Original Article
Stability of Glutamate-Aspartate Cardioplegia Additive Solution in Polyolefin IV Bags
Steven D. Rush, PharmD Candidate*; Stephanie E. Kim, PharmD*; Susan E. Hughes, RPh†; Justine M. Gilbert, RPh†; Peter P. Ciancaglini, PharmD†; and Fang Zhao, PhD*
Original Article
Stability of Glutamate-Aspartate Cardioplegia Additive Solution in Polyolefin IV Bags
Steven D. Rush, PharmD Candidate*; Stephanie E. Kim, PharmD*; Susan E. Hughes, RPh†; Justine M. Gilbert, RPh†; Peter P. Ciancaglini, PharmD†; and Fang Zhao, PhD*
Original Article
Stability of Glutamate-Aspartate Cardioplegia Additive Solution in Polyolefin IV Bags
Steven D. Rush, PharmD Candidate*; Stephanie E. Kim, PharmD*; Susan E. Hughes, RPh†; Justine M. Gilbert, RPh†; Peter P. Ciancaglini, PharmD†; and Fang Zhao, PhD*
Abstract
Objective: Glutamate-aspartate cardioplegia additive solution (GACAS) is used to enhance myocardial preservation and left ventricular function during some cardiac surgeries. This study was designed to evaluate the stability of compounded GACAS stored in sterile polyolefin intravenous (IV) bags. The goal is to extend the default USP beyond-use date (BUD) and reduce unnecessary inventory waste.
Methods: GACAS was compounded and packaged in sterile polyolefin 250 mL IV bags. The concentration was 232 mM for each amino acid. The samples were stored under refrigeration (2°C-8°C) and analyzed at 0, 1, and 2 months. At each time point, the samples were evaluated by pH measurement and visual inspection for color, clarity, and particulates. The samples were also analyzed by high-performance liquid chromatography (HPLC) for potency and degradation products. Due to the lack of ultraviolet (UV) chromophores of glutamate and aspartate, the samples were derivatized by ortho-phthalaldehyde prior to HPLC analysis.
Results: The time zero samples of GACAS passed the physical, chemical, and microbiological tests. Over 2 months of storage, there was no significant change in pH or visual appearance for any of the stability samples. The HPLC results also indicated that the samples retained 101% to 103% of the label claim strengths for both amino acids.
Conclusion: The physical and chemical stability of extemporaneously prepared GACAS has been confirmed for up to 2 months in polyolefin IV bags stored under refrigeration. With proper sterile compounding practice and microbiology testing, the BUD of this product can be extended to 2 months.
Key Words—aspartate, BUD, cardioplegia solution, compounding, glutamate, stability
Hosp Pharm—2015;50:522–525
Abstract
Objective: Glutamate-aspartate cardioplegia additive solution (GACAS) is used to enhance myocardial preservation and left ventricular function during some cardiac surgeries. This study was designed to evaluate the stability of compounded GACAS stored in sterile polyolefin intravenous (IV) bags. The goal is to extend the default USP beyond-use date (BUD) and reduce unnecessary inventory waste.
Methods: GACAS was compounded and packaged in sterile polyolefin 250 mL IV bags. The concentration was 232 mM for each amino acid. The samples were stored under refrigeration (2°C-8°C) and analyzed at 0, 1, and 2 months. At each time point, the samples were evaluated by pH measurement and visual inspection for color, clarity, and particulates. The samples were also analyzed by high-performance liquid chromatography (HPLC) for potency and degradation products. Due to the lack of ultraviolet (UV) chromophores of glutamate and aspartate, the samples were derivatized by ortho-phthalaldehyde prior to HPLC analysis.
Results: The time zero samples of GACAS passed the physical, chemical, and microbiological tests. Over 2 months of storage, there was no significant change in pH or visual appearance for any of the stability samples. The HPLC results also indicated that the samples retained 101% to 103% of the label claim strengths for both amino acids.
Conclusion: The physical and chemical stability of extemporaneously prepared GACAS has been confirmed for up to 2 months in polyolefin IV bags stored under refrigeration. With proper sterile compounding practice and microbiology testing, the BUD of this product can be extended to 2 months.
Key Words—aspartate, BUD, cardioplegia solution, compounding, glutamate, stability
Hosp Pharm—2015;50:522–525
Abstract
Objective: Glutamate-aspartate cardioplegia additive solution (GACAS) is used to enhance myocardial preservation and left ventricular function during some cardiac surgeries. This study was designed to evaluate the stability of compounded GACAS stored in sterile polyolefin intravenous (IV) bags. The goal is to extend the default USP beyond-use date (BUD) and reduce unnecessary inventory waste.
Methods: GACAS was compounded and packaged in sterile polyolefin 250 mL IV bags. The concentration was 232 mM for each amino acid. The samples were stored under refrigeration (2°C-8°C) and analyzed at 0, 1, and 2 months. At each time point, the samples were evaluated by pH measurement and visual inspection for color, clarity, and particulates. The samples were also analyzed by high-performance liquid chromatography (HPLC) for potency and degradation products. Due to the lack of ultraviolet (UV) chromophores of glutamate and aspartate, the samples were derivatized by ortho-phthalaldehyde prior to HPLC analysis.
Results: The time zero samples of GACAS passed the physical, chemical, and microbiological tests. Over 2 months of storage, there was no significant change in pH or visual appearance for any of the stability samples. The HPLC results also indicated that the samples retained 101% to 103% of the label claim strengths for both amino acids.
Conclusion: The physical and chemical stability of extemporaneously prepared GACAS has been confirmed for up to 2 months in polyolefin IV bags stored under refrigeration. With proper sterile compounding practice and microbiology testing, the BUD of this product can be extended to 2 months.
Key Words—aspartate, BUD, cardioplegia solution, compounding, glutamate, stability
Hosp Pharm—2015;50:522–525
Hosp Pharm 2015;50(6):522–525
2015 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj5006-522
Cardioplegia solutions induce rapid and complete diastolic arrest to minimize myocardial energy requirements during surgeries.1 This prevents ischemic damage during the arrest phase and minimizes reperfusion injury once coronary blood flow is restored.1 The cardiac consumption of glutamate and aspartate increases in the setting of hypoxia.2 During the first 4 hours following cardiopulmonary bypass, the heart does not use lipids or carbohydrate substrates and takes up amino acids, mainly glutamate.2 The amino acids glutamate and aspartate are Kreb cycle precursors that are used in cardioplegia solutions to improve myocardial metabolism.1 These amino acids can be metabolized in anaerobic conditions for energy production as well as to counteract the depletion of Kreb cycle intermediates during ischemia.1 Cardioplegia solutions rich in amino acids, glutamate and aspartate, enhance myocardial preservation and left ventricular function.2
Because the glutamate-aspartate cardioplegia additive solution (GACAS) is used only in selected cardiac surgeries, there are no commercial products available the United States. Typically this solution is prepared and sterilized by extemporaneous compounding in the hospital pharmacy. Due to the frequently unpredictable nature of cardiac surgery schedules, it is desirable to have this product available in the pharmacy inventory at all times. However, the default USP beyond-use date (BUD) of this high-risk level sterile product is only 3 days under refrigeration,3 which leads to significant inventory waste over time.
This study was initiated to evaluate the physical and chemical stability of the GACAS product in sterile polyolefin IV bags. These bags are selected as the packaging materials because of their low moisture permeability and minimal amount of leachable plasticizers. The goal is to establish an extended BUD of 2 months for storing the GACAS at refrigeration temperature (with passing sterility and endotoxin testing results for each batch).
MATERIALS AND METHOD
Materials
L-glutamic acid (monosodium and monohydrate) and L-aspartic acid (monosodium and monohydrate) were purchased from Sigma-Aldrich, St. Louis, MO, Cat# RES5063G-A701X and A6683, respectively. The empty sterile 250 mL polyolefin IV bags were purchased from Baxter, Round Lake, IL. Product# 2B8012 bags were completely di-(2-ethylhexyl)phthalate (DEHP) free, and Product# 2J8002 bags contain DEHP only in the polyvinyl chloride (PVC) layer of the administration and medication ports.
The ortho-phthalaldehyde (OPA) derivatization reagent (also contains 3-mercaptoproprionic acid) was purchased from Agilent, Palo Alto, CA, Part# 5061-3335. All other chemicals used for the HPLC analysis were purchased from Fisher Scientific. The borate buffer was prepared by adjusting 0.4 M boric acid to pH 10.2 with 10 M sodium hydroxide. Type I purified water was produced by a Milli-Q system (Millipore, Billerica, MA).
Stability Study
Cardioplegia solution containing 232 mM each of glutamate and aspartate was compounded extemporaneously using aseptic techniques. The amino acid additive solution was prepared by dissolving the L-glutamic acid and L-aspartic acid, each as the monosodium and monohydrate form, in USP Water for injection. The solution was cold-sterilized using a 0.2 micron filter and packaged in the 2 types of sterile polyolefin 250 mL IV bags — one completely PVC/DEHP free and another containing PVC/DEHP in the port. The label claim of each 250 mL IV bag was 58 mmol glutamate and 58 mmol aspartate.
The packaged bags were stored under refrigeration (2°C-8ºC). For each bag type, triplicate samples were pulled for stability evaluation at time zero, 1 month, and 2 months. The stability evaluation included visual inspection, pH measurement, and reverse phase HPLC (RP-HPLC) analysis. At each time point, a 10 mL aliquot was withdrawn, by syringe, from each bag and expelled into a 20 mL clear glass vial. The samples were visually inspected for clarity, color, and presence of particulate matter. The same samples were subjected to pH measurement by a Mettler-Toledo Seven Easy pH meter. Finally, a 970 µL aliquot from each sample was diluted to 250 mL with purified water followed by OPA derivatization and RP-HPLC analysis (3 replicate injections).
Time zero samples from the same stability batch were also submitted for routine endotoxin and sterility testing, and the results were satisfactory. Endotoxin testing was performed using Endosafe PTS system (Charles River Laboratories International, Model PTS100) with Limulus Amebocyte Lysate Test Cartridges (sensitivity 0.05 EU/mL, Product# PTS2005F). Sterility testing was performed using BBL Trypticase Soy Broth – Casein Digest Medium (Becton, Dickinson and Company, Cat# 221716), BBL Fluid Thioglycollate Medium (Becton, Dickinson and Company, Cat# 221196), and Potato Dextrose Agar (General Laboratory Products, Cat# 16T-2900-SL).
OPA Derivatization
Due to the absence of suitable UV chromophores for glutamate and aspartate, OPA derivatization was performed to enable RP-HPLC analysis of the stability samples. A manual procedure was developed according to the application note published by Agilent.4 For each diluted stability sample, a 25 µL -aliquot was first mixed with 25 µL of 0.4 M borate buffer solution in an HPLC vial. Five µL of OPA reagent was then added and gently mixed. Finally, 320 µL of purified water was added, and the sample was analyzed within 1 hour. The reaction of amino acids with OPA and 3-mercaptoproprionic acid forms an S-substituted isoindole functional group that has a unique UV chromophore at 338 nm.4,5
High-Performance Liquid Chromatography (HPLC)
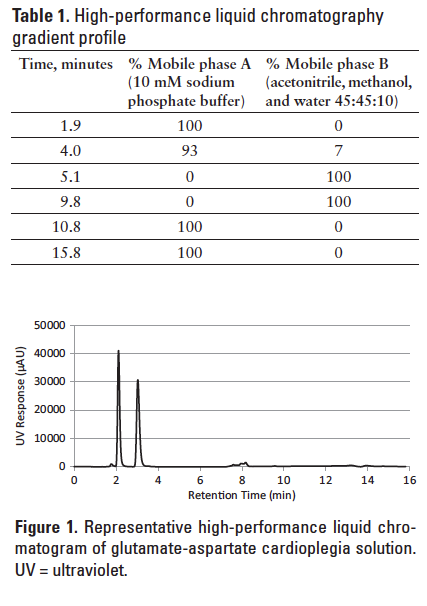
The HPLC analysis was performed using a Shimadzu model LC-2010A system (Shimadzu Scientific Instruments, Marlborough, MA). The chromatographic parameters were adapted from the application note published by Agilent.4 An Agilent Eclipse AAA C18 column (4.6 x 150 mm, 5 µm) was used and maintained at 40ºC. The mobile phase A was 10 mM sodium phosphate buffer (pH 7.8), and mobile phase B was acetonitrile, methanol, and water at 10:45:45 v/v/v. The gradient method of the mobile phase is listed in Table 1, with a constant flow rate of 0.8 mL/min. The sample injection volume was 10 µL, and the UV detection was set at 338 nm. Under these conditions, derivatized aspartate and glutamate were well separated with retention times of approximately 2.1 and 3.0 minutes, respectively (Table 1).
At each stability time point, 3 standard solutions each of glutamate and aspartate were prepared at 0.8, 0.9, and 1.0 mM for calibration purpose. This range encompassed the expected concentration of the diluted stability samples. Calibration curves were constructed by linear regression of the peak area against the glutamate and aspartate concentration, respectively. The curves were found to be linear over the concentration range of the standards with an R2 of 0.990 or better. The precision of the method was established by multiple injections of the mid-point (0.9 mM) standard on each analysis day. The intraday and interday coefficients of variation were all within 1.0%. The amino acid quantity in each stability sample was calculated based on the calibration result and converted to percent label claim. For this study, stability was defined as 90% to 110% of the label claim with no significant change in visual appearance or pH.
Typically a forced degradation study with extreme stress conditions is conducted to verify that the HPLC method can separate the potential degradation products and is therefore stability-indicating. However, this study was not carried out in this investigation, because the extreme stress conditions employed would interfere with the OPA derivatization process of the test samples. It was also considered unlikely that the potential degradation products would react with OPA in the same manner as the parent amino acids.
RESULTS AND DISCUSSION
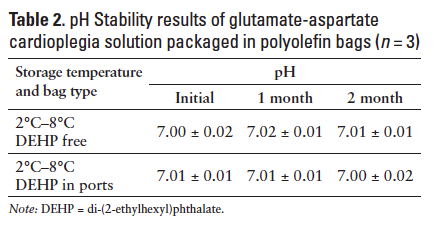
At the initiation of the stability study, the GACAS appeared as a clear and colorless liquid free of particulate matter. The initial sample pH and strength values are reported in Tables 2 and 3. Over 2 months of storage at refrigeration temperature (2°C-8°C), all samples remained clear, colorless, and free of visible particles. No significant change in pH was observed for any stability samples (Table 2).
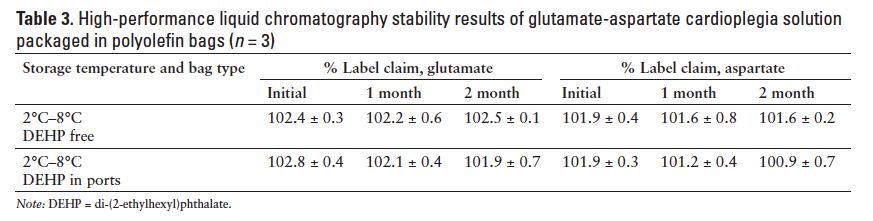
The HPLC analysis results also showed that all samples retained 101% to 103% of the label claim strengths for both glutamate and aspartate (Table 3).
This suggests that there was no significant loss of the amino acids due to chemical degradation or physical adsorption to the polyolefin infusion bag packaging materials.
The BUD assignment of a compounded sterile preparation is typically a 2-step process.3 First, the physical and chemical stability of the product at the recommended storage conditions needs to be established. Second, the risk of microbial contamination needs to be assessed and minimized for the compounding procedure. This study confirmed that the physical and chemical stability of the GACAS is -satisfactory for up to 2 months when stored at refrigeration conditions. With proper sterile compounding practice and microbiology testing, the BUD of GACAS can be safely assigned for 2 months.
Acknowledgments
Financial support/disclosures: The authors declare no conflicts of interest or financial interests in any product or service mentioned in this article, including grants, employment, gifts, stock holdings, or honoraria.
Additional contributions: The authors would like to acknowledge Dr. Ron Angona, Chief Cardiovascular Perfusionist at University of Rochester Medical Center, for his assistance with the article.
References
- Donnelly AJ, Djuric M. Carioplegia solutions. Am J Health Syst Pharm. 1991; 48:2444-2460.
- Arsenian M. Potential cardiovascular applications of glutamate, aspartate, and other amino acids. Clin Cardiol. 1998;21:620-624.
- General Chapter ⟨797⟩ Pharmaceutical Compounding-Sterile Preparations. In: United States Pharmacopeia and National Formulary, USP 36/NF 31. Rockville, MD: United States Pharmacopeial Convention, Inc.; 2013.
- Agilent Technologies. Application note: Automatic precolumn derivatization of amino acids and analysis by fast LC using the Agilent 1290 Infinity LC System [Internet]. Revised July 10, 2010. http://www.chem.agilent.com/Library/applications/5990-5599EN.pdf. Accessed December 20, 2013.
- Allison LA, Mayer GS, Shoup RE. o-Phthalaldehyde derivatives of amines for high-speed liquid chromatography/electrochemistry. Anal Chem.1984;56(7):1089-1096.

*Wegmans School of Pharmacy, St. John Fisher College, Rochester, New York; Department of Pharmacy, University of Rochester Medical Center, Rochester, New York. Corresponding author: Fang Zhao, PhD, Wegmans School of Pharmacy, St. John Fisher College, 3690 East Avenue, Rochester, NY 14618; fax: 585-385-5295; e-mail: fzhao@sjfc.edu