Accurate sulfur quantification in organic solvents using isotope dilution mass spectrometry



Glenn Woods
Agilent Technologies (UK) Ltd.
Based upon the published work “Accurate determination of S in organic matrices using isotope dilution ICP-MS/ MS” JAAS 2012 DOI: 10.1039/c2ja30265a by:
Lieve Balcaen and Frank Vanhaecke, Ghent University, Department of Analytical Chemistry, Ghent, Belgium
Martın Resano, University of Zaragoza, Department of Analytical Chemistry, Zaragoza, Spain
Glenn Woods, Agilent Technologies UK Ltd., 5500 Lakeside, Cheadle Royal Business Park, SK8 3GR, UK
Keywords
sulfur, ID-MS, biodiesel, environmental, ethanol, NIST SRM 2773, oxygen mass-shift
Introduction
Accurate measurement of sulfur in aqueous and organic media is relatively difficult for ICP-MS due to intense spectral interferences from polyatomic ions formed mainly from oxygen and nitrogen. Sulfur is an important element in environmental terms as it forms SOx when combusted, contributing to acid rain and photochemical smog. It is also a catalyst poison for some industrial processes and its accurate measurement can be critical.
Experimental
A quadrupole ICP-MS (ICP-QMS) with a collision/ reaction cell set up for O2 mass-shift reaction chemistry can be used to avoid the 16O2+ overlap on 32S+ by converting the S+ to SO+ reaction product ions that are then measured at a new mass (m/z 48) that is free from the O2+ overlap. However, in practice, this approach has been of relatively limited use, as ICP-QMS has no way to reject existing ions at the mass of the new analyte product ions, so not all of the interferences are eliminated, particularly when complex or variable matrices are investigated. There has also been some limited success reported by using Xe as a reaction gas to attenuate the O2-based interference particularly on the 34S isotope. Neither of these approaches reduces the backgrounds significantly enough to allow reliable trace level measurement of S, and they do not necessarily preserve the S isotopic abundances. In this investigation, ethanol was used as an example organic solvent and the Agilent 8800 ICP-QQQ was used to determine S by ID-MS in a biodiesel reference material to assess the measurement accuracy of MS/MS mode with O2 mass-shift for S determination.
Instrumentation: Agilent 8800 #100 with Micromist nebulizer (free aspiration). For organic solvent analysis, a narrow injector torch with id 1.0 mm (G3280-80005) and Pt cones were used. 20% O2 balanced in Ar was introduced via an option gas flow line to prevent carbon build up.
Plasma conditions: Plasma conditions were optimized manually.
(RF power = 1450 W,
CRGS flow rate = 0.98 L/min,
Option gas flow rate = 0.75 L/min and spray chamber temp. = -5˚C).
CRC conditions: O2 gas at 0.4 mL/min, Octopole bias = -9 V, KED = -8 V.
Sample: Biodiesel certified reference material NIST SRM 2773.
Results and discussion
When using mass-shift mode for sulfur (or any element) it is important to eliminate any potential interferences at the target mass of the reaction production, as well as on the primary element mass (the precursor ion). If the target mass suffers from interferences then the measurement would still be compromised. For sulfur, the corresponding isotopes are shifted as follows using M + 16 amu mass-shift:
32S → SO at 48 amu
33S → SO at 49 amu
34S → SO at 50 amu
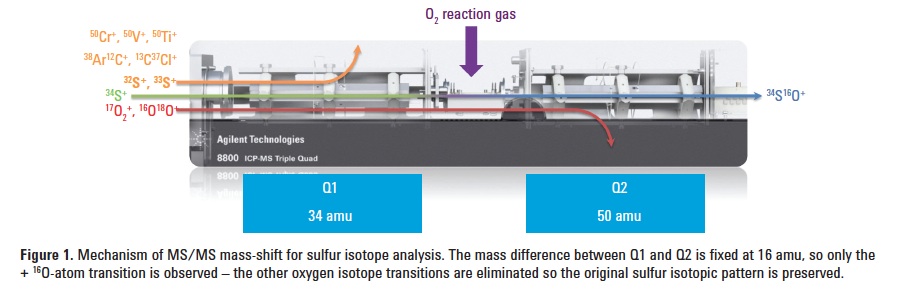
Unfortunately, the SO+ production masses (m/z 48, 49 and 50) can suffer from multiple interferences including Ca+, Cr+, V+, Ti+, ArC+ and CCl+ in natural samples. Furthermore the 33S and 34S isotopes can suffer from overlaps due to other combinations of SO+ product ions, as well as pre-existing ions at the target mass. For example, the 34S16O+ product ion formed at m/z 50 is overlapped by 32S18O+ and 33S17O+, as well as 50Cr+,50V+, 50Ti+,38Ar12C+, and 13C37Cl+. When operating the 8800 ICP-QQQ in MS/MS mass-shift mode, these overlaps are eliminated and the sulfur isotope pattern is preserved. Figure 1 provides a graphical representation of the ICP-QQQ setup and the method of interference elimination.
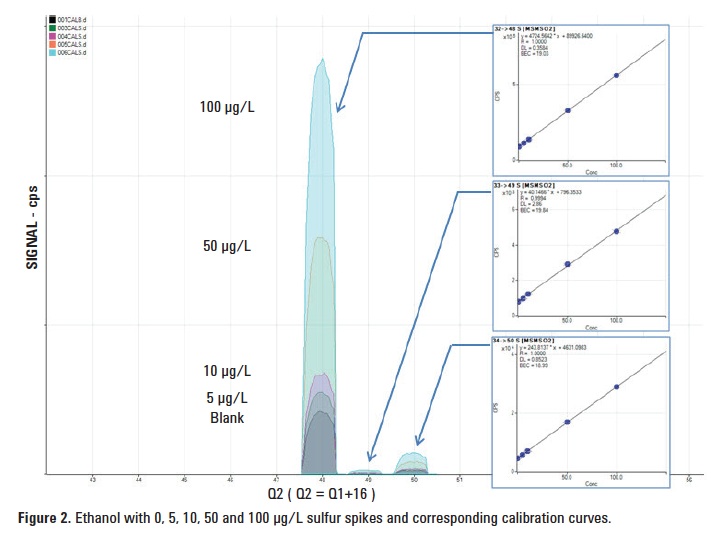
This method would not be useful if the reaction were not quantitative, so to check for linearity, a blank ethanol sample was spiked with sulfur – see Figure 2. Despite the wide variation in absolute sensitivity for the different S isotopes, the BEC was the same for all three isotopes, indicating that the background is due to sulfur in the ethanol.
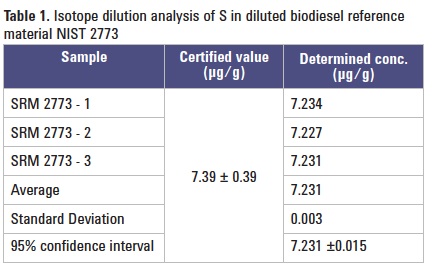
An isotope dilution (ID) method was used to evaluate the accuracy of the 8800 ICP-QQQ MS/MS method, using a biodiesel certified reference material (NIST SRM 2773) and an enriched 34S spike. The biodiesel sample was simply diluted into the ethanol solvent and the appropriate spike added. Reproducibility was tested by analyzing three separate samples of the CRM. The results are presented in Table 1. Repeat measurements were within the expected recovery limits for the material.
Conclusions
Until the introduction of ICP-QQQ with MS/MS capability, it was impossible to obtain reliable results for reaction chemistry methods combined with an ID approach, using a quadrupole-based ICP-MS. The novel QQQ configuration of the 8800 ICP-QQQ enables operation in MS/MS mode, which ensures precise control over the reaction chemistry in the cell. This allows the unique isotopic information of the analyte to be retained, while removing the interferences that could affect both precursor and product ions of the target analyte.