LEAD ISOTOPE ANALYSIS: REMOVAL OF 204Hg ISOBARIC INTERFERENCE ON 204Pb USING ICP-QQQ MS/MS REACTION CELL





Glenn Woods
Agilent Technologies (UK) Ltd.
Keywords
lead, isotope, ratio, geochronology, dating, mercury, artifacts, precious metals, food, ammonia, on-mass
Introduction
Lead isotope ratio analysis is important as it is used for Pb-Pb dating in geochronology, and to trace the origin of artifacts, precious metals and even foodstuffs. The natural isotopic pattern of lead varies more than any other element in the periodic table, because three of its isotopes are formed from the radioactive decay of uranium
(235U → 207Pb; 238U → 206Pb) and thorium (232Th → 208Pb). The Pb isotopic pattern can therefore vary depending upon the geology of the rocks and minerals from which the lead was extracted, and the age of the material. In geochronology, the constant rate of U/Th decay allows the Pb/Pb, U/Pb and Th/Pb ratios to be used to date the age of rocks using a so-called geological clock.
When Pb ratios are measured, it is often necessary to correct for the lead naturally present in the sample, and the only non-radiogenic isotope of Pb (204Pb; natural or common lead), is used for this purpose. For Pb-Pb dating, 204Pb is the reference isotope against which the radiogenic isotopes are compared (206Pb/204Pb; 207Pb/204Pb). Unfortunately 204Pb is directly overlapped by an isotope of Hg (204Hg), which makes accurate measurement of 204Pb impossible by ICP-MS. Mass resolution of
204Pb from 204Hg is far beyond the capability of any commercial high-resolution (HR-) ICP-MS system, and until recently there has been no reliable chemical means to remove the Hg interference, so mathematical correction has been employed, which introduces error. Mercury does however undergo a gas-phase charge-transfer reaction with ammonia gas (NH3), a reaction that can be utilized in the collision/reaction cell of a suitably equipped ICP-MS as follows:
Hg+ + NH3 → Hg0 + “NH3+”
This reaction offers the potential to remove the 204Hg interference from 204Pb, and could be applied to either solution or laser-based ICP-MS analysis.
Experimental
Instrumentation: Agilent 8800 #100.
Plasma conditions: Preset plasma/General purpose.
Ion lens tune: Soft extraction tune:
Extract 1 = 0 V, Extract 2 = -170 V.
CRC conditions: NH3 gas (10% in He) at 1.7 mL/min, Octopole bias = -8 V,
KED = -8 V.
Acquisition parameters: Three acquisition modes were compared:
- No gas: No reaction cell gas; Single Quad (SQ) mode with Q1 operating as an ion guide
- NH3 bandpass: Ammonia reaction gas; SQ mode with Q1 operating as a bandpass filter
- NH3 MS/MS: Ammonia reaction gas; MS/MS mode with Q1 operating as a mass filter at unit mass resolution
Results and discussion
Removal of 204Hg+ interference on 204Pb+
A preliminary study showed that Pb is almost unreactive with NH3 cell gas
(<0.5% loss of Pb signal) indicating that
on-mass sensitivity for Pb should be maintained. On-mass measurement of Pb in NH3 cell gas mode was therefore investigated in the presence of
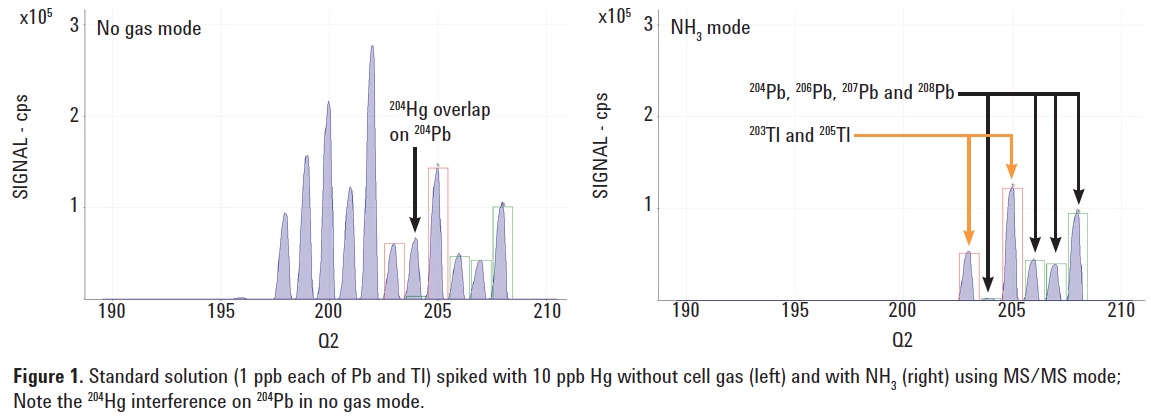
Hg at 10 ppb. Figure 1 displays the spectra obtained in no gas (left) and NH3 cell gas (right) modes. The 204Hg interference on 204Pb can be clearly seen in the no gas spectrum, while it has been completely removed under NH3 reaction mode with MS/MS. A perfect isotopic pattern match was confirmed for Pb in NH3 mode.
Effectiveness of MS/MS
The NH3 reaction that removes the 204Hg interference would also work in the reaction cell of a quadrupole ICP‑MS (ICP-QMS), but ammonia is a highly reactive gas and can produce many adduct cluster ions, for example from Rare Earth Elements (REEs), see Table 1. The complex matrix composition of many natural samples means that the results obtained with NH3 cell gas in ICP-QMS are often extremely unreliable. With the
8800 ICP-QQQ, MS/ MS mode allows all the co-existing matrix elements to be rejected by Q1, so only the target ions (204Pb and 204Hg) enter the CRC. The NH3 reactions are therefore controlled and consistent, and no overlapping reaction product ions are formed from other elements in the sample.
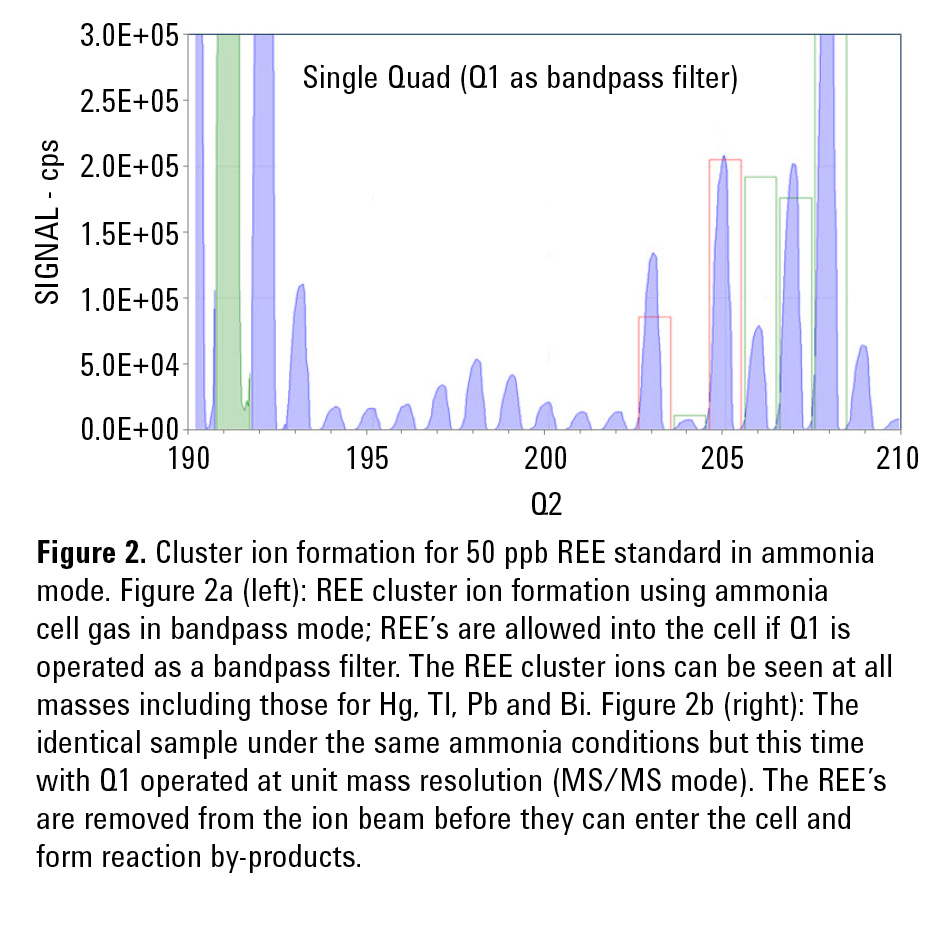
To check the formation of cluster ions, the ICP-QQQ was operated with NH3 cell gas; “Single Quad bandpass” and MS/MS modes were compared for the measurement of a 50 ppb REE mix. Figures 2a and 2b display the spectra obtained using bandpass and MS/MS conditions, respectively.
204Pb/208Pb isotope ratio analysis in presence of Hg
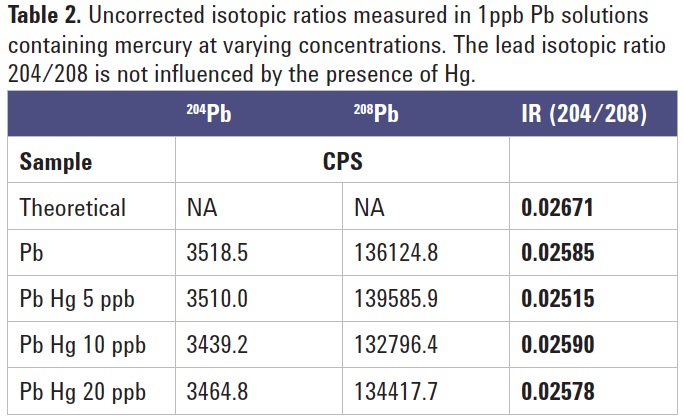
To check the effectiveness of the 204Hg removal, the 204Pb/208Pb ratio was measured in a 1 ppb lead solution spiked with increasing Hg concentration. Table 2 displays the measured Pb ratio results (without any mass bias correction), showing that the Pb isotope ratio remained constant, regardless of the Hg content.
Conclusions
With the successful removal of the 204Hg interference on the natural 204Pb isotope, ICP-QQQ displays great promise for Pb/Pb and U/Pb dating and for other applications where accurate measurement of 204Pb is required.
