Plastic Failure Through Molecular Degradation
Multiple mechanisms can attack polymer chains—here’s what can go wrong
Previous Article Next Article
By Jeffrey Jansen
The Madison Group, Madison, Wisconsin, USA
Plastic Failure Through Molecular Degradation
Multiple mechanisms can attack polymer chains—here’s what can go wrong
Previous Article Next Article
By Jeffrey Jansen
The Madison Group, Madison, Wisconsin, USA
Plastic Failure Through Molecular Degradation
Multiple mechanisms can attack polymer chains—here’s what can go wrong
Previous Article Next Article
By Jeffrey Jansen
The Madison Group, Madison, Wisconsin, USA

Figure 1: This nylon water-heater valve degraded through contact with chlorinated water at elevated temperatures.

Figure 1: This nylon water-heater valve degraded through contact with chlorinated water at elevated temperatures.


Figure 2: Reference and failed connectors, shown as received. The failed parts exhibited significant discoloration.

Figure 2: Reference and failed connectors, shown as received. The failed parts exhibited significant discoloration.
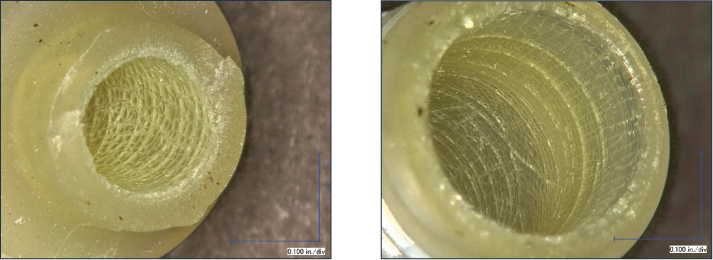
Figure 3: Photomicrographs showing features associated with brittle cracking on the transverse fracture surface of the connector (left) and an interconnecting series of cracks on the interior surfaces at both the nipple end (left) and threaded end (right).

Figure 3: Photomicrographs showing features associated with brittle cracking on the transverse fracture surface of the connector (left) and an interconnecting series of cracks on the interior surfaces at both the nipple end (left) and threaded end (right).

Figure 4: SEM image showing mud cracking, characteristic of severe molecular degradation, on the interior surface of connector.

Figure 4: SEM image showing mud cracking, characteristic of severe molecular degradation, on the interior surface of connector.
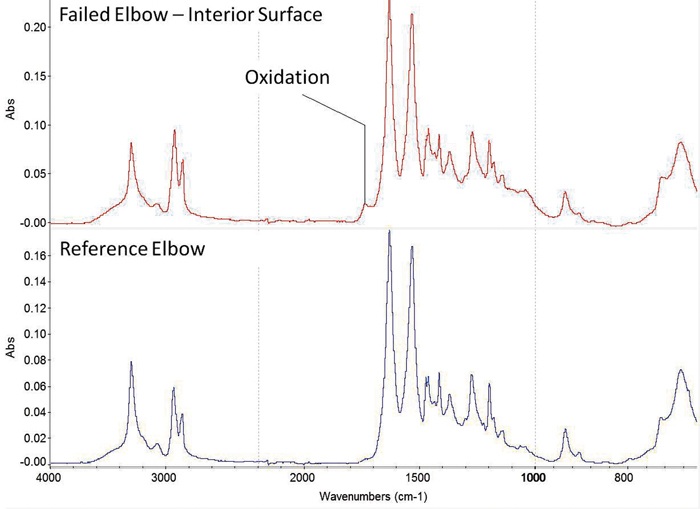
Figure 5: FTIR results on the failed elbow of the connector showed an absorption band indicative of oxidation.

Figure 5: FTIR results on the failed elbow of the connector showed an absorption band indicative of oxidation.
SPE webinar:
Degradation Failure of Plastics
Wednesday, February 18, 2015, 11:00 a.m. EST
Plastic materials offer a unique balance of strength and ductility associated with their inherent viscoelastic nature. However, they are susceptible to molecular degradation through a variety of exposures. This degradation can occur during compounding, processing, storage, or while in service.
Understanding the nature of degradation can help prevent failure. Thus, topics covered during this webinar will include:
- Introduction to plastic molecular degradation, including the various mechanisms
- Material susceptibility to degradation
- Stabilizers to prevent degradation
- Testing to assess the level of degradation
For more details or to register, go to www.4spe.org/Events/webinars.aspx.
[Note: The author will be presenting the SPE webinar “Degradation Failure of Plastics” on Feb. 18; see more details near the end of the article in the sidebar.—Ed.]
Molecular degradation is a leading cause of plastic component failure, with a study indicating that 17% of plastic failures are associated with a degradation mechanism.1 In generic terms, molecular degradation of a plastic is the deleterious alteration of the molecular structure within the polymer as the result of a chemical reaction. Importantly, degradation mechanisms principally involve a permanent reduction in molecular weight as a result of the chemical reaction. There are numerous molecular degradation mechanisms, but the most common are:
- thermal oxidation,
- ultraviolet radiation,
- chain scission, and
- hydrolysis.
Mechanisms of Degradation
The dominant mechanism of molecular degradation and the extent of degradation are dependent on the composition of the plastic resin and the surrounding environmental conditions. Significant property reduction can occur within most polymer families as a result of molecular degradation. All forms of degradation represent chemical reactions that result in molecular structural changes that are accompanied by noted decreases in physical properties. The reduction in molecular weight associated with degradation inherently leads to lower ductility due to the accompanying loss of entanglement of the now shortened polymer chains. This reduces the
energy required for disentanglement/ slippage to occur and shifts the preferred mechanism from ductile yielding to brittle fracture.
The manifestations of molecular degradation can include:
- loss of mechanical properties, like
– reduction in strength,
– embrittlement,
– cracking, and
– catastrophic failure;
- reduction in chemical resistance;
- aesthetic changes, like
– discoloration,
– chalking,
– loss of gloss, and
– clouding/loss of transparency;
- evolution of volatiles (including foul odor generation); and
- carbonyl formation (and loss of dielectric properties).
Molecular degradation can occur throughout the lifecycle of a plastic component. Regardless of when the degradation occurs, the effects can lead to premature failure of the component. (An example of a failure is shown in Figure 1, and some typical circumstances are listed in Table 1.)
Thermal oxidation
Thermal oxidation is the degradation of a polymeric material through contact with a chemical oxidizer. Most polymers are subject to oxidation, and it is the most common form of plastic degradation (consider Table 2).
Oxidation is a chemical reaction in which oxygen is introduced into the molecular structure of the polymer, creating a form of carbon-oxygen bonds known as carbonyl functionality. It produces a permanent change within the plastic, most often in the form of molecular weight reduction through shortening of the polymer chains. Oxidation takes place via a multi-step reaction based on free radical formation. Free radicals can be found within plastic formulations as unintentional residual byproducts of polymerization, as formulation additives, or as contaminants. These free radicals react with oxygen to attack the polymer backbone covalent bonds.
In most cases, the rate of the degradation mechanism will accelerate with time as the reaction proceeds to autocatalyze. Through thermal oxidation, the polymer chains are cleaved, and the resulting shortened chains are terminated with oxygenated functional groups, including carboxylic acids, esters, ketones, and aldehydes.
Photo-oxidation
Photo-oxidation, commonly referred to as ultraviolet (UV) degradation, is the degradation of a polymeric material through exposure to terrestrial light energy in conjunction with contact with a chemical oxidizer, such as air. Based upon their structure and the functional groups comprising the polymer, some plastics are inherently susceptible to photo-oxidation. Given enough light energy input, these susceptible functional groups, known as chromophores, cleave to produce free radicals.
Degradation through photo-oxidation is very similar to the thermal oxidation, and is driven by the production of free radicals. Essentially, the exposure to light energy results in the accelerated formation of free radicals, which speeds the initiation of the degradation reaction. Photo-oxidation is a chemical reaction facilitating the incorporation of oxygen into the backbone structure as carbonyl groups, resulting in molecular weight reduction of the polymer.
UV energy at short wavelengths is most severe toward polymeric materials, and can break the covalent molecular bonds forming the polymer backbone. The shortest UV wavelengths found within the Earth’s atmosphere are sufficient to produce this degradation. Most often, the chromophore that is susceptible to the UV energy is a functional group within the polymer; however, it can also be associated with an additive within the formulation. Bonds susceptible to photo-oxidation include carbon-nitrogen bonds such as nitrile, amide, and amine; carbon-oxygen bonds such as ether, ester, ketone, and carboxylic acid; carbon-chlorine bonds; oxygen-oxygen bonds such as peroxide; and nitrogen-hydrogen such as amide and amine.
Degradation damage is generally limited to the surface layer exposed to the UV radiation, to a depth of approximately 150 µm. The depth of the degradation penetration is determined by the extent of oxygen diffusion into the material. However, even damage to this minimal degree can produce a deleterious outcome. The radiation exposure degrades the polymeric material, which results in a brittle surface layer. This degraded layer is then subject to localized shrinkage, producing stresses, or the direct application of stress in the application. This stress, and the accompanying strain, makes the material more prone to further oxidation and results in the initiation of cracks within the surface layer. The cracking subsequently extends into the base material through brittle fracture.
Chain scission
Chain scission is the degradation of a polymeric material in the absence of a chemical agent, in particular without oxygen. As part of chain scission, the molecular structure of the polymer is altered solely based on energy, potentially as exposure to high levels of stress or elevated temperature. The covalent bonds forming the polymer backbone will break down randomly, resulting in rapid decreases in molecular weight.
Like thermal oxidation, chain scission proceeds via a free radical mechanism. As the polymer backbone chains are broken, carbon-carbon double bonds, known as unsaturation, are formed. The absence of oxygen drives this degradation mechanism. Normally, chain scission is caused by exposure to elevated temperatures in combination with high shear stress, and is often associated with processing techniques where air is not present.
Hydrolysis
Hydrolysis is the degradation of a polymeric material through contact with water, specifically the hydrogen cations (H+) or hydroxyl anions (OH-). Hydrolytic degradation can occur within plastic materials as a result of submersion in water, progression through condensation cycles, or by exposure to steam. It can also result from contact with acids (high H+ concentration) or bases (high OH- concentration), which can dramatically accelerate the process.
Some polymers are inherently susceptible to hydrolysis, given their molecular structure. Specifically, condensation polymers, such as those listed below, form water during the polymerization process, and as
such are susceptible to hydrolytic degradation:
- polyesters (like poly(butylene terephthalate), poly(ethylene terephthalate), and copolyester resins);
- polycarbonate;
- nylons (nylon 6, nylon 6/6, nylon 12);
- polyurethane (ether-based and ester-based); and
- polyacetal (homopolymers and copolymers).
Like the other types of molecular degradation reviewed here, hydrolysis represents a chemical reaction that results in a permanent change within the molecular structure of the polymer. The hydrolysis mechanism proceeds through the reaction of the polymer with water, resulting in the cleavage of the susceptible functional group, commonly into chemical species that resemble the initial reactants used in the original polymerization process. With some polymers, water is produced as a byproduct of the hydrolysis reaction, and in these cases the degradation proceeds relatively rapidly.
It is important to recognize that hydrolysis is different than water absorption. While water absorption can significantly alter the mechanical properties of a plastic, particularly nylon resins, the effect is transient and reversible.
Factors Influencing Degradation
The various forms of molecular degradation present some commonalities in regards to the factors that influence the type of degradation and the rate and degree of damage to the material. Generalized, the most important parameters affecting the extent of molecular degradation are as follows:
- Polymer type: The susceptibility to the specific types of molecular degradation is variable, and based on the polymer structure.
- Effectiveness of stabilization additives: The type, loading, and permanence of anti-degradant package used in the plastic formulation.
- Temperature: Higher temperatures result in increased reaction rates.
- Strength of reactant chemical or radiation: Stronger or more concentrated reactive agents will result in faster degradation.
- Stress level: Higher levels of stress results in more rapid degradation—internal and external stresses are combined.
A Case Illustration
What went wrong?
A plastic elbow connector used in a food service application failed while in service after approximately six months of use (Figure 2). A series of connectors installed within equipment at the same site had all failed prematurely, while parts at other locations had performed as expected. As part of normal operation, the systems are cleaned using a variety of commercial products. The elbow connector was stated to be produced from a nylon 6/6 resin.
Evaluation
Microscopic examination of the failed connector confirmed a catastrophic transverse crack within the nipple end of the elbow. The fracture surface displayed features associated with brittle cracking. Inspection of the interior surfaces of the elbow revealed widespread cracking in an intersecting pattern, commonly referred to as mud cracking (Figures 3-4).
The exterior surface of the failed part showed significant discoloration in the form of yellowing; however, cracking was not found. The extent and form of the cracking was indicative of molecular degradation of the elbow material initiating from the interior. Scanning electron microscopic (SEM) examination revealed further evidence indicating molecular degradation was severe and consistent with chemical attack associated with exposure to an aggressive, incompatible chemical agent
Fourier transform infrared spectroscopy (FTIR) was performed on the reference elbow, and the obtained results were consistent with a nylon resin (Figure 5). The interior surface of the failed elbow was also analyzed, and the resulting spectra contained absorption patterns associated with the nylon base material. However, additional absorption bands indicative of carbonyl functionality were also present, consistent with oxidative degradation.
Energy dispersive X-ray spectroscopy (EDS) was performed on the base material of the reference connector and the interior surface of the failed part. Both results showed relatively high levels of carbon, oxygen, and nitrogen, as expected for a nylon resin. Additionally, the interior surface of the failed part presented a low, but significant level of potassium.
Conclusion
It was the conclusion of the evaluation that the elbow connector failed as a result of severe molecular degradation of the nylon plastic resin. Degradation was indicated by the significant mud cracking and discoloration, as well as the oxidation indicated by FTIR.
The degradation, and subsequently the cracking, initiated on the interior surface of the connector, indicating that the chemical agent responsible for the degradation was contained within the equipment. Analysis of the interior surface of the failed part showed the presence of potassium, and because of the lack of other elements, this was thought to represent the presence of potassium hydroxide. Given the internal nature of the degradation, the general classes of materials that produce molecular degradation in nylon 6/6, and the presence of potassium, the likely potential source of the deleterious chemical agent was the cleaning products used in conjunction with sanitation of the food service equipment.
Reference
- Wright, D. Failure of Plastics and Rubber Products (Shawbury, Shrewsbury, Shropshire, UK: RAPRA Technology Ltd., Cambridge: 2001), p. 5.
About the author:
Jeffrey A. Jansen is a senior managing engineer and partner at The Madison Group (www.madisongroup.com; +1 608-231-1907), an independent plastics engineering and consulting firm. He specializes in failure analysis, material identification and selection, and aging studies for thermoplastic materials, and has been solving polymer-related problems for 23 years, during which he has performed over 1,525 failure investigations. Jansen is a regular presenter for SPE’s webinar series, covering a wide range of topics related to plastics failure, material performance, testing, and polymer technology.

