Original Article
Comparison of Inpatient Glycemic Control with Insulin Vials Versus Insulin Pens in General Medicine Patients
Juliann Horne, PharmD*; Rucha Bond, PharmD, CDE†; and Preeyaporn Sarangarm, PharmD, BCPS‡
Original Article
Comparison of Inpatient Glycemic Control with Insulin Vials Versus Insulin Pens in General Medicine Patients
Juliann Horne, PharmD*; Rucha Bond, PharmD, CDE†; and Preeyaporn Sarangarm, PharmD, BCPS‡
Original Article
Comparison of Inpatient Glycemic Control with Insulin Vials Versus Insulin Pens in General Medicine Patients
Juliann Horne, PharmD*; Rucha Bond, PharmD, CDE†; and Preeyaporn Sarangarm, PharmD, BCPS‡
Abstract
Background: The Institute for Safe Medication Practices has recommended against routine use of insulin pen devices for inpatients, but the quality of inpatient glycemic control that is achieved with insulin pens versus insulin vials and syringes has not been compared.
Objective: To evaluate the quality of glycemic control achieved with insulin vials versus insulin pens in type 2 diabetic general medicine patients.
Methods: This retrospective cohort study compared glycemic control between 2 groups of patients on rapid-acting insulin protocols: those receiving insulin via patient-specific pen devices and those receiving insulin from patient-specific vials. Patients on a prespecified subacute care floor with a diagnosis of type 2 diabetes and at least 24 hours of glucose monitoring while on an insulin protocol with insulin lispro were included. Glycemic control was compared by area under the curve (AUC) estimations of average overall glucose and average glucose above, below, and within goal range (70-180 mg/dL). Percentages of time above, below, and within goal range were also compared.
Results: The mean ± SD AUC-estimated average glucose for pens was 160 ± 39 mg/dL compared to 158 ± 45 mg/dL for vials (P = .752). The mean ± SD percentage time within goal range was 68.2% ± 28.1% in the pen group versus 69.4% ± 31.8% percent in the vial group (P = .825). No statistically significant differences were detected between those receiving pens or vials for any outcome before and after adjusting for baseline differences and significant covariates.
Conclusion: Glycemic control did not differ based on insulin delivery system.
Key Words—blood glucose, diabetes mellitus, type 2, drug delivery systems, injections, inpatient, insulin, subcutaneous
Hosp Pharm—2015;50:514–521
Abstract
Background: The Institute for Safe Medication Practices has recommended against routine use of insulin pen devices for inpatients, but the quality of inpatient glycemic control that is achieved with insulin pens versus insulin vials and syringes has not been compared.
Objective: To evaluate the quality of glycemic control achieved with insulin vials versus insulin pens in type 2 diabetic general medicine patients.
Methods: This retrospective cohort study compared glycemic control between 2 groups of patients on rapid-acting insulin protocols: those receiving insulin via patient-specific pen devices and those receiving insulin from patient-specific vials. Patients on a prespecified subacute care floor with a diagnosis of type 2 diabetes and at least 24 hours of glucose monitoring while on an insulin protocol with insulin lispro were included. Glycemic control was compared by area under the curve (AUC) estimations of average overall glucose and average glucose above, below, and within goal range (70-180 mg/dL). Percentages of time above, below, and within goal range were also compared.
Results: The mean ± SD AUC-estimated average glucose for pens was 160 ± 39 mg/dL compared to 158 ± 45 mg/dL for vials (P = .752). The mean ± SD percentage time within goal range was 68.2% ± 28.1% in the pen group versus 69.4% ± 31.8% percent in the vial group (P = .825). No statistically significant differences were detected between those receiving pens or vials for any outcome before and after adjusting for baseline differences and significant covariates.
Conclusion: Glycemic control did not differ based on insulin delivery system.
Key Words—blood glucose, diabetes mellitus, type 2, drug delivery systems, injections, inpatient, insulin, subcutaneous
Hosp Pharm—2015;50:514–521
Abstract
Background: The Institute for Safe Medication Practices has recommended against routine use of insulin pen devices for inpatients, but the quality of inpatient glycemic control that is achieved with insulin pens versus insulin vials and syringes has not been compared.
Objective: To evaluate the quality of glycemic control achieved with insulin vials versus insulin pens in type 2 diabetic general medicine patients.
Methods: This retrospective cohort study compared glycemic control between 2 groups of patients on rapid-acting insulin protocols: those receiving insulin via patient-specific pen devices and those receiving insulin from patient-specific vials. Patients on a prespecified subacute care floor with a diagnosis of type 2 diabetes and at least 24 hours of glucose monitoring while on an insulin protocol with insulin lispro were included. Glycemic control was compared by area under the curve (AUC) estimations of average overall glucose and average glucose above, below, and within goal range (70-180 mg/dL). Percentages of time above, below, and within goal range were also compared.
Results: The mean ± SD AUC-estimated average glucose for pens was 160 ± 39 mg/dL compared to 158 ± 45 mg/dL for vials (P = .752). The mean ± SD percentage time within goal range was 68.2% ± 28.1% in the pen group versus 69.4% ± 31.8% percent in the vial group (P = .825). No statistically significant differences were detected between those receiving pens or vials for any outcome before and after adjusting for baseline differences and significant covariates.
Conclusion: Glycemic control did not differ based on insulin delivery system.
Key Words—blood glucose, diabetes mellitus, type 2, drug delivery systems, injections, inpatient, insulin, subcutaneous
Hosp Pharm—2015;50:514–521
Hosp Pharm 2015;50(6):514–521
2015 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj5006-514
Hyperglycemia in hospitalized patients outside the intensive care unit (ICU) has been associated with increased hospital length of stay, infection, disability, and death.1-6 Similarly, inpatient hypoglycemia has been associated with poor outcomes.7,8 Though the ideal blood glucose targets remain a subject of debate, the most recent consensus guidelines for the inpatient management of hyperglycemia in the non–critical care setting recommend a premeal target of less than 140 mg/dL and random blood glucose targets less than 180 mg/dL for most patients.9,10 Subcutaneous insulin is the recommended medication used to achieve inpatient glycemic control.
Debate exists over the safest, most effective method of administering insulin to achieve inpatient glycemic control. Insulin pen devices can harbor biological materials in the pen cartridge, which may lead to bloodborne pathogen transmission if pens are inadvertently used on multiple patients, even if a different needle is used.11-13 Consequently, the Institute for Safe Medication Practices (ISMP) has called on hospitals to discontinue routine use of insulin pens for inpatient insulin administration.14 In accordance with ISMP recommendations, our institution transitioned from insulin administration via patient-specific insulin lispro pen devices (Humalog KwikPens, Lilly, USA, LLC, Indianapolis, IN) to patient-specific insulin lispro 3-mL vials (Humalog 3mL vial; Lilly USA, LLC, Indianapolis, IN). Concern regarding efficacy of insulin administered via vials and syringes compared to pens prompted this study evaluating the quality of glycemic control achieved with insulin vials versus insulin pens in type 2 diabetic general medicine patients. The study also identified patient-specific factors that may influence glycemic control.
METHODS
This retrospective cohort study was performed at an academic tertiary care medical center. The medical center’s institutional review board approved the study protocol. The internal medicine service, consisting of 8 medicine teams, managed the care of all patients using a standardized insulin lispro protocol. This protocol specifies a target average glucose value between 80 and 120 mg/dL with a goal of avoiding values exceeding 180 mg/dL or below 70 mg/dL. The protocol allows for selection between eating or nothing by mouth (NPO) status. The eating protocol includes 3 customizable components of insulin therapy: mealtime insulin lispro, correctional (sliding scale) insulin lispro, and basal insulin glargine. Providers may select from a low-, moderate-, or high-dose protocol, or they may enter a custom dose. Under the eating protocol, capillary blood glucose (CBG) is checked before each meal and at bedtime. The NPO protocol includes correctional insulin lispro with or without basal insulin glargine and specifies CBG testing every 4 hours.
Patients were selected from a prespecified hospital subacute care unit with stays during the same 4-month period in 2 consecutive years (pens: September 2012 through December 2012; vials: September 2013 through December 2013). Transition from pens to vials occurred on July 30, 2013; a 1-month period lapsed between the hospital’s transition from pens to vials before vial group patients were eligible for inclusion. Eligible patients were at least 18 years old, diagnosed with type 2 diabetes mellitus during or before admission, and on one of the previously described insulin lispro protocols. Patients were excluded if they were admitted for acute diabetic ketoacidosis, hyperosmolar hyperglycemic nonketotic syndrome, or hypoglycemia. Patients with type 1 diabetes or who had received octreotide, non-insulin antihyperglycemics, or greater than 24 hours of systemic steroids during admission were also excluded. Patients were ineligible if they refused greater than 15% of insulin doses, were transferred to the study unit from another inpatient unit, or received less than 24 hours of CBG monitoring.
Demographics, such as age, gender, ethnicity, and body mass index (BMI) upon admission, were collected for each patient. Potential confounding
variables were documented, including enteral or parenteral nutrition, eating or NPO insulin protocol, home insulin use, admission blood glucose, most recent hemoglobin A1C within previous year, medicine team, history of liver disease or chronic pancreatitis, current acute pancreatitis, percent of insulin doses refused, inpatient use of insulin glargine, antipsychotics (as needed or scheduled), and other nonprotocol insulin, such as regular insulin. Other variables were collected as an additional means of assessing glycemic control, including inpatient administration of glucagon, glucose, or dextrose and, if applicable, reason for transfer to the ICU. In addition, each patient’s first glycemic excursion (CBG 180 mg/dL) after their first within-goal CBG value was examined to evaluate appropriateness of the preceding insulin dose and time. Appropriateness was determined based on administration of insulin within 30 minutes of measured CBG at doses in concordance with the patient’s insulin orders at the time of the CBG result.
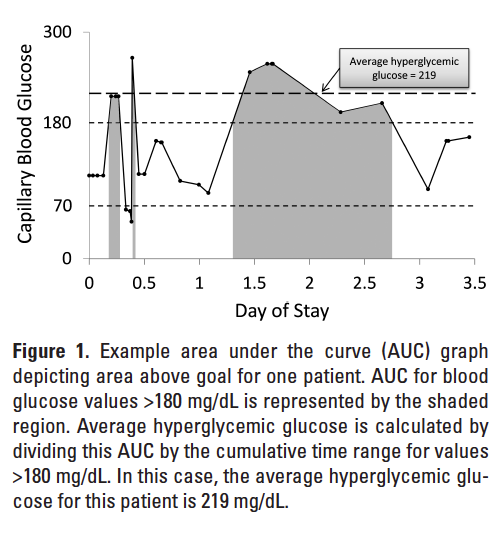
CBG values were collected for each patient along with date and time of measurement. Only CBG values taken after the patient first arrived on the study unit until the patient was discharged or transferred from the study unit were included. These values were plotted on a graph versus time. Area under the curve (AUC) was calculated for each patient for total area, area above goal, within goal, and below goal. Areas were calculated in R Statistics using the trapezoidal rule.15,16 Figure 1 shows a representative AUC graph for an example patient. AUC analysis allows for assessment of hyperglycemic and hypoglycemic excursions without placing improper weight on out-of-range values due to increased frequency of testing during periods of glycemic excursion. Area per unit time also provides a more accurate estimate of the average glucose than would a simple mean
or median.15
Outcomes and Statistical Analysis
The 2 primary outcomes for this study were estimated average glucose, which was determined by AUC divided by time, and percentage time within goal range. Goal range was defined as CBG between 70 and 180 mg/dL in accordance with our hospital insulin protocol and national guidelines.9,10 Hypoglycemia was defined as CBG 180 mg/dL. Secondary outcomes included percentage time hyper- and hypoglycemic as well as estimated average glucose above, within, and below goal range. All outcomes were standardized to patient length of stay on the study unit.
Statistical analyses were conducted using IBM SPSS Statistics (IBM Corp., Armonk, NY). P values less than .05 were considered statistically significant. Baseline characteristics were compared by independent t tests for continuous variables, Pearson’s chi-square analysis for categorical variables, and Fisher’s exact test for variables with expected values of less than 5. Primary and secondary outcomes were analyzed by independent t tests. Tests for normality were performed to ensure the normality assumption of the independent t test was met. Covariates were identified by testing all baseline characteristics against the
2 primary outcomes using Pearson correlation for continuous variables, linear regression for binary variables, and one-way analysis of variance (ANOVA) for variables with more than 2 categories. Variables with P values less than or equal to .1 in the univariate analysis were included in the multivariate analysis. Predictors for average blood glucose and percent time within, above, and below goal were determined using multiple linear regression.
RESULTS
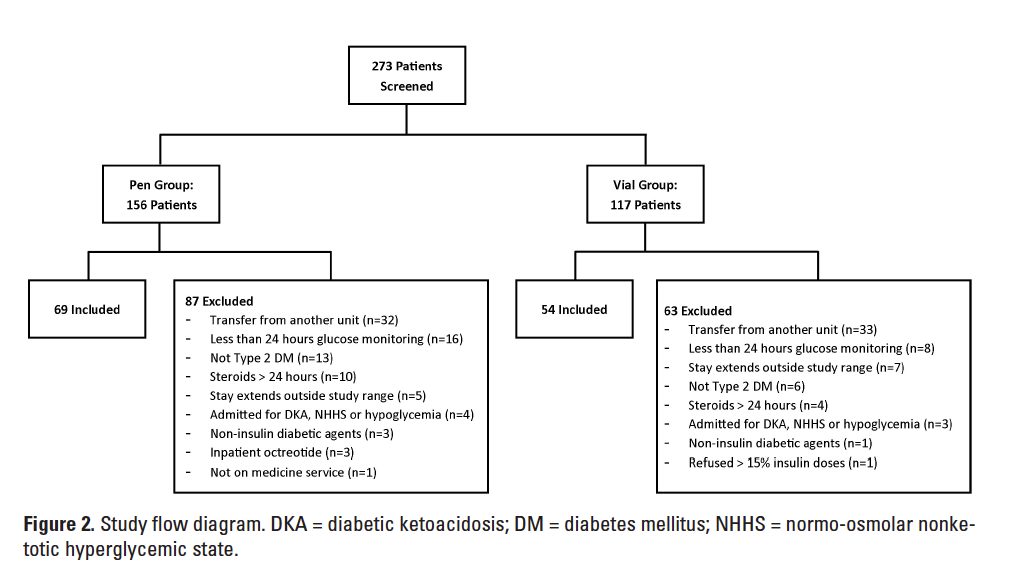
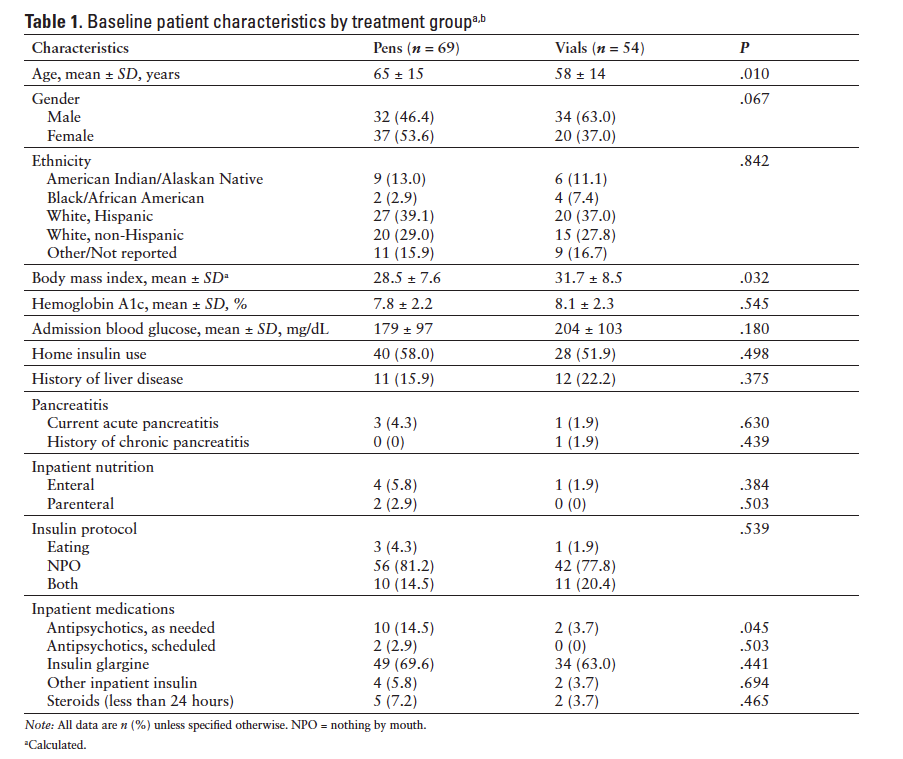
Of the 273 patients initially screened, 123 met our inclusion criteria. The final analysis included 69 patients in the pen group and 54 patients in the vial group. The most common reason for exclusion was transfer to the study unit from another inpatient unit (Figure 2). Patients in the pen group had a significantly higher mean age, lower mean BMI, and greater use of as-needed antipsychotics at baseline than patients in the vial group (Table 1). Nonsignificant differences were noted in gender and admission blood glucose. For the population overall, the average baseline A1c was 7.9% and the mean BMI was 29.9 kg/m2. Our study population was diverse, with 38.2% Hispanic Whites and 12.2% Native Americans. After excluding patients who refused greater than 15% of insulin doses, no patient refused more than 2 doses of insulin and the number of patients refusing doses was similar between groups.
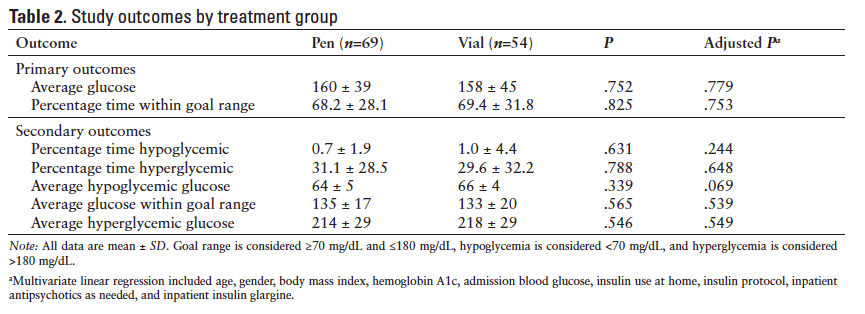
Results for the study outcomes are depicted in Table 2. The mean ± standard deviation (SD) overall average glucose for pens was 160 ± 39 mg/dL compared to 158 ± 45 mg/dL for vials (P = .752). The mean ± SD percentage time within goal range was 68.2% ± 28.1% in the pen group versus 69.4% ± 31.8% in the vial group (P = .825). No significant differences existed between pen and vial groups in average glucose above, within, or below goal or in percentage time above or below goal.
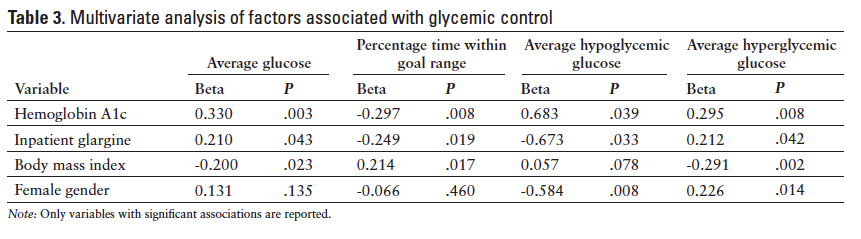
The univariate analyses of baseline characteristics to determine correlation with average glucose and percentage time within goal range detected significant associations for A1c, admission blood glucose, home insulin, and inpatient glargine. These variables were subsequently incorporated in the multiple linear regression, along with variables that differed between pen and vial groups at baseline (age, BMI, gender, and as-needed antipsychotics). After accounting for these variables, P values comparing pen and vial groups remained nonsignificant for all outcomes (Table 2). However, there was a trend toward greater average hypoglycemic glucose in the vial group compared to the pen group (P = .069). In the same multivariate analysis, female gender, low A1c, and inpatient glargine were associated with more severe hypoglycemia (Table 3). High A1c, low BMI, and inpatient glargine were associated with more severe hyperglycemia and increased percentage time hyperglycemic.
Glycemic Excursions
When the first glycemic excursion was examined for each patient, only 2 patients of the pen group and 4 patients of the vial group had received an inappropriate dose of insulin prior to the excursion. Only 1 patient of the pen group and 4 patients of the vial group received their dose at the inappropriate time. Doses were considered appropriate if they were given on schedule and according to the basal, mealtime, and correctional insulin ordered. Use of glucose or dextrose was required in 7 pen patients and 3 vial patients. In the pen group, 4 patients required transfer to the ICU compared to 2 patients in the vial group. Only 1 of these ICU transfers was related to glycemic control: 1 patient in the pen group was transferred due to diabetic ketoacidosis.
DISCUSSION
The results of our study suggest that no difference in glycemic control exists when insulin lispro is administered via patient-specific vials and syringes versus pen devices. Average glucose levels above, within, and below goal were similar between groups. Percentages of time above and below goal range were similar as well. Therefore, magnitude and duration of hypoglycemia and hyperglycemia did not differ between groups. The multivariate analysis revealed consistent results except for a trend toward significantly higher average hypoglycemic glucose in the vial group compared to the pen group. While this trend is not likely clinically significant, it may suggest an advantage for vials compared to pens due to milder hypoglycemia.
As expected, high A1c and inpatient insulin glargine were associated with greater hyperglycemia because patients with poorer control at baseline are likely to be more difficult to control in-hospital and are also likely to be placed on a basal insulin regimen. The inverse associations between BMI and average glucose and the positive association between BMI and time within the goal range are less intuitive. However, we hypothesize that providers may underestimate the insulin needs of patients with low BMI, resulting in greater hyperglycemia in this population. Patients on long-acting basal insulin experienced more severe hypoglycemia (lower average hypoglycemic glucose), which is a known risk of basal insulin in the hospital setting, given the frequent interruptions of regular meals. Female gender and low A1c were also associated with more severe hypoglycemia, possibly indicating a need to exercise caution in these individuals. In previous studies, female gender and baseline A1c have been shown to correlate with poor inpatient glycemic control.17,18
Hospitals use various strategies to administer insulin and achieve glycemic control in inpatients. Some hospitals have elected to provide insulin to inpatients via multidose pen devices because the pens allow for convenient insulin administration and dosage measurement. However, serious risk exists if pens are inadvertently used for more than one patient. Multiple studies have documented that biologic materials can travel through the needle to the pen cartridge and be delivered during subsequent uses.11-13
Thus, the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration (FDA) have recommended against sharing pen devices between patients due to the risk of transmitting bloodborne pathogens, even when different needles are used.19,20 The Centers for Medicare & Medicaid Services (CMS) considers sharing of insulin pens to be equivalent to the re-use of needles or syringes in regard to citations.21 Despite these warnings, patients continue to be at risk of exposure as demonstrated by at least 5 events resulting in patient notification of possible exposure to bloodborne pathogens through insulin pen sharing since the beginning of 2013.22-26 Because of the risk of inadvertent sharing of insulin pens, the ISMP urges hospitals not to use insulin pens routinely for inpatient insulin administration.14 However, the ISMP does acknowledge a role for pen use in training patients for home insulin use upon discharge. The American Society of Health-System Pharmacists recently convened an expert panel that considered the use of insulin pen devices for inpatients to be safe contingent upon appropriate policies, procedures, and staff education to prevent use of a single pen on multiple patients.27
In light of ISMP recommendations, concerns have been raised that the use of insulin vials and syringes may adversely affect inpatient glycemic control compared to the use of insulin pens. For example, nurses may be more prone to error in drawing up the correct dose when using insulin syringes and vials. Studies have shown that doses from insulin pens may be more accurate than insulin syringes, especially at doses of 5 units or less.28 Theoretically, these differences may result in increased hyperglycemia and/or hypoglycemia with insulin vials compared to pens. Additionally, insulin administration via syringe and vial may require more time and effort from nurses, resulting in delayed or neglected administration of correctional insulin doses.
However, insulin pens have drawbacks as well. Insulin pens must be primed before each use. In addition, depending on the specific product, the pen needle must be left in place for 5 to 10 seconds to ensure delivery of the entire dose.29-31 Due to difficulty depressing the pen plunger, pens may accidentally be withdrawn from the skin during the injection, resulting in leakage of a portion of the dose onto the skin surface.32 Thus pens may be prone to errors in injection technique, leading to insufficient dosing and hyperglycemia.
Hospitals also must consider cost when deciding between insulin formulations. Pens have the advantage of being sent home with patients upon discharge when appropriate, which may decrease waste. Each pen cartridge contains 3 mL of insulin at 100 units per mL. Depending on the product, some insulin vials may be available in 3 mL vials in addition to 10 mL vials, both at concentrations of 100 units per mL. Our institution’s acquisition cost for insulin lispro is roughly $13.30 per 3 mL vial, compared to about $16.60 per 3 mL pen. Based on quantities of product ordered for the entire hospital during the 4-month study periods, we estimate an annual savings of $15,000 with vials given the above acquisition costs. Therefore, inpatient insulin administration via vials may constitute a more economical choice compared to pens. However, it should be noted that our study was not designed to provide pharmacoeconomic analyses.
Our study is limited by its retrospective design, which restricts our ability to control for confounding variables. For example, differences in nursing staff between groups could result in variability in insulin administration practices. Although the insulin protocol remained constant, differences in medical residents comprising the medicine service may have caused variations in glycemic management strategies. Our study did not control for some medications that may exert a chronic effect on glycemic control, such as antiretroviral agents. However, these medications are unlikely to exert an effect on acute inpatient control. Overall, we must assume that patients in both groups provide a representative sample of type 2 diabetic general medical patients and have similar characteristics. Generalizability is limited by the single-center design. Moreover, the small study population led to wide standard deviations and decreased power to detect significant differences between groups. However, based on statistical power estimations for the average glucose values ± SD obtained in this study (pens: 160 ± 39; vials: 158 ± 45), an estimated 400 patients in each group would be required to achieve a power of 80%. Given the small differences between groups, our conclusions would likely not change if the study were extended to a larger population. Finally, the retrospective nature, small sample size, and lack of follow-up prevented analysis of clinical outcomes such as infection, length of stay, and mortality. Although mortality was not analyzed, a previous study has shown an association between AUC-estimated average glucose and mortality in patients hospitalized for acute myocardial infarction.33
Despite both theoretical and documented differences between insulin vials and pens, our data suggest that glycemic control is not likely to differ based on use of insulin pen devices versus vials and syringes in an inpatient setting. Given the recommendation from the ISMP to avoid routine administration of insulin to inpatients with pen devices, we hope that hospitals currently using pen devices will be encouraged to transition to vials and syringes for improved patient safety.
ACKNOWLEDGMENTS
Financial support/disclosures: This project was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health through grant number 8UL1TR000041, The University of New Mexico Clinical and Translational Science Center. The authors declare no conflicts of interest.
Additional contributions: Ron Schrader, PhD, was instrumental in guiding the statistical design of the study and calculating the glycemic outcomes for each patient.
REFERENCES
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: An independent marker of in-hospital mortality in patients with undiagnosed diabetes.
J Clin Endocrinol Metab. 2002;87(3):978-982. - Baker EH, Janaway CH, Philips BJ, et al. Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax. 2006;61(4):284-289.
- Bruno A, Gregori D, Caropreso A, Lazzarato F, Petrinco M, Pagano E. Normal glucose values are associated with a lower risk of mortality in hospitalized patients. Diabetes Care. 2008;31(11):2209-2210.
- Noordzij PG, Boersma E, Schreiner F, et al. Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. Eur J Endocrinol. 2007;156(1):137-142.
- Pomposelli JJ, Baxter JK, 3rd, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. J Parenter Enteral Nutr. 1998;22(2):77-81.
- McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28(4):810-815.
- Turchin A, Matheny ME, Shubina M, Scanlon JV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care. 2009;32(7):1153-1157.
- Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33(6):1389-1394.
- Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16-38.
- Standards of medical care in diabetes--2014. Diabetes Care. 2014;37(suppl 1):S14-80.
- Le Floch JP, Herbreteau C, Lange F, Perlemuter L. Biologic material in needles and cartridges after insulin injection with a pen in diabetic patients. Diabetes Care. 1998;21(9):1502-1504.
- Sonoki K, Yoshinari M, Iwase M, et al. Regurgitation of blood into insulin cartridges in the pen-like injectors. Diabetes Care. 2001;24(3):603-604.
- Herdman ML, Larck C, Schliesser SH, Jelic TM. Biological contamination of insulin pens in a hospital setting. Am J Health Syst Pharm. 2013;70(14):1244-1248.
- Institute for Safe Medication Practices. Ongoing concern about insulin pen reuse shows hospitals need to consider transitioning away from them. ISMP Medication Safety Alert! [serial online]. 2013;18(3):1-2.
- Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18(2):245-250.
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. Br Med J. 1990;300(6719):230-235.
- Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552.
- Maruyama S, Sakura H, Kanno H, Iwamoto Y. Factors associated with glycemic control after an inpatient program. Metabolism. 2009;58(6):843-847.
- The Centers for Disease Control and Prevention. CDC clinical reminder: Insulin pens must never be used for more than one person. http://www.cdc.gov/injectionsafety/clinical-reminders/insulin-pens.html. Accessed August 1, 2013.
- Information for healthcare professionals: Risk of transmission of bloodborne pathogens from shared use of insulin pens. US Food and Drug Administration Postmarket Drug Safety Information for Patients and Providers. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm133352.htm. Accessed August 18, 2013.
- Centers for Medicare & Medicaid Services. Memorandum: Use of insulin pens in health care facilities. May 18, 2012. Office of Clinical Standards and Quality/Survey & Certification Group. http://www.cms.gov/Medicare/Provider-Enrollment-and-certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-12-30.pdf. Accessed April 7, 2014.
- Ochs R. South Nassau Hospital alerting 4,247 patients of possible blood contamination. Newsday. March 11, 2014. http://www.newsday.com/news/health/south-nassau-hospital-alerting-4-247-patients-of-possible-blood-contamination-1.7359969. Accessed April 7, 2014.
- McCarthy D. Olean General alerts patients to possible insulin pen re-use. Press release, Olean General Hospital. January 24, 2013. http://www.ogh.org/programs-and-services/diabetes/Press%20Release-OGH%20Alerts%20Patients%20to%20Possible%20Insulin%20Pen%20Re-use.pdf. Accessed April 7, 2014.
- Whitman V. Catskill Hospital warns that insulin pens may have been reused. Times Herald-Record. May 21, 2013. http://www.recordonline.com/apps/pbcs.dll/article?AID=/20130521/NEWS/130529929. Accessed April 7, 2014.
- Cook E. Misuse of insulin pens at VA leads to testing. Salisbury Post. March 8, 2013. http://www.salisburypost.com/article/20130308/SP01/130309740/. Accessed April 7, 2014.
- N.Y. hospital patients potentially exposed to HIV, hepatitis through reused insulin pens. CBS News. January 24, 2013. http://www.cbsnews.com/news/ny-hospital-patients-
potentially-exposed-to-hiv-hepatitis-through-reused-insulin-pens/. Accessed April 7, 2014. - Cobaugh DJ, Maynard G, Cooper L, et al. Enhancing insulin-use safety in hospitals: Practical recommendations from an ASHP Foundation expert consensus panel. Am J Health Syst Pharm. 2013;70(16):1404-1413.
- Luijf YM, DeVries JH. Dosing accuracy of insulin pens versus conventional syringes and vials. Diabetes Technol Ther. 2010;12(suppl 1):S73-77.
- Instructions for use HUMALOG KwikPen insulin lispro injection (rDNA origin). http://pi.lilly.com/us/humalog-kwikpen-um.pdf. Accessed August 14, 2013.
- Novolog (insulin aspart [rDNA origin] injection) [package insert]. Bagsvaerd, Denmark: Novo Nordisk A/S; October 2013.
- How to use your Lantus Solostar pen. 2013. Sanofi-Aventis U.S. LLC. http://www.lantus.com/docs/pdf/SoloSTAR-QRG.pdf. Accessed April 18, 2014.
- Institute for Safe Medication Practices. Considering insulin pens for routine hospital use? Consider this… ISMP Medication Safety Alert! Acute Care Newsletter [serial online]. May 8, 2008; Doc no. 1.
- Kosiborod M, Inzucchi SE, Krumholz HM, et al. Glucometrics in patients hospitalized with acute myocardial infarction: Defining the optimal outcomes-based measure of risk. Circulation. 2008;117(8):1018-1027.

*PGY2 Pharmacy Resident, University of New Mexico College of Pharmacy, Albuquerque, New Mexico; †Assistant Professor - Clinician Educator, University of New Mexico College of Pharmacy, Albuquerque, New Mexico; ‡ED/ICU Clinical Pharmacy Lead, University of New Mexico Hospitals, Albuquerque, New Mexico. Corresponding author: Juliann Horne, PharmD, Pharmacy Administration, 2211 Lomas Blvd NE, Albuquerque, NM 87106; phone: 505-272-4926; e-mail address: jmhorne@salud.unm.edu