Radium Ra 223 Dichloride Therapy in the Private Practice Environment
Jeffrey C. Seeber, MS, DABR
A.M.P. Radiation Oncology, Syracuse, NY
[Rev Urol. 2016;18(2):106-109 doi: 10.3909/riu0720]
© 2016 MedReviews®, LLC
Radium Ra 223 Dichloride Therapy in the Private Practice Environment
Jeffrey C. Seeber, MS, DABR
A.M.P. Radiation Oncology, Syracuse, NY
[Rev Urol. 2016;18(2):106-109 doi: 10.3909/riu0720]
© 2016 MedReviews®, LLC
Radium Ra 223 Dichloride Therapy in the Private Practice Environment
Jeffrey C. Seeber, MS, DABR
A.M.P. Radiation Oncology, Syracuse, NY
[Rev Urol. 2016;18(2):106-109 doi: 10.3909/riu0720]
© 2016 MedReviews®, LLC

Figure 1. Sample hot laboratory layout.

Figure 1. Sample hot laboratory layout.
Independent urology groups are currently availing themselves of the numerous life-extending therapies that have been US Food and Drug Administration–approved for patients with metastatic castration-resistant prostate cancer (mCRPC). As they bolster their groups’ capabilities to impact and extend patient lives, they are expanding their account offerings by integrating infusion suites (sipuleucel-T), dispensing pharmacies (abiraterone, enzalutamide), and hot laboratories (radium-223 [223Ra]). Each of these medicines has a unique mechanism of action that allows physicians to tailor treatment to an individual’s disease characteristics.
223Ra is an isotope of radium that has an 11.4-day half-life. 223Ra decays primarily via four α emissions (~ 94%), in addition to β (4%) and photon emissions (2%). Radium Ra 223 dichloride is commercially available, and is approved for extending life in patients with mCRPC, symptomatic bone metastases, and no known visceral metastatic disease. The decay characteristics of 223Ra make it a relatively simple radionuclide to administer in a freestanding, private radiation oncology practice.
The use of byproduct material is regulated by the United States Nuclear Regulatory Commission (NRC), or by the various radiation control agencies in Agreement States. NRC regulations are found in Title 10, Chapter 1, Part 35 of the Code of Federal Regulations: “Medical Use of Byproduct Material” (10 CFR 35).1 An institution seeking to use ionizing radiation in the practice of medicine must obtain a license from the appropriate regulatory agency. The application for a radioactive materials license requires that the licensee provide program-specific information including—but not limited to—specifics about the radiation safety program; instrumentation and calibration; procedures for ordering, receiving, opening, and handling radioactive materials; procedures for handling spills and performing area surveys; a description of the radioactive waste disposal program; the personnel monitoring program; and the As Low As Reasonably Achievable (ALARA) program. Additionally, the application must include the training and qualifications of the individuals listed as the Radiation Safety Officer, Authorized User, and Authorized Medical Physicist on the license. Outsourced resources are also available to assist with amendments, applications, and health physics/radiation safety programs (eg, CardinalHealth™ Nuclear Pharmacy Services [Dublin, OH], Nuclear Diagnostic Products [Cherry Hill, NJ]).
Administration of radium Ra 223 dichloride requires a written directive from, and must be performed under the supervision of, an Authorized User. There are two methods by which a physician can be considered eligible for Authorized User status: board certification or a combination of training and experience. The NRC recognizes certain specialty boards that require candidates to complete a residency in radiation therapy, nuclear medicine, or a related medical field.1 Additionally, the certifying board must require physicians to pass an examination that tests knowledge and competence in radiation safety, radionuclide handling, quality assurance, and clinical use of unsealed byproduct material for which a written directive is required. Physicians of other specialties who wish to achieve Authorized User status must follow the training and experience pathway. The training and experience required is the same that is achieved by physicians who complete the approved residencies previously mentioned. The requirements are listed in 10 CFR 35 and include classroom and laboratory training in radiation physics and instrumentation, radiation protection, and radiation biology. Extensive work experience under the supervision of an Authorized User in the use, handling, measurement, and administrative control procedures of radium Ra 223 dichloride is also necessary for individuals requesting Authorized User status. Training, experience, and preceptor attestation is documented using NRC form 313A, or an Agreement State equivalent.
Facilities that use radium Ra 223 dichloride may be required to have a Radiation Protection Program. The purpose of the program is to ensure compliance with the applicable regulations and to protect patients, workers, and members of the public. The Radiation Safety Officer, appointed by the licensee’s management, implements and directly oversees the Radiation Protection Program. Commonly, an Authorized User or Authorized Medical Physicist (AMP) serves as the Radiation Safety Officer. AMP status is achieved in a manner similar to that of the Authorized User, with training specific to the practice of therapeutic medical physics. A Radiation Safety Committee, having specific requirements and responsibilities, is mandatory for most NRC and Agreement State licenses; however, if 223Ra is the only radionuclide requiring a written directive at the facility, this may not be necessary (depending on the state).
Receipt, possession, calibration, and storage of radium Ra 223 dichloride requires a secure, designated area that provides accountability of the product and offers protection to workers and members of the public. This is commonly known as the hot laboratory. The license application requires a design of the hot laboratory, detailing the floor plan, and incorporated shielding. Calculations of anticipated absorbed doses must be provided to demonstrate compliance with regulatory tolerances. The α emissions from 223Ra deposit their energy over very short distances and can easily be absorbed by a sheet of paper.2 Assuming radium Ra 223 dichloride is the only radiopharmaceutical administered at the facility, and a reasonable distance is provided between the hot laboratory and any uncontrolled occupied areas, additional shielding materials should not be necessary. Figure 1 provides an example of a radium Ra 223 dichloride hot laboratory in which no additional shielding is required. The footprint for such a hot laboratory can be relatively small.
Proper ventilation must be ensured in the hot laboratory. However, a radioisotope fume hood is not required for the exclusive use of radium Ra 223 dichloride, as it is not volatile or easily respirable. The immediate daughter radionuclide of 223Ra is radon-219 (219Rn). 219Rn has a half-life of 3.96 seconds, which prevents significant migration of the gas from the source of 223Ra (the diffusion time for 219Rn to come out of solution is much longer than its physical half-life).2
The hot laboratory includes a nonporous working surface for preparation and measurement of radium Ra 223 dichloride. The accurate measurement of radium Ra 223 dichloride activity is assured by adjustment of dose calibrators using a National Institute of Standards and Technology–traceable reference standard, which is provided to the licensee.
The calibration factor (dial setting) for 223Ra must be determined by the end user. To determine this dial setting, the licensee receives radium Ra 223 dichloride solution. The activity of radium Ra 223 dichloride is provided, along with instructions for determining an accurate dial setting.
The short half-life of 223Ra allows for a decay-in-storage program for radium Ra 223 dichloride therapy. The NRC allows licensees to hold byproduct material for decay in storage if that material has a physical half-life of 120 days or less.1 Radium Ra 223 dichloride waste must be surveyed at the surface prior to its disposal. Documentation that its radioactivity cannot be distinguished from background levels is necessary. In principle, for a radioactive material to reach background levels, it should decay for 10 half-lives. Thus, the waste generated from radium Ra 223 dichloride procedures must be decayed for approximately 4 months prior to disposal. The amount of radioactive waste that must be held for decay for each radium Ra 223 dichloride procedure is minimal. A lockable cabinet within the hot laboratory may provide enough storage for the program. Accurate record keeping of each disposal is required per NRC regulations.
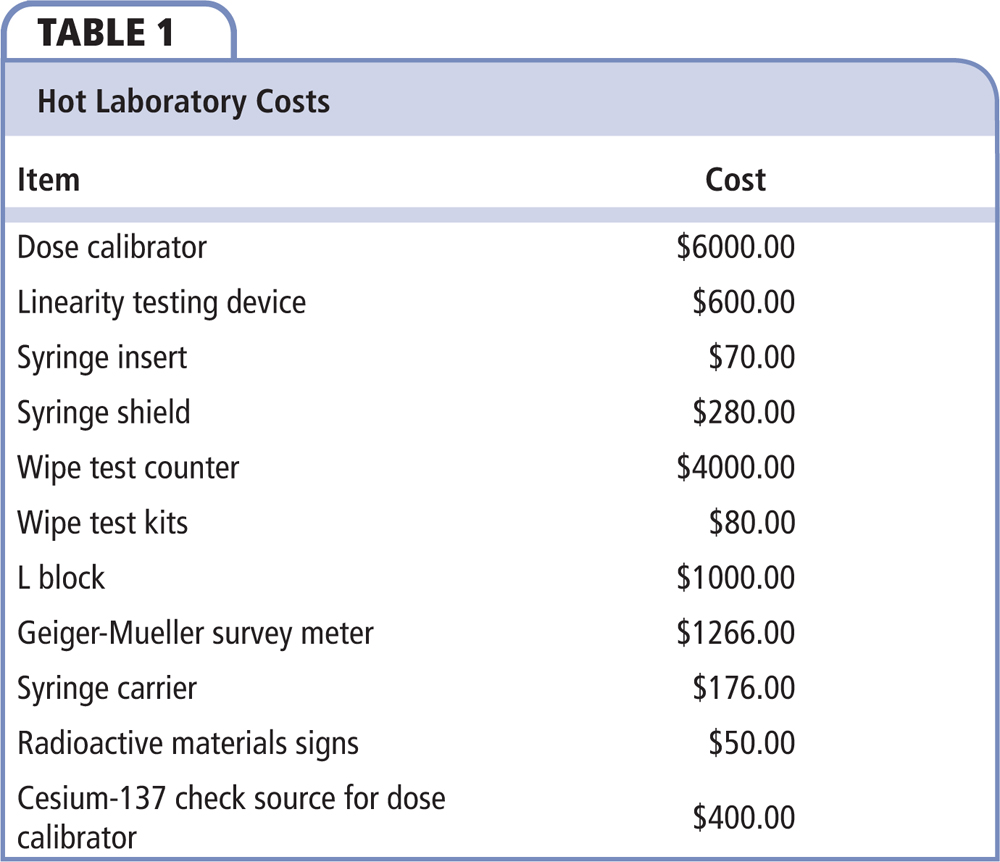
The essential items for a hot laboratory, along with approximate costs, are provided in Table 1. Used equipment can be purchased to reduce cost. The cost of design and construction of the hot laboratory varies, and is not included in Table 1. A Geiger-Mueller–style survey meter is required to detect any spilled or leaked radioactive material. It may also be used to survey patients prior to their release to the public. Wipe test kits are useful in investigating any suspected spills in the hot laboratory, and are necessary for the periodic leakage testing of the cesium-137 source, which is used for daily constancy checks on the facility’s dose calibrator. Syringe shields may not be necessary per institutional protocol.
Establishment of a radium Ra 223 dichloride therapy program in a private practice is a process that involves understanding the requirements of the NRC, or individual Agreement State. These specifications are provided in detail in 10 CFR 35, or applicable Agreement State documents. During the radioactive materials license application process the prospective licensees must address the major components of implementing their radium Ra 223 dichloride program: policies and procedures and hot laboratory design. Existing private radiation oncology practices already have the resources and expertise available to easily incorporate radium Ra 223 dichloride therapy into their programs.
Independent community-based practices, which develop long-term relationships with patients and caregivers, offer continuity with a care team that understands the histories of their conditions, treating them in familiar and comfortable settings. The integration of further services for men with mCRPC represents an opportunity to extend life and offer hope. ![]()
References
- Medical Use of Byproduct Material. Title 10, Chapter 1, Part 35. Code of Federal Regulations. United States Nuclear Regulatory Commission website. www.nrc.gov/reading-rm/doc-collections/cfr/part035/. Accessed June 15, 2016.
- Radium-223 Dichloride: Bayer Responses to NRC Questions. United States Nuclear Regulatory Commission website. http://www.nrc.gov/docs/ML1232/ML12320A450.pdf. Accessed June 15, 2016.