By Vida Poursorkhabi1, Amar K. Mohanty2, and Manjusri Misra3
1,2,3school of Engineering, University of Guelph, Guelph, Ontario, Canada 1,2Bioproducts Discovery and Development Center, University of Guelph
By Vida Poursorkhabi1, Amar K. Mohanty2, and Manjusri Misra3
1,2,3school of Engineering, University of Guelph, Guelph, Ontario, Canada 1,2Bioproducts Discovery and Development Center, University of Guelph
By Vida Poursorkhabi1, Amar K. Mohanty2, and Manjusri Misra3
1,2,3school of Engineering, University of Guelph, Guelph, Ontario, Canada 1,2Bioproducts Discovery and Development Center, University of Guelph
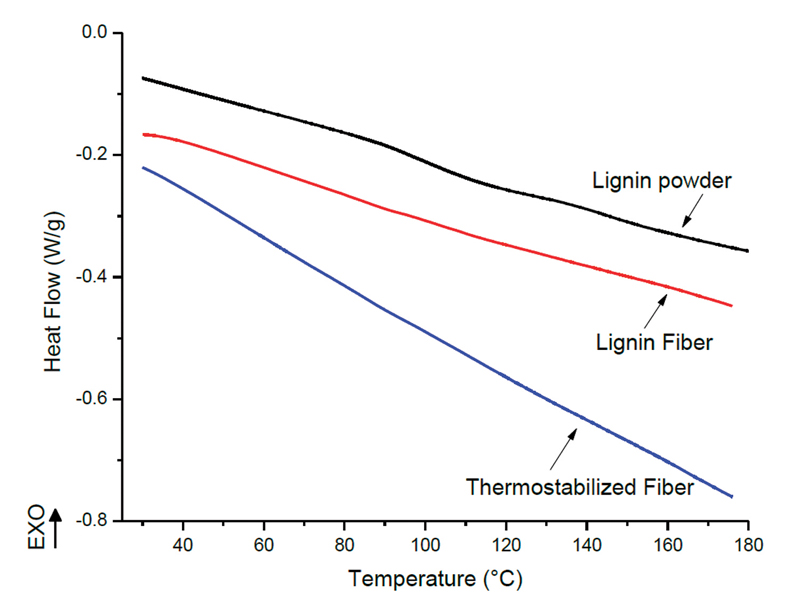
Figure 1: DSC thermograms of lignin powder, and its electrospun fibers before and after thermostabilization.
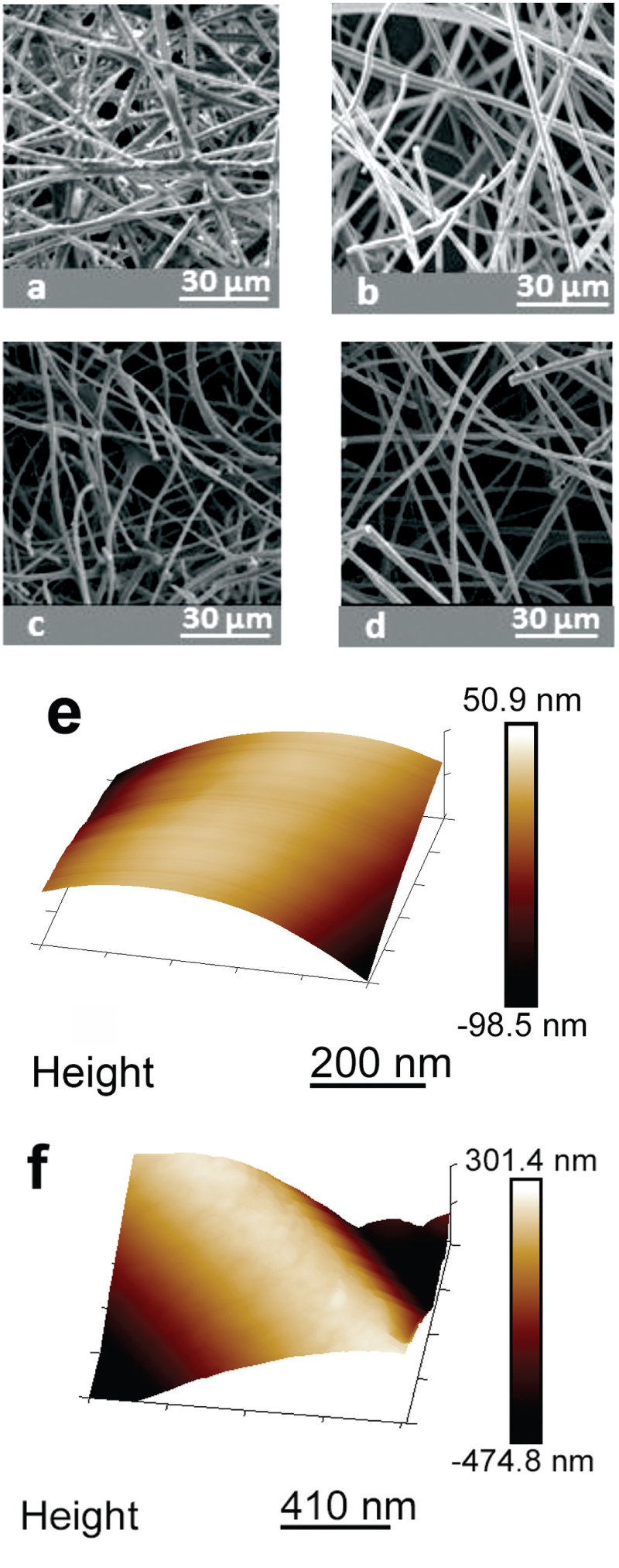
Figure 2: SEM images of electrospun lignin/PEO:95/5 fibers: (a) as-spun fibers, (b) thermostabilized fibers, (c) carbonized fibers (1), and (d) carbonized fibers (2). Below these are AFM images of electrospun lignin/PEO 95:5 fibers: (e) as-spun fibers and (f) carbonized fibers (2).
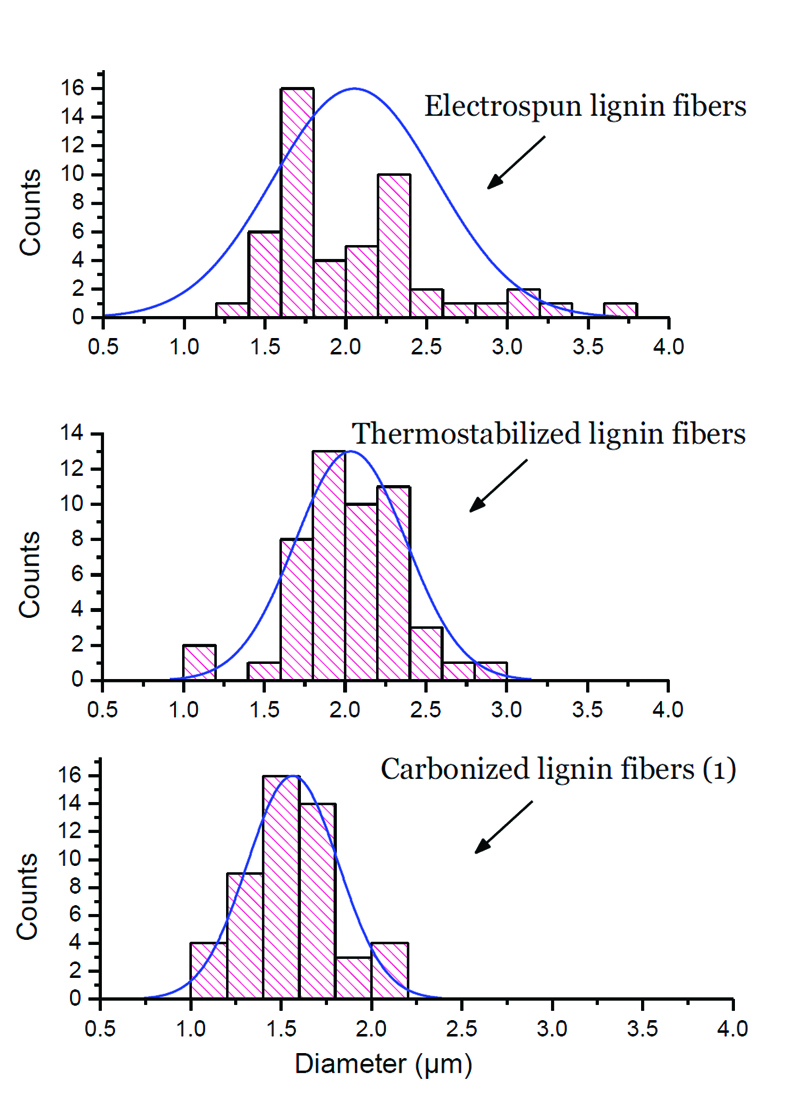
Figure 3: Fiber diameter distribution.
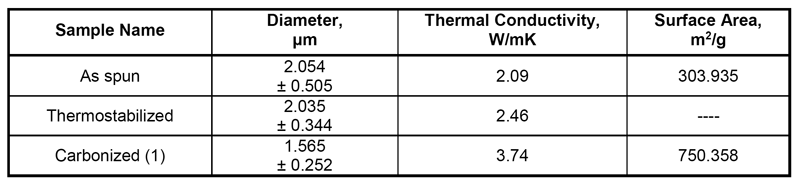
Table 1: Properties of As-Spun, Thermostabilized, and Carbonized Lignin/PEO Fibers
Note: The lead author was awarded the Ken J. Braney International Award for her student poster presentation on this same topic at ANTEC® Orlando 2015. The original version of this paper was also presented at the conference.
Lignin is a polyphenolic polymer which is naturally synthesized in lignocellulosic biomass. This polymer is linked to cellulose and hemicellulose and provides the structural integrity of the cell wall. The lignin content of biomass depends on the type of biomass, and is around 18-25% for hardwoods. Annually, a large amount of lignin is produced by its separation from cellulose and only a limited amount of it has commercial application. Lignin is a highly available source of renewable carbon, and research is going on to produce carbonized fibers from lignin for applications in composites or electronic devices.1-4
The isolation of lignin from biomass is a pretreatment step in the paper and cellulosic ethanol industries. Multiple processes are used to break and separate lignin from cellulose and hemicellulose. Lignin remains in the byproduct stream of the process.2
Annually around 20 billion tons of lignin are produced. Most of it is either burned to produce energy or discarded in landfills. Only a limited amount of this material is used in commercial products.5,6 The low molecular weight of lignin and the variability of properties based on the extraction method are some of the limiting factors of lignin utilization in commercial products.
One of the promising areas of application of lignin is in the production of carbonized fibers. Lignin has a high carbon content which is comparable to polyacrylonitrile (PAN), the commercial precursor of carbon fiber.6,7 The melt spinning and electrospinning of lignins have been studied to produce precursor fibers for carbonization.8 While melt-spun fibers are mainly for composite applications, electrospun fibers have diameters in the range of submicron to a few microns that are mostly targeted for energy-storage applications.
Electrospinning is a versatile and convenient process for producing nano-/micro-diameter fibers with a high surface area. This spinning technology can be scaled up for large-scale production.9,10 It also enables the spinning of polymers which are sensitive to heat or can degrade during melt spinning.
Electrospinning of blends of lignin with PAN, polyethylene oxide (PEO), polyvinyl alcohol (PVA), chitosan, and cellulose acetate has been reported.9,11-16 The majority of research has been done on fibers spun from solutions of lignin blends in N,N-dimethyl formamide (DMF) or co-electrospinning of organosolv lignin in ethanol, and a few studies have been done on spinning of aqueous solutions.12
In general, using high volumes of toxic organic solvents to dissolve the polymer is one of the drawbacks of the electrospinning process. For electrospinning of lignin, the other drawback is the low solution elasticity, due to the low molecular weight and broad molecular weight distribution of lignin. The solution needs to be modified by blending lignin with a second polymer or by using only high molecular weight fractions of lignin.
This research investigates the electrospinning of a low molecular weight organosolv hardwood lignin. Alkaline aqueous solutions were prepared by blending a low percentage of PEO (5%) with lignin. The interactions between lignin and PEO in this system enhanced the spinnability and allowed reduction of PEO content.17 The fibers were successfully carbonized and characterized.
Materials
Organosolv hardwood lignin (Mw = 1551 g/mol, from the producer’s datasheet) was provided by Lignol Innovations Ltd. Sodium hydroxide and PEO (Mv = 2,000,000) were purchased from Fisher Scientific and Sigma-Aldrich, respectively.
Methods
First, lignin and PEO were separately dissolved in alkaline aqueous solution and distilled water, respectively. The solutions were vigorously stirred for one hour at 70°C. Then they were mixed and stirred for another 15 min. The solution was electrospun in a NANON-01 electrospinning instrument (MECC Co.). The weight percentage of PEO in spun fibers was 5%, compared to lignin. The electrospinning voltage and feed rate were 20 kV and 0.7 mL/hr, respectively. The distance between the needle and collector was kept at 20 cm. Temperature and humidity of the spinning chamber were 30°C and 36-40%, respectively.5
Electrospun fibers were thermostabilized in air by heating the fibers to 250°C at a heating rate of 0.5°C /min., followed by holding at 250°C for 2 hr. Thermostabilized fibers were carbonized in nitrogen atmosphere in a tube furnace (Carbolite, 1200°C, G-range). Carbonized fibers were produced in two different conditions: “carbonized (1)” fibers heated to 900°C at a heating rate of 3°C/min. and held at 900°C for 2.5 hr, and “carbonized (2)” fibers heated to 1100°C at a heating rate of 2°C/min. and held at 1100°C for 3 hr.
The glass transition temperature of the fibers before and after thermostabilization was studied by differential scanning calorimetry (DSC), and it was compared with lignin powder (DSC-Q200, TA Instruments). For the DSC experiments, samples were heated to 90°C and held isothermally for 10 min. to remove the moisture. Then the samples went through a heat/cool/heat cycle from 0 to 200°C.
Fiber morphology was studied by scanning electron microscopy (SEM; Inspect S50–FEI Company) and atomic force microscopy (AFM; Multi-mode 8 from Bruker Nano Inc). A Hot Disk TPS 500 Thermal Constants Analyzer (ThermTest, Inc.) was used to measure the thermal conductivity of the fibers. Surface area measurement (BET analysis) of the fibers was done from the nitrogen gas sorption at 77.3 K measured by a NOVA 4200e (Quantachrome Instruments). Raman spectra were collected with a Renishaw Raman imaging microscope with a laser wavelength of 785 nm. All spectra were obtained with extended scans between 500 and 2000 cm−1.
Results
The carbonization yield was measured by heating hardwood lignin powders in the same conditions that fibers were carbonized. The percentage of weight loss was 57%.
Figure 1 shows the DSC graphs of the lignin powder, its electrospun fibers, and thermostabilized fibers from the second heating of the samples. The Tg of the lignin powder was determined to be around 96°C. The Tg for the fibers is difficult to detect by conventional DSC; however, it was observed that orientation of the polymer chains affected the transition points for the fibers. After thermostabilization, the glass transition point was not detected, which shows the fibers are suitable for carbonization.
SEM images of the fibers are shown in Figure 2. Electrospun fibers from alkaline aqueous solutions had larger diameters compared to fibers spun from DMF solutions. This is due to the resistance of the blend to elongational forces during spinning.17 The results show carbonization of the fibers without melting or deformation. Higher-magnification SEM images and also AFM images show the surface of fibers are relatively smooth. After carbonization, the average fiber diameter was reduced due to the mass loss of lignin during carbonization.
Figure 3 shows the distribution of fiber diameters. Thermostabilization narrows the diameter distribution but does not have a significant effect on the average diameters. The reduction in average fiber diameter that occurred after carbonization suggests that by modifying the spinning conditions, it is feasible to get carbonized fibers with sub-micron diameters.
Properties of the fibers, i.e., diameter, thermal conductivity, and surface area are reported in Table 1. Thermal conductivity of the fibers increased after thermostabilization and carbonization. This is due to the removal of volatile materials and formation of a carbonized structure.
The surface area of the fibers increased after carbonization, which is due to the diameter reduction of the fibers. This value is lower than the surface area of carbonized fibers obtained from pitch and pure PAN fibers, but it is comparable to and higher than other precursors tested for carbon fiber production and also PAN fibers with fillers.18
Raman spectroscopy was used to study the extent of ordered carbon in the carbonized sample. The ratio of the peak at 1350 cm-1 (D band), which is due to amorphous carbon, to the intensity of the peak at 1580 cm-1 (G band), which is due to the graphitic carbon, was 1.37. This value is comparable to the values obtained for PAN-based electrospun carbon fibers.18
Conclusions
Electrospun lignin fibers were produced by spinning alkaline aqueous blends of low molecular weight hardwood lignin (95 wt%) and 5 wt% PEO. The fibers were thermostabilized and carbonized without fusion of fibers. The average fiber diameter was ~2.1 ± 0.5 µm, which reduced to ~1.6 ± 0.3 µm after carbonization. The carbonized fibers had twice the surface area and higher thermal conductivity compared to the as-spun fibers. A comparison of the results with PAN-based electrospun carbon fibers shows the high potential of low molecular weight lignin to be a renewable precursor for carbonized fiber production.
Acknowledgements
The authors are thankful for the financial support from the Natural Sciences and Engineering Research Council (NSERC), Canada, for the Discovery grants individual (project #400320), NSERC NCE AUTO21 (project # 460373); the Ontario Ministry of Economic Development and Innovation (MEDI) for the Ontario Research Fund (ORF) Research Excellence (RE) Round-4 (Project # 050289); and the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA) University of Guelph Bioeconomy Industrial uses research program (Project # 200371). The authors are also thankful to Lignol Innovations Ltd. for providing the Organosolv Lignin samples.
References
- P. Sannigrahi, Y. Pu, and A. Ragauskas, Curr. Opin. Environ. Sustainability, 2, 383, (2010).
- Y. Zeng, S. Zhao, S. Yang, and S.-Y. Ding, Curr. Opin. Biotechnol., Energy Biotechnology, Environmental Biotechnology, 27, 38 (2014).
- M.N.S. Kumar, A.K. Mohanty, L. Erickson, and M. Misra, J. Biobased Mater. Bioenergy, 3, 1 (2009).
- V. Poursorkhabi, M. Misra, and A.K. Mohanty, BioResources, 8, 5083 (2013).
- S. Laurichesse, and L. Avérous, Prog. Polym. Sci., Topical Issue on Biomaterials, 39, 1266 (2014).
- W.-J. Liu, H. Jiang, and H.-Q. Yu, Green Chem., 17, 4888 (2015).
- Suhas, P.J.M. Carrott, and M.M.L.R. Carrott, Bioresource Technol., 98, 2301 (2007).
- D.A. Baker, and T.G. Rials, J. Appl. Polym. Sci., 130, 713 (2013).
- N.-Y. Teng, I. Dallmeyer, and J.F. Kadla, J. Wood Chem. Technol., 33, 299 (2013).
- L. Persano, A. Camposeo, C. Tekmen, and D. Pisignano, Macromol. Mater. Eng., 298, 504 (2013).
- I. Dallmeyer, F. Ko, and J.F. Kadla, J. Wood Chem. Technol., 30, 315 (2010).
- M. Lallave, J. Bedia, R. Ruiz-Rosas, J. Rodrίguez-Mirasol, T. Cordero, J.C. Otero, M. Marquez, A. Barrero, and I.G. Loscertales, Adv. Mater., 19, 4292 (2007).
- M. Ago, K. Okajima, J.E. Jakes, S. Park, and O.J. Rojas, Biomacromolecules, 13, 918 (2012).
- D.K. Seo, J.P. Jeun, H.B. Kim, and P.H. Kang, Rev. Adv. Mater. Sci., 28, 31 (2011).
- M. Schreiber, S. Vivekanandhan, A.K. Mohanty, and M. Misra, Adv. Mater. Lett., 4, 476 (2012).
- M. Schreiber, S. Vivekanandhan, P. Cooke, A. Mohanty, and M. Misra, J. Mater. Sci., 49, 7949 (2014).
- V. Poursorkhabi, A.K. Mohanty, and M. Misra, J. Appl. Polym. Sci., 132, 41260 (2015).
- M. Inagaki, Y. Yang, and F. Kang, Adv. Mater., 24, 2547 (2012).
About the authors

Vida Poursorkhabi (pictured) is an engineering Ph.D. candidate at the Bioproducts Discovery and Development Center (BDDC) in the University of Guelph’s School of Engineering. Her research interests cover the electrospinning of biopolymers (especially lignin), characterization of nanofibers, and production and characterization of carbonized fibers for applications in energy-storage devices. Dr. Amar K. Mohanty is the director of the BDDC and a professor at Guelph’s School of Engineering, Department of Plant Agriculture. He’s an international expert in the fields of bioplastics, biobased materials, and biorefineries, with a focus in engineering new sustainable materials. Dr. Manju Misra ( mmisra@uoguelph.ca) is also a professor at Guelph’s Department of Plant Agriculture; her current research focuses primarily on novel bio-based composites from agricultural and forestry resources for the sustainable bio-economy.