however, administration with an antacid (Maalox Advanced Maximum Strength) reduced the dolutegravir AUC by 74%. Dolutegravir should be administered 2 hours before or 6 hours after antacids.33
Dosage adjustments are not necessary when dolutegravir is combined with atazanavir, atazanavir/ritonavir, darunavir/ritonavir, lopinavir/ritonavir, fosamprenavir/ritonavir, or tenofovir.30 Nevirapine use with dolutegravir should be avoided because nevirapine reduces dolutegravir levels.30 Administration of the NNRTI etravirine alone with dolutegravir resulted in reduced exposure to dolutegravir but did not significantly affect dolutegravir pharmacokinetics when etravirine and dolutegravir were administered in conjunction with lopinavir/ritonavir or darunavir/ritonavir.34 Pharmacokinetic drug interactions were not observed with coadministration of dolutegravir and the NNRTI rilpivirine.35
Coadministration of dolutegravir with drugs that inhibit UGT1A1, CYP3A4, UGT1A3, UGT1A9, BCRP, and P-gp may increase dolutegravir plasma concentration.1 Coadministration of atazanavir, a potent inhibitor of UGT1A1, produced a modest increase in dolutegravir exposure. The dolutegravir AUC was increased 91% and the Cmax was increased 50%. Administration of dolutegravir with atazanavir/ritonavir was associated with a 62% increase in dolutegravir AUC and a 34% increase in dolutegravir Cmax.36 Administration of dolutegravir with fosamprenavir/ritonavir, a UGT1A1 inducer, resulted in a 35% decrease in dolutegravir AUC and a 24% decrease in dolutegravir Cmax; however, these small reductions are not likely to be clinically important.37
Rifampin reduced exposure to dolutegravir; but when dolutegravir dosing was increased to twice daily in conjunction with rifampin, the dolutegravir levels were similar to those achieved with once-daily dosing without rifampin. Rifabutin had minimal impact on dolutegravir pharmacokinetics.38 Other enzyme inducers, such as barbiturates, phenytoin, phenobarbital, carbamazepine, modafinil, pioglitazone, and St. John’s wort, may also reduce dolutegravir levels.30 Prednisone did not have clinically important effects on dolutegravir pharmacokinetics.39
Recommended Monitoring
HIV-1 RNA and CD4 count should be monitored to assess response. Patients should also be monitored for immune reconstitution syndrome.3 In patients with underlying hepatic disease, serum liver biochemistries should be assessed prior to initiating therapy and monitoring for hepatotoxicity should be undertaken during therapy.1
Dosing
The recommended dolutegravir dosage for treatment-naive and treatment-experienced INSTI-naive patients is 50 mg once daily. For treatment-naive and treatment-experienced INSTI-naive patients receiving concomitant potent UGT1A/CYP3A inducers (eg, efavirenz, fosamprenavir/ritonavir, tipranavir/ritonavir, rifampin), the recommended dolutegravir dosage is 50 mg twice daily. In patients who are INSTI-experienced with INSTI-associated resistance substitutions or clinically suspected INSTI resistance, the recommended dosage is also 50 mg twice daily. In this population combination, regimens that do not include metabolic inducers should be considered when possible.1
The dosage for pediatric patients 12 years and older weighing at least 40 kg is the same as the adult dose; however, safety and efficacy have not been established in pediatric patients who are INSTI-experienced with documented or clinically suspected resistance to other INSTIs.1
Dolutegravir can be administered without regard to meals.1
Product Availability and Storage
Dolutegravir received US Food and Drug Administration approval August 2013. It is available as 50 mg tablets supplied in bottles of 30 tablets. It should be stored at a controlled room temperature of 25°C (77°F) with excursions permitted from 15° to 30°C (59° to 86°F).1
Drug Safety / Risk Evaluation and Mitigation Strategy (REMS)
No REMS is required for dolutegravir.
Conclusion
Dolutegravir is an integrase inhibitor that offers a once-daily alternative to raltegravir. It exhibited activity in treatment-naive and treatment-experienced patients, demonstrating response rates comparable with raltegravir in treatment-naive patients and greater than raltegravir in treatment-experienced patients. The adverse event and drug interaction profiles of dolutegravir and raltegravir appear comparable.
References
- Tivicay [package insert]. Research Triangle Park, NC: ViiV Healthcare; August 2013.
- Isentress [package insert]. Whitehouse Station, NJ: Merck & Co Inc.; August 2013.
- Integrase inhibitors. Drug Facts and Comparisons. Facts & Comparisons [database online]. St. Louis, MO: Wolters Kluwer Health Inc; 2013. Accessed June 11, 2013.
- Hare S, Smith SJ, Métifiot M, et al. Structural and functional analyses of the second-generation integrase strand transfer inhibitor dolutegravir (S/GSK1349572). Mol Pharmacol. 2011;80(4):565-572.
- Hightower KE, Wang R, Deanda F, et al. Dolutegravir (S/GSK1349572) exhibits significantly slower dissociation than raltegravir and elvitegravir from wild-type and integrase inhibitor-resistant HIV-1 integrase-DNA complexes. Antimicrob Agents Chemother. 2011;55(10):4552-4559.
- Garrido C, Soriano V, Geretti AM, et al. Resistance associated mutations to dolutegravir (S/GSK1349572) in HIV-infected patients—impact of HIV subtypes and prior raltegravir experience. Antiviral Res. 2011;90(3):164-167.
- Canducci F, Ceresola ER, Boeri E, et al. Cross-resistance profile of the novel integrase inhibitor dolutegravir
(S/GSK1349572) using clonal viral variants selected in patients failing raltegravir. J Infect Dis. 2011;204(11):1811-1815. - Malet I, Wirden M, Fourati S, et al. Prevalence of resistance mutations related to integrase inhibitor S/GSK1349572 in HIV-1 subtype B raltegravir-naive and -treated patients.
J Antimicrob Chemother. 2011;66(7):1481-1483. - Saladini F, Meini G, Bianco C, et al; ARCA Collaborative Group. Prevalence of HIV-1 integrase mutations related to resistance to dolutegravir in raltegravir naïve and pretreated patients. Clin Microbiol Infect. 2012;18(10):E428-E430.
- Vavro C, Hasan S, Madsen H, et al. Prevalent polymorphisms in wild-type HIV-1 integrase are unlikely to engender drug resistance to dolutegravir (S/GSK1349572). Antimicrob Agents Chemother. 2013;57(3):1379-1384.
- Underwood MR, Johns BA, Sato A, Martin JN, Deeks SG, Fujiwara T. The activity of the integrase inhibitor dolutegravir against HIV-1 variants isolated from raltegravir-treated adults. J Acquir Immune Defic Syndr. 2012;61(3):297-301.
- Min S, Sloan L, DeJesus E, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1–infected adults. AIDS. 2011;25(14):1737-1745.
- Min S, Song I, Borland J, et al. Pharmacokinetics and safety of S/GSK1349572, a next-generation HIV integrase inhibitor, in healthy volunteers. Antimicrob Agents Chemother. 2010;54(1):254-258.
- Castellino S, Moss L, Wagner D, et al. Metabolism, excretion, and mass balance of the HIV-1 integrase inhibitor dolutegravir in humans. Antimicrob Agents Chemother. 2013;57(8):3536-3546.
- Patel P, Song I, Borland J, et al. Pharmacokinetics of a dolutegravir pediatric granule formulation in healthy adult subjects [abstract]. Presented at: 19th Conference on Retroviruses and Opportunistic Infections; March 5-8, 2012; Seattle, WA. Abstract 985.
- Song I, Borland J, Chen S, et al. Effect of food on the pharmacokinetics of the integrase inhibitor dolutegravir. Antimicrob Agents Chemother. 2012;56(3):1627-1629.
- Letendre S, Mills A, Tashima K, et al. Distribution and antiviral activity in cerebrospinal fluid of the integrase inhibitor, dolutegravir: ING116070 week 16 results [abstract]. Presented at: 20th Conference on Retroviruses and Opportunistic Infections; March 3-6, 2013; Atlanta, GA. Abstract 178LB.
- Reese MJ, Savina PM, Generaux GT, et al. In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. Drug Metab Dispos. 2013;41(2):353-361.
- Koteff J, Borland J, Chen S, et al. A phase 1 study to evaluate the effect of dolutegravir on renal function via measurement of iohexol and para-aminohippurate clearance in healthy subjects. Br J Clin Pharmacol. 2013;75(4):990-996.
- Song I, Borland J, Savina P, et al. Pharmacokinetics of dolutegravir in subjects with moderate hepatic impairment [abstract]. Presented at: 19th Conference on Retroviruses and Opportunistic Infections; March 5-8, 2012; Seattle, WA. Abstract 608.
- US Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1–infected adults and adolescents. AIDSinfo Web site. http://aidsinfo.nih.gov/guidelines/html/1/ adult-and-adolescent-arv-guidelines/0. Updated February 12, 2013. Accessed June 11, 2013.
- van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: Planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis. 2012;12(2):111-118.
- AIDS. 2013;27(11):1771-1778.
- Walmsley S, Antela A, Clumeck N, et al. Dolutegravir (DTG; S/GSK1349572) + abacavir/lamivudine once daily statistically superior to tenofovir/emtricitabine/efavirenz: 48-week results—SINGLE (ING114467) [abstract]. Presented at: 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy; September 9-12, 2012; San Francisco, CA. Abstract H-556b.
- Raffi F, Rachlis A, Stellbrink HJ, et al; SPRING-2 Study Group. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381(9868):735-743.
- Raffi F, Jaeger H, Motta D, et al. Dolutegravir is non-inferior to raltegravir and shows durable response through 96 weeks: Results from the SPRING-2 trial [abstract]. Presented at: 7th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; June 30-July 3, 2013; Kuala Lumpur, Malaysia. Abstract TULBPE17.
- Eron JJ, Clotet B, Durant J, et al; VIKING Study Group. Safety and efficacy of dolutegravir in treatment-experienced
subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING study. J Infect Dis. 2013;207(5):740-748. - Nichols G, Mills A, Grossberg R, et al. Antiviral activity of dolutegravir in subjects with failure on an integrase inhibitor-based regimen: Week 24 phase 3 results from VIKING-3 [abstract]. J Int AIDS Soc. 2012;15(suppl 4):18112.
- Cahn P, Pozniak AL, Mingrone H, et al; Extended SAILING Study Team. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: Week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382(9893):700-708.
- Fantauzzi A, Turriziani O, Mezzaroma I. Potential benefit of dolutegravir once daily: Efficacy and safety. HIV/AIDS (Auckl). 2013;5:29-40.
- Pozniak A, Mingrone H, Shuldyakov A, et al. Dolutegravir vs raltegravir in ART-experienced, integrase-naive subjects: 24-week interim results from SAILING (ING111762) [abstract]. Presented at: 20th Conference on Retroviruses and Opportunistic Infections; March 3-6, 2013; Atlanta, GA. Abstract 179LB.
- Song I, Mark S, Borland J, et al. Dolutegravir has no effect on pharmacokinetics of methadone or oral contraceptives with norgestimate and ethinyl estradiol [abstract]. Presented at: 20th Conference on Retroviruses and Opportunistic Infections; March 3-6, 2013; Atlanta, GA. Abstract 535.
- Patel P, Song I, Borland J, et al. Pharmacokinetics of the HIV integrase inhibitor S/GSK1349572 co-administered with acid-reducing agents and multivitamins in healthy volunteers. J Antimicrob Chemother. 2011;66(7):1567-1572.
- Song I, Borland J, Min S, et al. Effects of etravirine alone and with ritonavir-boosted protease inhibitors on the pharmacokinetics of dolutegravir. Antimicrob Agents Chemother. 2011;55(7):3517-3521.
- Ford SL, Gould E, Chen S, et al. Lack of pharmacokinetic (PK) interaction between rilpivirine and the integrase inhibitors dolutegravir and S/GSK1265744 [abstract]. Presented at: 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy; September 9-12, 2012; San Francisco, CA. Abstract A-1249.
- Song I, Borland J, Chen S, et al. Effect of atazanavir and atazanavir/ritonavir on the pharmacokinetics of the next-generation HIV integrase inhibitor, S/GSK1349572. Br J Clin Pharmacol. 2011;72(1):103-108.
- Song I, Borland J, Chen S, et al. Effects of fosamprenavir/ritonavir on the pharmacokinetics of the integrase inhibitor, dolutegravir, in healthy subjects [abstract]. Presented at: 51st Interscience Conference on Antimicrobial Agents and Chemotherapy; September 17-20, 2011; Chicago, IL. Abstract A1-1727.
- Dooley KE, Sayre P, Borland J, et al. Safety, tolerability, and pharmacokinetics of the HIV integrase inhibitor dolutegravir given twice daily with rifampin or once daily with rifabutin: Results of a phase 1 study among healthy subjects. J Acquir Immune Defic Syndr. 2013;62(1):21-27.
- Song IH, Borland J, Chen S, Savina P, Peppercorn AF, Piscitelli S. Effect of prednisone on the pharmacokinetics of the integrase inhibitor dolutegravir. Antimicrob Agents Chemother. 2013;57(9):4394-4397.

*Founder and Contributing Editor, The Formulary; †Clinical Associate Professor of Pharmacotherapy, Drug Information Center, Washington State University, Spokane, Washington; ‡Director, Drug Information Center, and Professor of Pharmacy Practice, College of Pharmacy, Washington State University Spokane, PO Box 1495, Spokane, Washington 99210-1495. The authors indicate no relationships that could be perceived as a confl ict of interest.
