Current FDA-Related Drug Information
Approvals, Submission, and Important Labeling Changes for US Marketed Pharmaceuticals
Danial E. Baker, PharmD, FASHP, FASCP*
This monthly feature will help readers keep current on new drugs, new indications, dosage forms, and safety-related changes in labeling or use. Efforts have been made to ensure the accuracy of this information; however, if there are any questions, please let me know at danial.baker@wsu.edu.
Hosp Pharm 2014;49(2):199–205
2014 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj4902-199









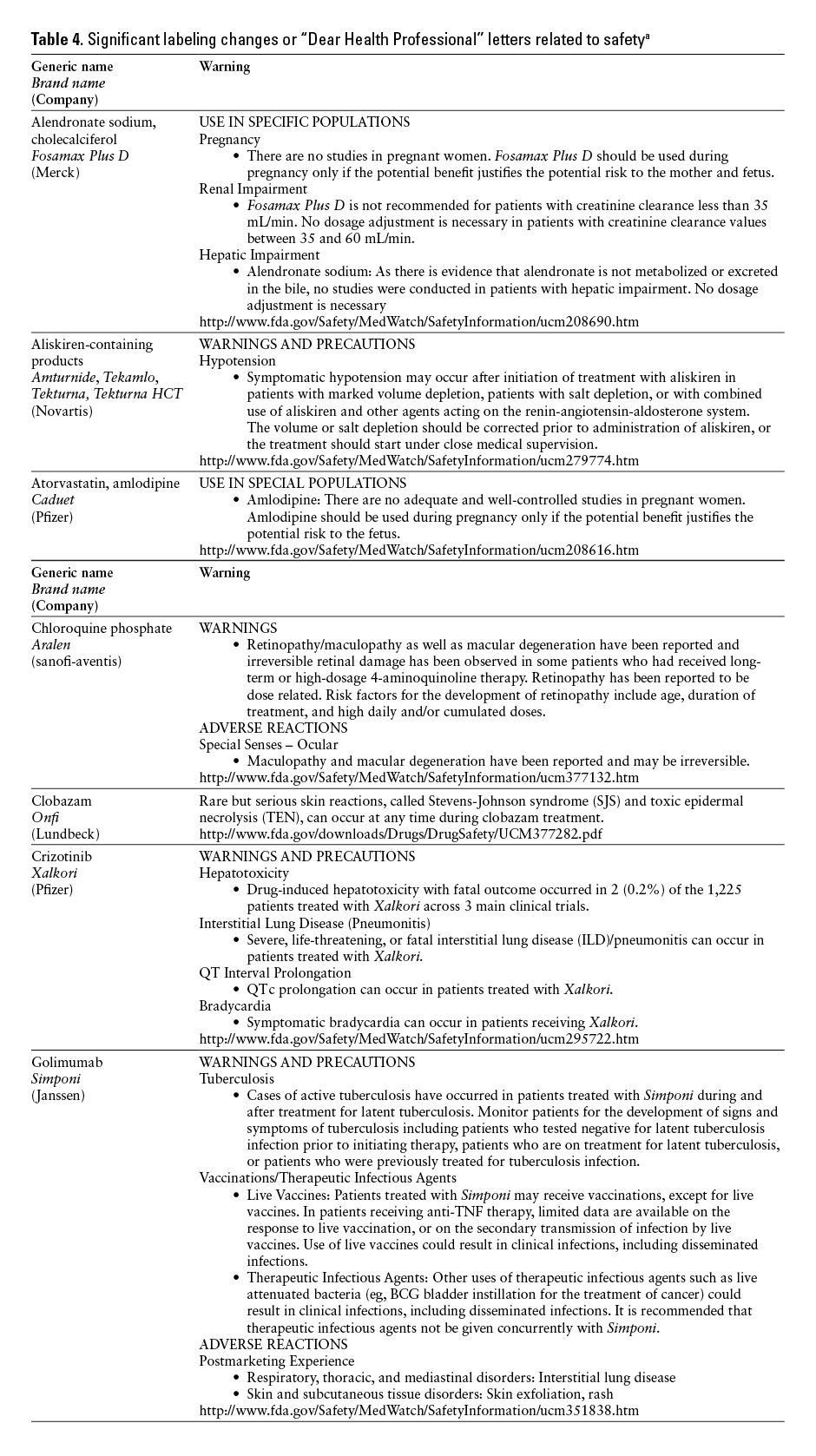


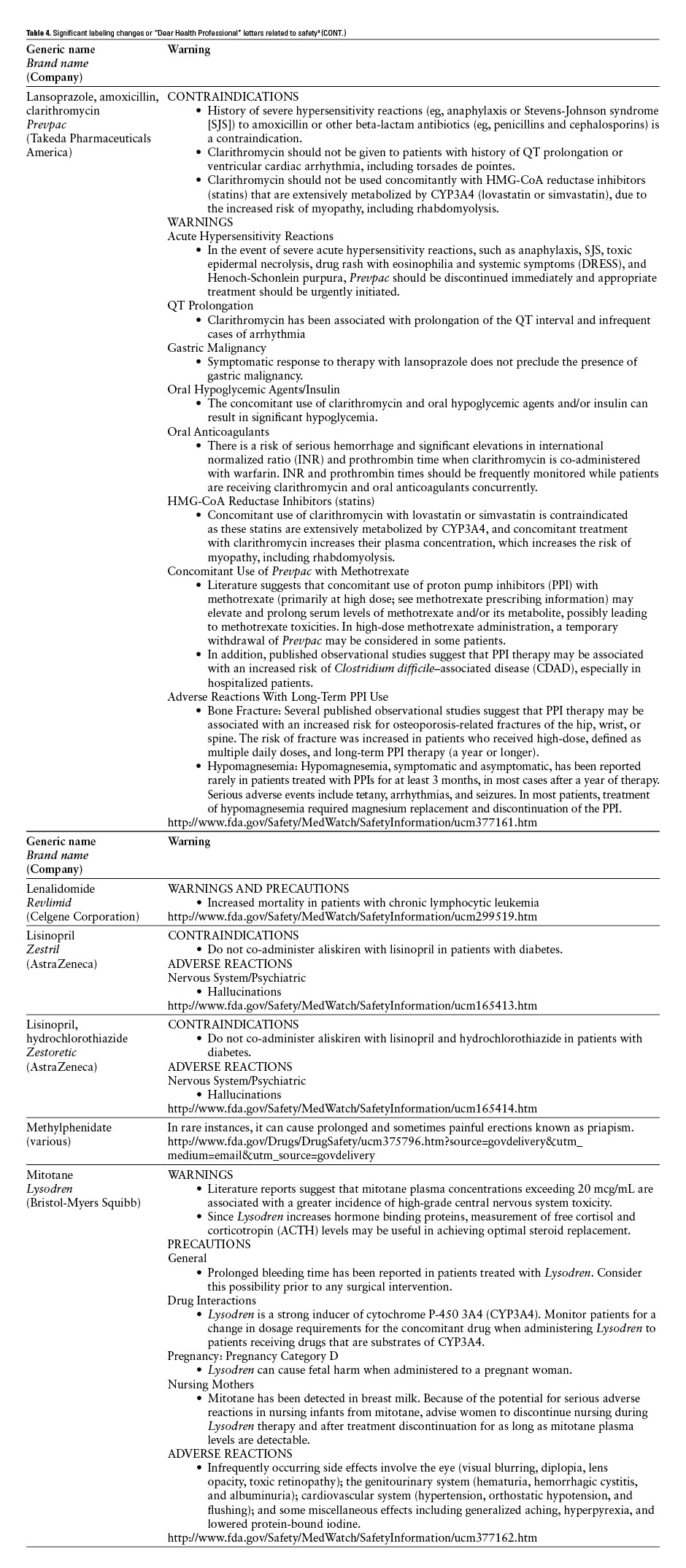


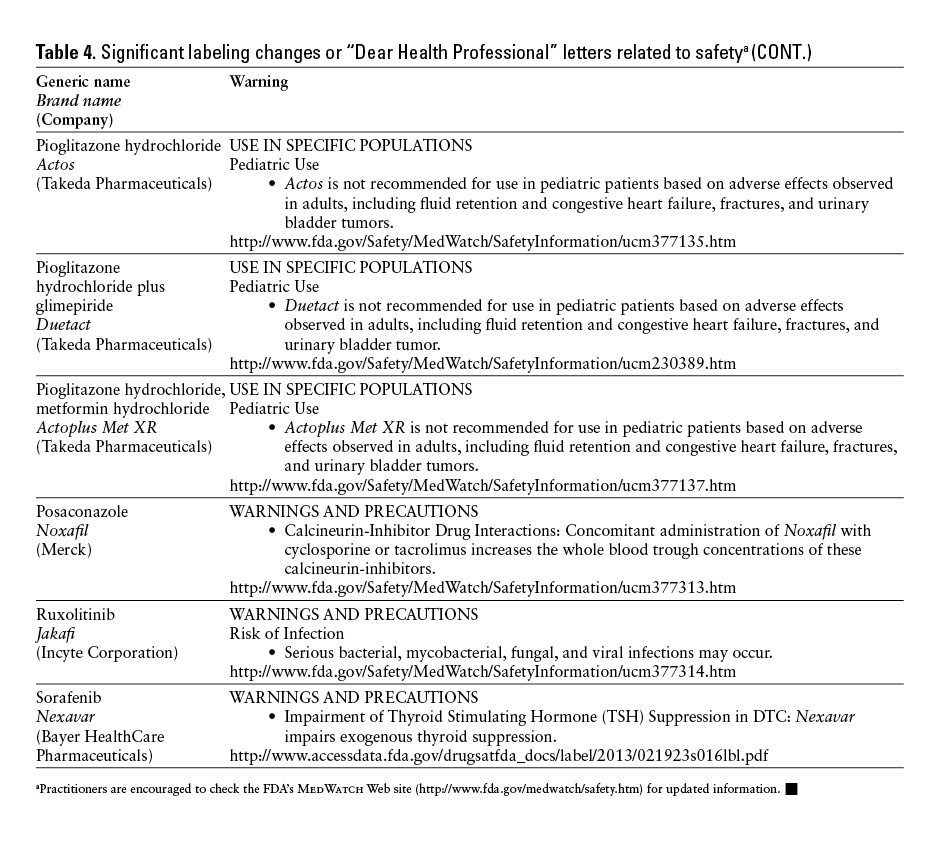


*Practitioners are encouraged to check the FDA’s MedWatch Web site (http://www.fda.gov/medwatch/safety.htm) for updated information. ![]()
*Director, Drug Information Center, College of Pharmacy, Washington State University Spokane, PO Box 1495, Spokane, WA 99210-1495.