Urethral Strictures and Stenoses Caused by Prostate Therapy
Mang L. Chen, MD,1 Andres F. Correa, MD,2 Richard A. Santucci, MD2
1California Pacific Medical Center, Davies Campus, San Francisco, CA; 2Department of Urology, University of Pittsburgh School of Medicine, Pittsburgh, PA; 3Michigan State College of Medicine, The Center for Urologic Reconstruction, Detroit Medical Center, Detroit, MI
The number of patients with prostate cancer and benign prostatic hyperplasia is on the rise. As a result, the volume of prostate treatment and treatment-related complications is also increasing. Urethral strictures and stenoses are relatively common complications that require individualized management based on the length and location of the obstruction, and the patient’s overall health, and goals of care. In general, less invasive options such as dilation and urethrotomy are preferred as first-line therapy, followed by more invasive substitution, flap, and anastomotic urethroplasty.
[Rev Urol. 2016;18(2):90-102 doi: 10.3909/riu0685]
© 2016 MedReviews®, LLC
Urethral Strictures and Stenoses Caused by Prostate Therapy
Mang L. Chen, MD,1 Andres F. Correa, MD,2 Richard A. Santucci, MD2
1California Pacific Medical Center, Davies Campus, San Francisco, CA; 2Department of Urology, University of Pittsburgh School of Medicine, Pittsburgh, PA; 3Michigan State College of Medicine, The Center for Urologic Reconstruction, Detroit Medical Center, Detroit, MI
The number of patients with prostate cancer and benign prostatic hyperplasia is on the rise. As a result, the volume of prostate treatment and treatment-related complications is also increasing. Urethral strictures and stenoses are relatively common complications that require individualized management based on the length and location of the obstruction, and the patient’s overall health, and goals of care. In general, less invasive options such as dilation and urethrotomy are preferred as first-line therapy, followed by more invasive substitution, flap, and anastomotic urethroplasty.
[Rev Urol. 2016;18(2):90-102 doi: 10.3909/riu0685]
© 2016 MedReviews®, LLC
Urethral Strictures and Stenoses Caused by Prostate Therapy
Mang L. Chen, MD,1 Andres F. Correa, MD,2 Richard A. Santucci, MD2
1California Pacific Medical Center, Davies Campus, San Francisco, CA; 2Department of Urology, University of Pittsburgh School of Medicine, Pittsburgh, PA; 3Michigan State College of Medicine, The Center for Urologic Reconstruction, Detroit Medical Center, Detroit, MI
The number of patients with prostate cancer and benign prostatic hyperplasia is on the rise. As a result, the volume of prostate treatment and treatment-related complications is also increasing. Urethral strictures and stenoses are relatively common complications that require individualized management based on the length and location of the obstruction, and the patient’s overall health, and goals of care. In general, less invasive options such as dilation and urethrotomy are preferred as first-line therapy, followed by more invasive substitution, flap, and anastomotic urethroplasty.
[Rev Urol. 2016;18(2):90-102 doi: 10.3909/riu0685]
© 2016 MedReviews®, LLC
Key words
Prostate cancer • Benign prostatic hyperplasia • Stricture • Stenosis • Complications
Key words
Prostate cancer • Benign prostatic hyperplasia • Stricture • Stenosis • Complications
Penile urethral strictures may be due to compression and to insufficient use of lubricant, which can cause frictional injury.


Urethral strictures and bladder neck stenoses are the most common long-term complications of HoLEP.
Radical prostatectomy had a higher risk than radiation therapy for stricture formation within the first 24 months. However, with longer follow-up, radiation therapy had higher stricture rates than surgery.
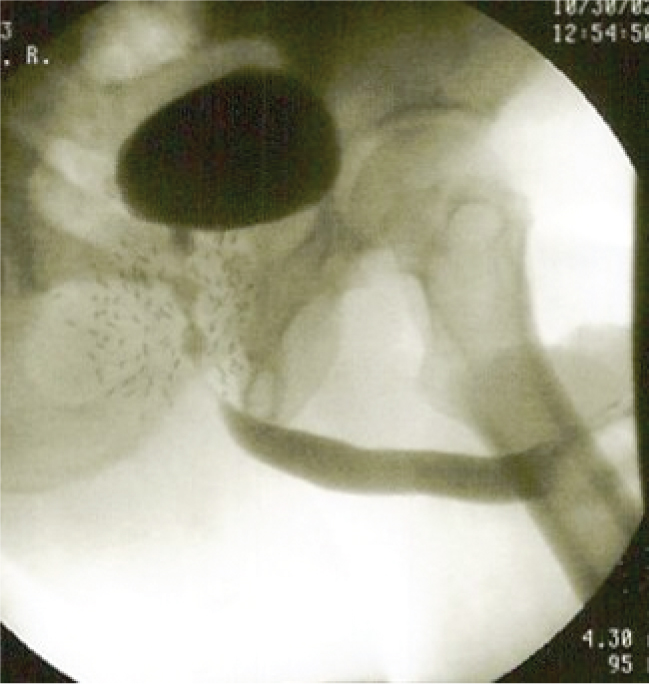
Figure 1. Brachytherapy overdosage to the bulbar and membranous urethra can result in strictures and stenoses in these areas.

Figure 1. Brachytherapy overdosage to the bulbar and membranous urethra can result in strictures and stenoses in these areas.
Patients treated with HIFU or cryotherapy may develop symptoms immediately after catheter removal from urethral sloughing, or within the first year after treatment from development of prostatic urethral stenoses.

For the most recalcitrant prostatic urethral obstruction, open reconstruction with prostatectomy and anastomotic urethroplasty to the bladder neck may be necessary.
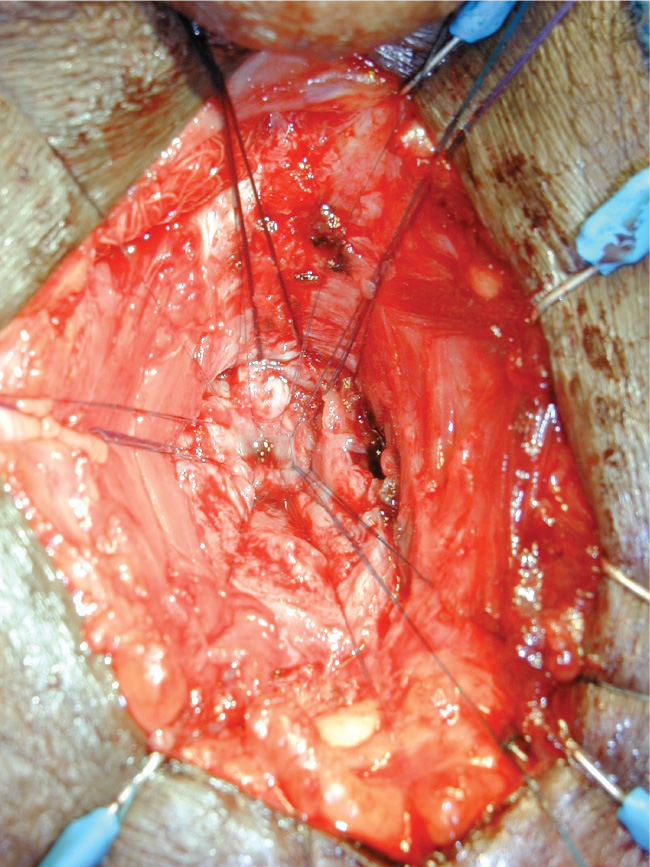
Figure 2. Urethroplasty for bulbomembranous urethral stricture can be technically challenging, requiring deep dissection and anastomosis to the prostatic apex. The figure shows interrupted sutures placed in the proximal urethral segment during an anastomotic urethroplasty. Posturethroplasty stress incontinence should be expected. We often avoid this surgery because of unwillingness by the patient to be rendered profoundly incontinent, and preference for either suprapubic diversion or repeat dilations is acknowledged.

Figure 2. Urethroplasty for bulbomembranous urethral stricture can be technically challenging, requiring deep dissection and anastomosis to the prostatic apex. The figure shows interrupted sutures placed in the proximal urethral segment during an anastomotic urethroplasty. Posturethroplasty stress incontinence should be expected. We often avoid this surgery because of unwillingness by the patient to be rendered profoundly incontinent, and preference for either suprapubic diversion or repeat dilations is acknowledged.
Main Points
• The number of patients with prostate cancer and benign prostatic hyperplasia is on the rise. As a result, the volume of prostate treatment and treatment-related complications is also increasing. Urethral strictures and stenoses are relatively common complications that require individualized management.
• Benign prostatic hyperplasia (BPH) resistant to pharmacotherapy is primarily managed with endoscopic incision, resection, or ablation.). Various factors may individually or cooperatively lead to stricture/stenosis formation in endoscopic prostatic surgery, including excessive resection, circumferential resection, mechanical failure with stray current, urinary extravasation, ischemia from compression of a large resectoscope on a narrow urethra, ischemia from large urethral catheters, and infection.
• Radiation therapy causes strictures and stenoses in 1% to 18% of patients. Combined radiation and time lead to higher rates of stricture and stenosis development.
• The management of BPH-related strictures and stenoses begin with dilation, direct visual internal urethrotomy, and/or bladder neck incision or resection.
• Cryotherapy and HIFU have high urethral complication rates, prompting preventative measures such as urethral warming and prophylactic measures such as concomitant TURP.
• Treatment for urethral strictures and stenoses depends on stricture location, etiology, and individual surgical goals. In general, endoscopic treatment may be attempted first followed by anastomotic or substitution urethroplasty.
Main Points
• The number of patients with prostate cancer and benign prostatic hyperplasia is on the rise. As a result, the volume of prostate treatment and treatment-related complications is also increasing. Urethral strictures and stenoses are relatively common complications that require individualized management.
• Benign prostatic hyperplasia (BPH) resistant to pharmacotherapy is primarily managed with endoscopic incision, resection, or ablation.). Various factors may individually or cooperatively lead to stricture/stenosis formation in endoscopic prostatic surgery, including excessive resection, circumferential resection, mechanical failure with stray current, urinary extravasation, ischemia from compression of a large resectoscope on a narrow urethra, ischemia from large urethral catheters, and infection.
• Radiation therapy causes strictures and stenoses in 1% to 18% of patients. Combined radiation and time lead to higher rates of stricture and stenosis development.
• The management of BPH-related strictures and stenoses begin with dilation, direct visual internal urethrotomy, and/or bladder neck incision or resection.
• Cryotherapy and HIFU have high urethral complication rates, prompting preventative measures such as urethral warming and prophylactic measures such as concomitant TURP.
• Treatment for urethral strictures and stenoses depends on stricture location, etiology, and individual surgical goals. In general, endoscopic treatment may be attempted first followed by anastomotic or substitution urethroplasty.
With an aging population and widespread acceptance of prostate-specific antigen (PSA) screening, benign prostatic hyperplasia (BPH) and prostate adenocarcinoma are becoming more prevalent. According to autopsy reports, at least half of men ≥ 50 years have histologic evidence of BPH, and men in their 80s have a 90% chance of having BPH.1 Although first-line therapy for BPH is α blockade and 5-α reductase inhibition, medically refractory BPH is treated primarily with endoscopic incision, ablation, or resection. For very large prostates, simple prostatectomies or perhaps even better—staged transurethral resection of the prostate (TURP)—can be performed. Known urethral complications from these procedures include strictures and stenoses. Bladder neck stenoses (also known as contractures) may also occur.
In 2010, the American Cancer Society estimated that over 210,000 men were diagnosed with prostate cancer; in 2013, over 230,000 were diagnosed.2 Prostate cancer therapies depend greatly on risk stratification of both the disease and the patient. In general, patients unfit for surgery are observed or undergo radiation therapy. Young patients with localized prostate cancer are often offered definitive therapy such as radiation or surgery. Active surveillance is an option for low-volume and low-risk disease. For intermediate- and high-risk disease, treatment options include radiation (brachytherapy [BT] and external beam radiation therapy), cryotherapy, high-intensity focused ultrasound (HIFU) ablation, and radical prostatectomy. Radiation and various ablative therapies (prostate in situ therapies) can cause urethral strictures and stenoses. After removal of the prostate, the bladder is anastomosed to the membranous urethra, which can lead to vesicourethral anastomotic stenoses (VUAS).
In this review, stricture is the term used for narrowing of the portion of urethra surrounded by the corpus spongiosum—specifically, the fossa navicularis, penile urethra, and bulbar urethra. Stenoses occur in the membranous and prostatic urethra, the bladder neck, and at the anastomosis after prostatectomy. Stenosis is the more pathologically descriptive and accurate term and is used in place of contracture.3 This review covers the epidemiology, risk factors, evaluation, and management of urethral strictures and stenoses identified after treatment of prostatic hyperplasia and malignancy.
Benign Prostatic Hyperplasia–associated Strictures and Stenoses
Epidemiology and Etiology
BPH resistant to pharmacotherapy is primarily managed with endoscopic incision, resection, or ablation. Examples include thermal ablative therapy (microwave, laser, bipolar button, or mushroom), transurethral incision of the prostate (TUIP), TURP, holmium laser enucleation of the prostate (HoLEP), or the newer transurethral bipolar enucleation (TUBE). Strictures occur in 1.7% to 11.7% of patients,4-7 and the rate of bladder neck stenoses ranges from 0.3% to 9.7%, as shown in Table 1. Various factors may individually or cooperatively lead to stricture/stenosis formation in endoscopic prostatic surgery. This includes excessive resection, circumferential resection, mechanical failure with stray current, urinary extravasation, ischemia from compression of a large resectoscope on a narrow urethra, ischemia from large urethral catheters, and infection. Obstruction from strictures or stenoses usually occurs within the first 6 to 12 months postoperatively. However, open prostatectomy rarely causes strictures.
Transurethral Resection of the Prostate and Transurethral Incision of the Prostate
Urethral stricture disease associated with TURP may present anywhere in the urethra. The most common location is the bulbomembranous urethra, followed by the fossa navicularis and penile urethra.6-8 The prostatic urethra is rarely involved. True prostatic urethral stenosis is seldom encountered. A comprehensive review by Rassweiler and colleagues9 evaluated TURP-related complications over the past three decades. The risks of TURP syndrome, blood loss, and clot retention improved over time, but the risk of urethral stricture remained the same. This lack of improvement is explained by the persistent need for large-caliber sheaths for TURP. The large-caliber endoscopic sheath may cause pressure ischemia to the fixed bulbomembranous urethra and narrow caliber fossa navicularis, increasing stricture formation in these regions. Penile urethral strictures may be due to compression and to insufficient use of lubricant, which can cause frictional injury.6 The TURP endoscope can move back and forth within the urethra hundreds of times during a single procedure.10
Bipolar TURP has become more popular given the reduction in symptomatic hyponatremia. Numerous randomized trials between bipolar and monopolar resections have been performed that showed equivalent efficacy and an overall safer morbidity profile for bipolar resection.11-13 However, bipolar technology may have a higher incidence of urethral strictures.11,13 Several reports approximate twice as many urethral strictures with bipolar TURP compared with monopolar resections of 6.1% to 8.3% versus 1.9% to 4.2%, respectively.11-13 Follow-up was limited at 12 to 24 months. The increase in stricture rate may be due to urethral thermal injury associated with the higher cutting current of the bipolar technology (270W) than the conventional monopolar technique (175W). As a result, today’s bipolar cutting current is set at 200W. Of note, a recent study by Falahatkar and colleagues report only a 2% (1/49) risk of urethral stricture disease after bipolar TURP with a cutting current of 280W.14 The authors compared bipolar transurethral ablation of the prostate with bipolar TURP and found that bipolar ablation had no strictures (0/39), but higher risks of urinary retention (3/39 vs 0/49) and repeat surgery (1/39 vs 0/49). Ablative techniques can decrease operative time as it reduces bleeding and eliminates the need to irrigate out pieces of prostate. Given these findings, it is unclear whether bipolar TURP actually increases the risk of urethra-related complications.
Bladder neck stenosis (BNS) is a well-known late complication of TURP, with a reported incidence ranging from 0.14% to 9.6%.9 BNS is more common after resection of small prostate glands measuring < 40 g.4,5,15,16 Given the higher incidence of BNS in smaller glands and fear of retrograde ejaculation in younger patients, Orandi17 introduced TUIP in 1973. TUIP does not remove prostatic adenoma, but it decreases flow resistance by destroying the sympathetic innervation of the prostatic fascia through bilateral deep incisions. Although ineffective in patients with prostates > 40 g or with prominent median lobes, TUIP provides an effective treatment for symptomatic patients with glands < 40 g with little to no risk of BNS.18
Holmium Laser Enucleation, Photoselective Vaporization, Transurethral Microwave Thermotherapy, and Transurethral Bipolar Enucleation
In an effort to minimize morbidity further for BPH treatment, other endoscopic techniques have been developed, including HoLEP, photoselective vaporization (PVP), transurethral microwave thermotherapy (TUMT), and TUBE. Of these minimally invasive treatments, HoLEP and PVP have been the most popular and most studied. Use of both techniques has increased because of their safety profile and their versatility in treating prostate glands of various sizes.19 HoLEP uses a holmium:yttrium aluminum garnet (Ho:YAG) laser, which is highly and rapidly absorbed by water. Given that water constitutes 60% to 70% of the prostate, the laser vaporizes prostatic tissue and simultaneously coagulates small blood vessels via local heat dissipation.19 The surgical technique in HoLEP involves total nucleation of the prostate by creating cleavage planes between adenoma and capsule with the laser. The prostatic pieces are then excised and retrieved by using a cystoscopic tissue morcellator. Urodynamic assessment suggests that HoLEP is superior to TURP and equivalent to open prostatectomy for the management of large prostate glands (> 40).20 Urethral strictures and bladder neck stenoses are the most common long-term complications of HoLEP. The rate of urethral stricture disease ranges between 1.4% and 3.0%, whereas the incidence of bladder neck stenosis is between 0.6% and 5.4%.21 HoLEP-related complications are also associated with prostate size.22 Prostates > 100 g had a higher incidence of bulbar urethral strictures. BNS was rarely seen after HoLEPs performed for smaller glands.
PVP is generated by passing a neodymium:yttrium aluminum garnet (Nd:YAG) laser through a frequency doubling crystal composed of potassium titanyl phosphate (80W) and lithium triborate (120W), which reduces the wavelength from 1054 nm to 532 nm.19 At this wavelength, the laser is predominantly absorbed by hemoglobin rather than water, thus enhancing coagulation. The improved coagulation compared with TURP shows a significant reduction in postoperative blood transfusions and is ideal for patients with bleeding disorders or on anticoagulation medications. When outcomes were compared with TURP and open prostatectomy, PVP had lower postoperative complication rates, decreased need for catheterization, and shorter hospital stays.19 In comparative studies with long-term follow-up, patients treated with PVP had overall improved urinary symptoms, but the maximum flow rates were higher and reoperation rates lower after TURP and open prostatectomy.19 The most common complication of PVP is irritative urinary symptoms, which can occur in as many as 25% of patients. These symptoms are usually self-limiting and frequently respond to anti-inflammatory or anticholinergic pharmacotherapy. Urethral strictures develop in 1.7% to 5.2% of post-PVP patients; BNS occurs in 1.4% to 3.6% of patients.
TUBE is a newer method that uses a technique very similar to HoLEP. The prostatic lobes are enucleated, often using the resectoscope in a blunt manner as one would use a dissecting finger in an open prostatectomy. The technique has an advantage over HoLEP because after the lobes are 90% dissected the resectoscope can be used to shave them out, eliminating the need for morselization. This technique is new enough that the rate of urethral and bladder neck complications has not been established.23
TUMT has been used in very high-risk patients for the treatment of BPH. This provides short-term relief and has a high reoperation rate. Nevertheless, TUMT is an attractive treatment for BPH because it is a 1-hour office procedure with a high safety profile.24 The most common postoperative complications include urinary retention and infection. What has been described as “prostatic urethral stenoses” occur approximately 10% of the time after TUMT.25 This is likely from direct thermal injury to the prostatic urethra during adenoma treatment.
Open Simple Prostatectomy
Open simple prostatectomy was historically the primary treatment for symptomatic BPH. It is efficacious in treating BPH, with excellent long-term control of obstructive urinary symptoms along with a low retreatment rate.21 However, open prostatectomy has a higher rate of morbidity and longer convalescence compared with transurethral surgery. For this reason, it has been largely abandoned in developed countries as the main treatment for BPH.26 We prefer to treat large prostates with a single bipolar TURP, resecting only the median lobe and right lobe in the first stage. Rarely, a second TURP is required to resect the remaining left lobe. In the past 10 years, three large contemporary series evaluated the use of open prostatectomy for the treatment of BPH in western countries.27-29 The most common late complication was bladder neck stenosis, which occurred at a rate of 3.3% to 5.3%. The rate of stricture disease was low, at 0.6%.4
Prevention of BPH-associated Strictures and Stenoses
Sciarra and coworkers8 investigated the use of cyclooxygenase (COX)-2 inhibitors for stricture prevention after TURP. The theory behind COX-2 inhibitor use was the discovery of COX-2 messenger RNA expression in prostatic and urethral tissues. COX-2, a known proinflammatory substance, can theoretically decrease postoperative inflammation and subsequent scar formation. In their study, 96 men were randomized to COX-2 inhibitor (rofecoxib, 25 mg/d) or placebo for 25 days following catheter removal after TURP. The diagnosis of stricture was confirmed cystoscopically if the patient presented with a maximum flow rate (Qmax) < 15 mL/sec. At 12-month follow-up, the overall stricture rate was 8.3%, with all cases seen in the placebo group and none in the COX-2 inhibitor group (P < .0001). Unfortunately, the use of COX-2 inhibitors for prevention of urethral strictures has not been validated in other trials.
Other agents that have been studied for the prevention of urethral strictures include halofuginone, rapamycin, and docetaxel. Of these, the most investigated was halofuginone—a plant alkaloid originally isolated from Dichroa febrifuga. Halofuginone inhibits collagen type I deposition and therefore decreases fibrosis. Nagler and colleagues30 showed the beneficial effect of halofuginone in urethral stricture formation by directly injecting 0.03% halofuginone solution into rat urethras that strictured. The study showed that it prevented collagen type I deposition at the urethrotomy site. To date there have been no published human trials on the use of halofuginone for urethral stricture prevention.
Lee and coworkers15 introduced the use of transurethral incision of the capsule at the end of TURP to decrease BNS rates. They performed a retrospective review of 1135 patients, of whom 667 underwent TURP and 468 underwent TURP plus transurethral incision (TUI), with a median follow-up of 38 months. The overall incidence of bladder neck stenosis was 12.3% for the TURP group versus 6.0% for the TURP plus TUI group (P < .001). In glands < 30 g, the incidence of BNS in the TURP vs the TURP plus TUI group was 19.3% and 7.7%, respectively (P < .05). In prostate glands < 30 g, the overall incidence of BNS in the TURP plus TUI was zero compared with 17.7% in the TURP group. Mean time to BNS diagnosis was 18.5 months with 50% of patients diagnosed by 6 months.
Strictures and Stenoses Associated With Prostate Cancer Therapy
Epidemiology
The incidence of urethral strictures and stenoses requiring treatment after prostate cancer therapy (surgery and radiation) is 5.2% (344 of 6597 patients) based on the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) database.31 Radical prostatectomy had a higher risk than radiation therapy for stricture formation within the first 24 months. However, with longer follow-up, radiation therapy had higher stricture rates than surgery. Curiously, 4 out of 378 (1.1%) patients undergoing watchful waiting developed a urethral stricture. Table 2 summarizes the etiology and associated incidence of urethral strictures and stenoses after prostate cancer therapy.
Radiation Therapy
Radiation treatments include BT and external beam radiation therapy (EBRT). Because the bladder neck, prostatic urethra, and bulbomembranous urethra are intimately associated with the prostate, they become innocent bystanders from purposeful destruction of prostate cancer.
Brachytherapy
Physicians deliver BT by high-dose nonpermanent seed implantation or low-dose permanently implanted seeds. Acute obstruction after BT is related to urethral inflammation and is usually self limited. Chronic obstruction is associated with urethral scarring. BT causes strictures and/or stenoses in as many as 12% of patients, with a higher percentage of patients affected over time.32-34 The most common location of stricture disease is the bulbomembranous urethra, with mean time to development of approximately 2 years.33,35,36 When combined with EBRT, stricture and stenosis rates increase.35,37 The CaPSURE database revealed that strictures after BT alone were 1.8% versus 5.2% for combination BT and EBRT.31 With longer follow-up of 4 years, the stricture/stenosis rate for combination radiotherapy rose to 16%. Risk factors for brachytherapy-associated stricture development include combination therapy with EBRT, higher radiation doses to the bulbomembranous urethra or prostate apex (Figure 1), and prior history of TURP.38,39
External Beam Radiation Therapy
External beam radiation therapy causes strictures and stenoses in 1% to 13% of patients.40 As with BT, the risk of EBRT-related strictures/stenoses rises with time: < 10% occur within 5 years of follow-up; this increases to 10% to 18% within 5 to 10 years.41,42 Radiation in the adjuvant or salvage setting after radical prostatectomy causes strictures in approximately 3% of patients.43 This rate can be minimized by delaying adjuvant radiation therapy delivery > 9 months.44 However, postponing adjuvant treatment for high-risk prostate cancer may increase the risks of bone-related events and prostate cancer-specific mortality.
Cryotherapy
Freezing temperatures cause cytotoxicity via protein denaturation, cell membrane rupture, intracellular concentration of toxic substances, and other cellular changes that induce apoptosis and necrosis.45,46 With improved delivery systems and lack of lifetime dose limitations, cryotherapy has emerged as both a primary treatment alternative and a salvage therapy for prostate cancer.47 Before urethral warming protocols were used during cryotherapy, urethral-associated complication rates were high and included prostatic urethral sloughing and stenosis, causing obstruction in up to 55% of patients.45 Adding a continuous infusion of warm saline through the urethra to keep the neighboring tissue at 38°C has decreased sloughing risk to approximately 5%, and now stenoses are rare.40,48 When stenoses occur, they affect the bladder neck and/or the prostatic urethra. Approximately 5% of patients will require transurethral therapies for bladder outlet obstruction caused by cryotherapy, which include dilations, urethrotomies, bladder neck incisions, and resections.45
High-intensity Focused Ultrasound
HIFU technology heats the target tissue, causing coagulative necrosis and thermal tissue ablation.49 Transrectal HIFU, like cryotherapy, has no dose limitations and has been used for salvage or primary therapy for prostate cancer. Primary HIFU therapy for prostate cancer has an obstruction rate near 25%. Common complications of primary and adjuvant HIFU include urinary retention (1%-20%), urinary tract infections (2%-48%), urinary incontinence (1%-34%), erectile dysfunction (20%-82%), and rectourethral fistulas (< 2%).50 Prostatic urethral stenosis occurs in up to 40% of patients.51 This high rate is secondary to edema and sloughing of necrotic tissue in the prostatic urethra, prompting some urologists to leave postprocedure catheters (suprapubic and urethral) for prolonged periods (> 2 wk); others perform concomitant TURP.52 Long-term follow-up is required because outlet obstruction can present years later, with bladder neck stenosis occurring in as many as 5% of patients. Concomitant TURP does not decrease BNS rates.40
Radical Prostatectomy
Radical retropubic prostatectomy (RRP) causes VUAS in 0.5% to 30% of patients.53-56 Some believe that robotic assisted laparoscopic radical prostatectomy (RALP) can yield overall lower VUAS rates.57-60 Radical perineal prostatectomies are also thought to have less postoperative VUAS.61 However, no matter which modality is used (RRP, RALP, RPP), surgeon experience remains the most important factor in minimizing anastomotic stenosis rates.62 Other risk factors include obesity, prior TURP, cigarette smoking, older age, excess blood loss, postoperative urinary extravasation, and adjuvant radiation therapy.59,62-66 Data from CaPSURE showed that age, lower PSA, lower clinical risk cancer, and higher body mass index increased the risk of VUAS.31 CapSURE also showed that when stenoses occur, most develop within 6 months postoperatively, and rarely after 24 months. In general, radical prostatectomies performed before the PSA screening era yielded higher anastomotic stenosis rates of at least 10% due to higher-grade prostate cancer.62,63 Subsequent studies of prostatectomies performed on lower-risk cancer revealed stenosis rates < 5.5%.60,61,67
Prevention of Prostate Cancer Therapy-related Strictures and Stenoses
There are several ways to minimize the risk of urethral strictures and stenoses. For EBRT and BT, decreasing the radiation dose to the prostate and delaying the administration of adjuvant radiation after prostatectomy decreases the incidence of stricture disease.35,44 For prostatectomy, everting the bladder neck mucosa may theoretically prevent VUAS formation, but no clear difference was noted.66 Only surgeon volume and experience improved outcomes.62 For cryotherapy, urethral warming devices have drastically decreased stenoses rates.45 Concomitant TURP may be helpful for HIFU.
Diagnosis and Evaluation
Urinary symptoms associated with urethral strictures and stenoses are primarily obstructive, with weak stream, straining to void, hesitancy, and incomplete bladder emptying. Irritative symptoms such as dysuria and frequency often accompany obstructive symptoms, especially when patients are treated with radiation. More serious sequelae include urinary tract infections and urinary retention. Rarely, strictures and stenoses can lead to renal failure. In postradiation therapy patients or patients with a history of several previous TURPs, who have persistent pelvic, suprapubic, groin, and/or thigh pain with recurrent urinary tract infections, pubic osteomyelitis must be considered. A thorough history and physical examination will help identify strictures and stenoses. Postbrachytherapy patients with new obstructive urinary symptoms several years after treatment are predicted to have a bulbomembranous stricture. Stenoses that truly affect the membranous urethra are rare in the absence of radiation. The radiation field aimed at the prostate can damage surrounding tissue, with the membranous urethra affected first, followed by the bulbar urethra. Patients treated with HIFU or cryotherapy may develop symptoms immediately after catheter removal from urethral sloughing, or within the first year after treatment from development of prostatic urethral stenoses.40 Postprostatectomy patients may have anastomotic stenoses.
Additional diagnostic tools include urinalysis and urine culture, uroflowometry, bladder scanner postvoid residual, retrograde urethrogram, serum PSA level, and cystoscopy. Renal ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), and urodynamics are useful tests for specific cases. PSA helps assess for cancer recurrence. Cystoscopy rules out penile/bulbar stricture disease and confirms the location of urethral obstruction. Urethroscopy with a narrow-caliber rigid ureteroscope can be helpful with navigating through small openings to confirm length and location of the stricture, especially in relation to the striated sphincter. Additionally, it facilitates luminal placement of a guidewire for subsequent dilation if required. Renal sonography is helpful for patients with incomplete bladder emptying and azotemia. CT and MRI can assess for a urinary tract abscess, fistula, and osteomyelitis. Urodynamics can elucidate the cause of irritative and obstructive voiding symptoms. Sonography may be used to assess for urethral fibrosis in the penile urethra but has a limited role for the more proximal strictures and stenosis.
Treament Overview
The management of prostate therapy-related strictures and stenoses requires a flexible and individualized approach. The various treatment modalities lead to varying obstructive diseases from short, mild annular strictures and stenoses to panurethral strictures and obliterative panprostatic urethral stenoses. Furthermore, the proximity to the striated sphincter and compromised tissue vascularity inevitably complicate surgical repair and threaten urinary continence. Patients are initially managed endoscopically. The most severe prostate-related strictures that fail endoscopic management are temporized with suprapubic catheter drainage followed by open surgical reconstruction.
The first step is to understand the anatomy perfectly, as patients may have any mix of bladder neck stenoses, postprostatectomy anastomotic stenosis, true bulbar strictures with sparing of the membranous urethra, bulbomembranous strictures, or even the very rare prostatic urethra stenosis. The treatments for these various entities are all different (Table 3).
Endoscopic Management
The management of BPH-related strictures and stenoses begin with dilation, direct visual internal urethrotomy (DVIU), and/or bladder neck incision or resection. Pansadoro and Emiliozzi68 showed a 92% overall success rate with median 63-month follow-up in endoscopic management of 122 patients with iatrogenic prostatic urethral and bladder neck stenoses. Success rates were higher (98%; 45/46) in patients with mid-prostatic urethral stenosis; they were lower in patients with bladder neck stenosis (91%; 54/59) and complete stenosis of the prostatic urethra (76%; 13/17). We have difficulty reconciling these and other very high success rates with the much lower success rates seen in our own North American referral population. If the real world success rates of simple treatments were truly over 90%, we would not see such a large number of referral patients with multiple failed treatments at our hospital (may reflect definition of stricture).
A separate study by Ramirez and colleagues69 looked at recurrent BNS treated with deep lateral TUI.8 The reporting surgeons balloon dilated the stenosis followed by deep bipolar cuts at the 3- and 9-o’clock positions until circular bladder muscle fibers and perivesical fat were visible. Of 15 total BPH patients, 3 (20%) failed during a mean follow-up of 13 months.
Radiation-associated urethral strictures and stenoses are difficult to manage, primarily because of poor tissue vascularization and proximity to the striated sphincter. These patients can be managed endoscopically with dilation with a recurrence rate of approximately 50% within the first 16 to 60 months after treatment.36,70,71 We avoid DVIU because of concern for damage to the nearby/involved striated sphincter. Approximately 10% of patients are eventually placed on intermittent self-catheterization for recalcitrant disease, and another 10% develop new urinary incontinence. For brachytherapy patients who develop urinary retention or obstructive urinary symptoms, approximately 3% (79/2495) will require channel TURP.72 Of those 79 patients, 20 (25%) will develop urinary incontinence regardless of TURP timing after brachytherapy.
Prostatectomy-related VUAS are also preferentially managed endoscopically due to the morbidity of open repair. Most VUASs occur within 6 months and success rates of dilation/DVIU with minimal follow-up range from 38% to 100%.56,63,64,73-80 Ramirez and associates69 describe their 5-year follow-up data for endoscopic management of recurrent VUASs with deep lateral transurethral incisions. VUASs treated with this technique had an approximately 70% (24/33) stenosis-free rate with mean 13-month follow-up. Brede and coworkers81 treated 63 postprostatectomy VUAS patients with bladder neck incisions, resulting in 46 patients (73%) remaining stenosis free after 11 months of follow-up.
For stenoses that fail dilation/DVIU/TUI, transurethral resection of scar tissue may be of benefit at the cost of higher incontinence rates.82 In general, we favor multiple attempts of endoscopic management/mitomycin C of VAUSs prior to consideration for complex open repair.
Attempts to decrease stricture and stenosis rates after endoscopic dilation/DVIU/TUI/TUR include adjunct injection of scar modulators including mitomycin C, steroids, and onabotulinum toxin. Mitomycin C is an antiproliferative agent that can be injected into DVIU sites for urethral strictures and stenoses.83 Vanni and coauthors84 noted a 72% 1-year patency rate for radial urethrotomy of VUAS with intralesional injection of mitomycin C. Repeat radial urethrotomy with mitomycin C injection yielded an overall higher stenosis-free rate. Steroid injection was used in conjunction with laser DVIU by Eltahawy and colleagues85 with favorable results. Of the 24 patients with VUAS, 19 (79%) followed for a mean of 24 months remained stenosis free after holmium laser deep urethrotomy at the 3- and 9-o’clock positions with subsequent triamcinolone injection at the incision sites. Other investigators noted anecdotal improvement after DVIU if concomitant onabotulinum toxin A injection is used.86 We found a doubling of the stricture-free interval when 100 U of onabotulinum toxin A were injected into the urethra after DVIU in six patients who were not candidates for urethroplasty (R. Santucci, unpublished data, 2016).
Prostatic urethral stents were introduced by Fabian87 in 1980. The initial experience with prostatic stents was plagued with complications including stent migration, recurrent urinary tract infections, and urethral lumen obstruction.88 The newer biocompatible stent systems have been more promising, the most studied of which is the Memotherm stent (Bard, Murray Hill, NJ) in Europe and the UroLume stent (American Medical Systems, Minnetonka, MN) in the United States. Both are permanent stents that have been used for the management of BPH in high-risk patients. In a review by Armitage and colleagues,89 it was concluded that stent systems provide good short-term relief of bladder outlet obstruction symptoms from BPH with minimal immediate postoperative complications. However, long-term, complication rates rise, prompting high stent explantation rates. Shah and coworkers90 analyzed their stent explantation experience over a 7-year period. Of the 126 patients who underwent stent placement for treatment of BPH, 29 (23%) required stent explanation. The most common reasons were worsening voiding symptoms in 13 (45%) followed by stent migration in 7 (24%). All 29 stents were removed using a monopolar cutting current to transurethrally excise epithelialized tissue followed by gentle extraction into the cystoscope sheath with the use of alligator forceps. We no longer use prostatic stents.
Despite their problems, urethral stents remain a reasonable option for recalcitrant VUAS. Magera and colleagues91 studied their experience with urethral stents for VUAS failing a median of three endoscopic treatments. Patency rates after stent placement were > 50% with a median follow-up of 2.9 years, and the need to place additional stents to obtain patency was seen in 6 of 25 patients (24%). Due to concurrent or resultant stress urinary incontinence in stented patients, artificial urinary sphincter placement was often offered after confirmation of stent epithelialization and lack of stenosis.91,92 Urethral stents cause many problems, including perineal pain, stent migration, stone formation, and tissue ingrowth. Consequently, stents are rarely offered for long-term management, although some authors find utility in stent placement for patients unfit for or refusing open reconstruction.67,93
Endourethroplasty involves transurethral resection of the VUAS scar tissue with subsequent graft harvest and placement with a combined percutaneous and endoscopic approach.92,94,95 This option requires specialized equipment and training, with success rates < 60%, making it an unattractive treatment modality. Innovations may make this a better option in the future.
Open Surgical Management
For the most recalcitrant prostatic urethral obstruction, open reconstruction with prostatectomy and anastomotic urethroplasty to the bladder neck may be necessary. Postprostatectomy patients with recurrent anastomotic stenoses are offered scar excision and anastomotic urethroplasty with or without pubectomy and bladder neck reconstruction. Occasionally, local fasciocutaneous, muscle, and omental pedicle flaps are required.96 These techniques can vary from patient to patient and depend largely on prior interventions, radiation history, and amount of scar tissue encountered. Due to the high risk of urinary incontinence, artificial urinary sphincters may subsequently be required. Some patients opt out of surgery altogether and accept chronic suprapubic catheter bladder drainage or self-catheterization regimens. Urinary diversion or catheterizable stomas are offered as last resorts.
For patients with radiation-induced urethral strictures, urethroplasty may be a viable therapy (Figure 2).97 Hofer and colleagues performed transecting anastomotic urethroplasties in 72 men with these radiation strictures and successfully repaired 46 men (69.7%).98 Time to recurrence was often within 1 year and incontinence occurred in 12 patients (18.5%). Various urethroplasties (anastomotic, buccal graft, perineal flap) were reported by Glass and colleagues99 for radiation therapy-related strictures and stenoses. The investigators noted a 90% success rate over a median 40 months of follow-up with new incontinence in 7% of patients.
Another study by Elliott and associates100 looked at 32 patients with urethral strictures after treatment for prostate cancer100; 11 had radical prostatectomy and 21 received radiation, with urethroplasty type depending on disease location and length. For patients with short bulbar strictures, an anastomotic urethroplasty or buccal mucosa graft onlay procedure was performed. For long or penile urethral strictures, management depended on whether patients received radiation; those who had radiation underwent a perineal urethrostomy and those with healthier nonirradiated tissue had a staged urethroplasty or a fasciocutaneous graft repair. Their overall success rate given this treatment algorithm was 73% (23/32). Although the incontinence rates are overall low for urethroplasty, we must emphasize that periodic dilation and/or conservative DVIUs for membranous urethral or anastomotic stenoses are preferred over open repair to minimize postoperative morbidity and incontinence.
Conclusions
There are numerous types of urethral strictures and stenoses, with their associated etiologies, that must all be taken into account when planning for reconstruction. Management can vary from minimally invasive dilation and urethrotomy to complicated open reconstruction. Critical to patient and practitioner satisfaction is an adaptable and individualized outlook on treatment of each type of urethral stricture and stenosis because life expectancy, treatment goals, and expectations will vary from patient to patient. Avoiding iatrogenic new or worsening incontinence should be a major treatment goal. Although repetitive and ultimately hopeless dilation and DVIU should be avoided in preference to potentially curative urethroplasty, we must be aware that repeat dilations one to two times a year in some radiated patients may be the best treatment. Patients seeking permanent solutions with lower restenosis rates benefit from open urethral reconstruction but risk higher incontinence rates. Strictures and stenoses that occur after treatment of prostate diseases are often complex and should be managed by experts in the field. ![]()
References
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474-479.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30.
- Latini JM, McAninch JW, Brandes SB, et al. Epidemiology, etiology, anatomy, and nomenclature of urethral stenoses, strictures, and pelvic fracture urethral disruption injuries. Urology. 2014;83(3 suppl):S1-S7.
- Varkarakis J, Bartsch G, Horninger W. Long-term morbidity and mortality of transurethral prostatectomy: a 10-year follow-up. Prostate. 2004;58:248-251.
- Zwergel U, Wullich B, Lindenmeir U, et al. Long-term results following transurethral resection of the prostate. Eur Urol. 1998;33:476-480.
- Michielsen DP, Coomans D. Urethral strictures and bipolar transurethral resection in saline of the prostate: fact or fiction? J Endourol. 2010;24:1333-1337.
- Balbay MD, Ergen A, Sahin A, et al. Development of urethral stricture after transurethral prostatectomy: a retrospective study. Int Urol Nephrol. 1992;24:49-53.
- Sciarra A, Salciccia S, Albanesi L, et al. Use of cyclooxygenase-2 inhibitor for prevention of urethral strictures secondary to transurethral resection of the prostate. Urology. 2005;66:1218-1222.
- Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP)—incidence, management, and prevention. Eur Urol. 2006;50:969-979; discussion 980.
- Mundy AR, Andrich DE. Urethral strictures. BJU Int. 2011;107:6-26.
- Seckiner I, Yesilli C, Akduman B, et al. A prospective randomized study for comparing bipolar plasmakinetic resection of the prostate with standard TURP. Urol Int. 2006;76:139-143.
- Ho HS, Yip SK, Lim KB, et al. A prospective randomized study comparing monopolar and bipolar transurethral resection of prostate using transurethral resection in saline (TURIS) system. Euro Urol. 2007;52:517-522.
- Tefekli A, Muslumanoglu AY, Baykal M, et al. A hybrid technique using bipolar energy in transurethral prostate surgery: a prospective, randomized comparison. J Urol. 2005;174(4 Pt 1):1339-1343.
- Falahatkar S, Mokhtari G, Moghaddam KG, et al. Bipolar transurethral vaporization: a superior procedure in benign prostatic hyperplasia: a prospective randomized comparison with bipolar TURP. Int Braz J Urol. 2014;40:346-355.
- Lee YH, Chiu AW, Huang JK. Comprehensive study of bladder neck contracture after transurethral resection of prostate. Urology. 2005;65:498-503; discussion 503.
- Floratos DL, Kiemeney LA, Rossi C, et al. Long-term followup of randomized transurethral microwave thermotherapy versus transurethral prostatic resection study. J Urol. 2001;165:1533-1538.
- Orandi A. Transurethral incision of the prostate. J Urol. 1973;110:229-231.
- Orandi A. Transurethral resection versus transurethral incision of the prostate. Urol Clin North Am. 1990;17:601-612.
- Naspro R, Bachmann A, Gilling P, et al. A review of the recent evidence (2006-2008) for 532-nm photoselective laser vaporisation and holmium laser enucleation of the prostate. Eur Urol. 2009;55:1345-1357.
- Kuntz RM, Lehrich K, Ahyai SA. Holmium laser enucleation of the prostate versus open prostatectomy for prostates greater than 100 grams: 5-year follow-up results of a randomised clinical trial. Eur Urol. 2008;53:160-166.
- Reich O, Gratzke C, Stief CG. Techniques and long-term results of surgical procedures for BPH. Eur Urol. 2006;49:970-978; discussion 978.
- Shah HN, Sodha HS, Kharodawala SJ, et al. Influence of prostate size on the outcome of holmium laser enucleation of the prostate. BJU Int. 2008;101: 1536-1541.
- Geavlete B, Stanescu F, Iacoboaie C, Geavlete P. Bipolar plasma enucleation of the prostate vs open prostatectomy in large benign prostatic hyperplasia cases - a medium term, prospective, randomized comparison. BJU Int. 2013;111:793-803.
- Lau KO, Li MK, Foo KT. Long-term follow-up of transurethral microwave thermotherapy. Urology. 1998;52:829-833.
- Sall M, Bruskewitz RC. Prostatic urethral strictures after transurethral microwave thermal therapy for benign prostatic hyperplasia. Urology. 1997;50:983-985.
- Tubaro A, de Nunzio C. The current role of open surgery in BPH. EAU-EBU Update Series. European Association of Urology website. http://eu-acme.org/europeanurology/upload_articles/The%20current%20role.pdf. Accessed May 3, 2016.
- Varkarakis I, Kyriakakis Z, Delis A, et al. Long-term results of open transvesical prostatectomy from a contemporary series of patients. Urology. 2004;64: 306-310.
- Helfand B, Mouli S, Dedhia R, McVary KT. Management of lower urinary tract symptoms secondary to benign prostatic hyperplasia with open prostatectomy: results of a contemporary series. J Urol. 2006;176(6 Pt 1):2557-2561; discussion 2561.
- Serretta V, Morgia G, Fondacaro L, et al; Members of the Sicilian-Calabrian Society of Urology. Open prostatectomy for benign prostatic enlargement in southern Europe in the late 1990s: a contemporary series of 1800 interventions. Urology. 2002;60:623-627.
- Nagler A, Gofrit O, Ohana M, et al. The effect of halofuginone, an inhibitor of collagen type i synthesis, on urethral stricture formation: in vivo and in vitro study in a rat model. J Urol. 2000;164:1776-1780.
- Elliott SP, Meng MV, Elkin EP, et al. Incidence of urethral stricture after primary treatment for prostate cancer: data From CaPSURE. J Urol. 2007;178:529-534; discussion 534.
- Ragde H, Blasko JC, Grimm PD, et al. Interstitial iodine-125 radiation without adjuvant therapy in the treatment of clinically localized prostate carcinoma. Cancer. 1997;80:442-453.
- Zelefsky MJ, Hollister T, Raben A, et al. Five-year biochemical outcome and toxicity with transperineal CT-planned permanent I-125 prostate implantation for patients with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2000;47:1261-1266.
- Zelefsky MJ, Wallner KE, Ling CC, et al. Comparison of the 5-year outcome and morbidity of three-dimensional conformal radiotherapy versus transperineal permanent iodine-125 implantation for early-stage prostatic cancer. J Clin Oncol. 1999;17:517-522.
- Merrick GS, Butler WM, Wallner KE, et al. Risk factors for the development of prostate brachytherapy related urethral strictures. J Urol. 2006;175:1376-1380; discussion 1381.
- Sullivan L, Williams SG, Tai KH, et al. Urethral stricture following high dose rate brachytherapy for prostate cancer. Radiother Oncol. 2009;91:232-236.
- Chen AB, D’Amico AV, Neville BA, Earle CC. Patient and treatment factors associated with complications after prostate brachytherapy. J Clin Oncol. 2006;24:5298-5304.
- Seymore CH, el-Mahdi AM, Schellhammer PF. The effect of prior transurethral resection of the prostate on post radiation urethral strictures and bladder neck contractures. Int J Radiat Oncol Biol Phys. 1986;12:1597-1600.
- Deger S, Boehmer D, Roigas J, et al. High dose rate (HDR) brachytherapy with conformal radiation therapy for localized prostate cancer. Eur Urol. 2005;47:441-448.
- Herschorn S, Elliott S, Coburn M, et al. SIU/ICUD Consultation on Urethral Strictures: Posterior urethral stenosis after treatment of prostate cancer. Urology. 2014;83(3 suppl):S59-S70.
- Lawton CA, Bae K, Pilepich M, et al. Long-term treatment sequelae after external beam irradiation with or without hormonal manipulation for adenocarcinoma of the prostate: analysis of radiation therapy oncology group studies 85-31, 86-10, and 92-02. Int J Radiat Oncol Biol Phys. 2008;70:437-441.
- Gardner BG, Zietman AL, Shipley WU, et al. Late normal tissue sequelae in the second decade after high dose radiation therapy with combined photons and conformal protons for locally advanced prostate cancer. J Urol. 2002;167:123-126.
- Macdonald OK, Lee RJ, Snow G, et al. Prostate-specific antigen control with low-dose adjuvant radiotherapy for high-risk prostate cancer. Urology. 2007;69:295-299.
- Kowalczyk KJ, Gu X, Nguyen PL, et al. Optimal timing of early versus delayed adjuvant radiotherapy following radical prostatectomy for locally advanced prostate cancer. Urol Oncol. 2014;32:303-308.
- Rodriguez SA, Arias Funez F, Bueno Bravo C, et al. Cryotherapy for primary treatment of prostate cancer: intermediate term results of a prospective study from a single institution. Prostate Cancer. 2014;2014:571576.
- Cooper IS, Hirose T. Application of cryogenic surgery to resection of parenchymal organs. N Engl J Med. 1966;274:15-18.
- Friedlander DF, Gu X, Prasad SM, et al. Population-based comparative effectiveness of salvage radical prostatectomy vs cryotherapy. Urology. 2014;83: 653-657.
- Han KR, Cohen JK, Miller RJ, et al. Treatment of organ confined prostate cancer with third generation cryosurgery: preliminary multicenter experience. J Urol. 2003;170(4 Pt 1):1126-1130.
- Crouzet S, Rouviere O, Martin X, Gelet A. High-intensity focused ultrasound as focal therapy of prostate cancer. Curr Opin Urol. 2014;24:225-230.
- Cordeiro ER, Cathelineau X, Thuroff S, et al. High-intensity focused ultrasound (HIFU) for definitive treatment of prostate cancer. BJU Int. 2012;110: 1228-1242.
- Ahmed HU, Zacharakis E, Dudderidge T, et al. High-intensity-focused ultrasound in the treatment of primary prostate cancer: the first UK series. Br J Cancer. 2009;101:19-26.
- Uchida T, Ohkusa H, Nagata Y, et al. Treatment of localized prostate cancer using high-intensity focused ultrasound. BJU Int. 2006;97:56-61.
- Park R, Martin S, Goldberg JD, Lepor H. Anastomotic strictures following radical prostatectomy: insights into incidence, effectiveness of intervention, effect on continence, and factors predisposing to occurrence. Urology. 2001;57:742-746.
- Popken G, Sommerkamp H, Schultze-Seemann W, et al. Anastomotic stricture after radical prostatectomy. Eur Urol. 1998;33:382-386.
- Tomschi W, Suster G, Höltl W. Bladder neck strictures after radical retropubic prostatectomy: still an unsolved problem. Br J Urol. 1998;81:823-826.
- Dalkin BL. Endoscopic evaluation and treatment of anastomotic strictures after radical retropubic prostatectomy. J Urol. 1996;155:206-208.
- Hu JC, Nelson RA, Wilson TG, et al. Perioperative complications of laparoscopic and robotic assisted laparoscopic radical prostatectomy. J Urol. 2006;175:541-546; discussion 546.
- Fischer B, Engel N, Fehr JL, John H. Complications of robotic assisted radical prostatectomy. World J Urol. 2008;26:595-602.
- Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA. 2009;302:1557-1564.
- Carlsson S, Nilsson AE, Schumacher MC, et al. Surgery-related complications in 1253 robot-assisted and 485 open retropubic radical prostatectomies at the Karolinska University Hospital, Sweden. Urology. 2010;75:1092-1097.
- Gillitzer R, Thomas C, Wiesner C, et al. Single center comparison of anastomotic strictures after radical perineal and radical retropubic prostatectomy. Urology. 2010;76:417-422.
- Hu JC, Gold KF, Pashos CL, et al. Role of surgeon volume in radical prostatectomy outcomes. J Clin Oncol. 2003;21:401-405.
- Borboroglu PG, Sands JP, Roberts JL, Amling CL. Risk factors for vesicourethral anastomotic stricture after radical prostatectomy. Urology. 2000;56:96-100.
- Surya BV, Provet J, Johanson KE, Brown J. Anastomotic strictures following radical prostatectomy: risk factors and management. J Urol. 1990;143:755-758.
- Besarani D, Amoroso P, Kirby R. Bladder neck contracture after radical retropubic prostatectomy. BJU Int. 2004;94:1245-1247.
- Srougi M, Paranhos M, Leite KM, et al. The influence of bladder neck mucosal eversion and early urinary extravasation on patient outcome after radical retropubic prostatectomy: a prospective controlled trial. BJU Int. 2005;95:757-760.
- Erickson BA, McAninch JW, Eisenberg ML, et al. Management for prostate cancer treatment related posterior urethral and bladder neck stenosis with stents. J Urol. 2011;185:198-203.
- Pansadoro V, Emiliozzi P. Iatrogenic prostatic urethral strictures: classification and endoscopic treatment. Urology. 1999;53:784-789.
- Ramirez D, Zhao LC, Bagrodia A, et al. Deep lateral transurethral incisions for recurrent bladder neck contracture: promising 5-year experience using a standardized approach. Urology. 2013;82: 1430-1435.
- Bullock TL, Brandes SB. Adult anterior urethral strictures: a national practice patterns survey of board certified urologists in the United States. J Urol. 2007;177:685-690.
- Gómez-Iturriaga Piña A, Crook J, Borg J, et al. Median 5 year follow-up of 125iodine brachytherapy as monotherapy in men aged,<or=55 years with favorable prostate cancer. Urology. 2010;75:1412-1416.
- Mock S, Leapman M, Stock RG, et al. Risk of urinary incontinence following post-brachytherapy transurethral resection of the prostate and correlation with clinical and treatment parameters. J Urol. 2013;190:1805-1810.
- Ramchandani P, Banner MP, Berlin JW, et al. Vesicourethral anastomotic strictures after radical prostatectomy: efficacy of transurethral balloon dilation. Radiology. 1994;193: 345-349.
- Geary ES, Dendinger TE, Freiha FS, Stamey TA. Incontinence and vesical neck strictures following radical retropubic prostatectomy. Urology. 1995;45:1000-1006.
- Park R, Martin S, Goldberg JD, Lepor H. Anastomotic strictures following radical prostatectomy: insights into incidence, effectiveness of intervention, effect on continence, and factors predisposing to occurrence. Urology. 2001;57:742-746.
- Thiel DD, Igel TC, Brisson TE, Heckman MG. Outcomes with an alternative anastomotic technique after radical retropubic prostatectomy: 10-year experience. Urology. 2006;68:132-136.
- Gonzalgo ML, Pavlovich CP, Trock BJ, et al. Classification and trends of perioperative morbidities following laparoscopic radical prostatectomy. J Urol. 2005;174:135-139; discussion 139.
- Hayashi T, Yoshinaga A, Ohno R, et al. Successful treatment of recurrent vesicourethral stricture after radical prostatectomy with holmium laser: report of three cases. Int J Urol. 2005;12:414-416.
- Lagerveld BW, Laguna MP, Debruyne FM, De La Rosette JJ. Holmium:YAG laser for treatment of strictures of vesicourethral anastomosis after radical prostatectomy. J Endourol. 2005;19:497-501.
- Yurkanin JP, Dalkin BL, Cui H. Evaluation of cold knife urethrotomy for the treatment of anastomotic stricture after radical retropubic prostatectomy. J Urol. 2001;165:1545-1548.
- Brede C, Angermeier K, Wood H. Continence outcomes after treatment of recalcitrant postprostatectomy bladder neck contracture and review of the literature. Urology. 2014;83:648-652.
- Anger JT, Raj GV, Delvecchio FC, Webster GD. Anastomotic contracture and incontinence after radical prostatectomy: a graded approach to management. J Urol. 2005;173:1143-1146.
- Mazdak H, Meshki I, Ghassami F. Effect of mitomycin C on anterior urethral stricture recurrence after internal urethrotomy. Eur Urol. 2007;51:1089-1092; discussion 1092.
- Vanni AJ, Zinman LN, Buckley JC. Radial urethrotomy and intralesional mitomycin C for the management of recurrent bladder neck contractures. J Urol. 2011;186:156-160.
- Eltahawy E, Gur U, Virasoro R, et al. Management of recurrent anastomotic stenosis following radical prostatectomy using holmium laser and steroid injection. BJU Int. 2008;102:796-798.
- Khera M, Boone TB, Smith CP. Botulinum toxin type A: a novel approach to the treatment of recurrent urethral strictures. J Urol. 2004;172: 574-575.
- Fabian KM. [The intra-prostatic “partial catheter” (urological spiral)]. Urologe. A. 1980;19:236-238.
- Gesenberg A, Sintermann R. Management of benign prostatic hyperplasia in high risk patients: long-term experience with the Memotherm stent. J Urol. 1998;160:72-76.
- Armitage JN, Rashidian A, Cathcart PJ, et al. The thermo-expandable metallic stent for managing benign prostatic hyperplasia: a systematic review. BJU Int. 2006;98:806-810.
- Shah DK, Kapoor R, Badlani GH; North American Study Group. Experience with urethral stent explantation. J Urol. 2003;169:1398-1400.
- Magera JS Jr, Inman BA, Elliott DS. Outcome analysis of urethral wall stent insertion with artificial urinary sphincter placement for severe recurrent bladder neck contracture following radical prostatectomy. J Urol. 2009;181:1236-1241.
- Elliott DS, Boone TB. Combined stent and artificial urinary sphincter for management of severe recurrent bladder neck contracture and stress incontinence after prostatectomy: a long-term evaluation. J Urol. 2001;165:413-415.
- McNamara ER, Webster GD, Peterson AC. The UroLume stent revisited: the Duke experience. Urology. 2013;82:933-936.
- Kuyumcuoglu U, Eryildirim B, Tarhan F, et al. Antegrade endourethroplasty with free skin graft for recurrent vesicourethral anastomotic strictures after radical prostatectomy. J Endourol. 2010;24:63-67.
- Chiou RK, Howe S, Morton JJ, et al. Treatment of recurrent vesicourethral anastomotic stricture after radical prostatectomy with endourethroplasty. Urology. 1996;47:422-425.
- Wessells H, Morey AF, McAninch JW. Obliterative vesicourethral strictures following radical prostatectomy for prostate cancer: reconstructive armamentarium. J Urol. 1998;160:1373-1375.
- Chi AC, Han J, Gonzalez CM. Urethral strictures and the cancer survivor. Curr Opin Urol. 2014;24:415-420.
- Hofer MD, Zhao LC, Morey AF, et al. Outcomes after urethroplasty for radiotherapy induced bulbomembranous urethral stricture disease. J Urol. 2014;191:1307-1312.
- Glass AS, McAninch JW, Zaid UB, et al. Urethroplasty after radiation therapy for prostate cancer. Urology. 2012;79:1402-1405.
- Elliott SP, McAninch JW, Chi T, et al. Management of severe urethral complications of prostate cancer therapy. J Urol. 2006;176(6 Pt 1):2508-2513.
- Naspro R, Suardi N, Salonia A, et al. Holmium laser enucleation of the prostate versus open prostatectomy for prostates >70 g: 24-month follow-up. Eur Urol. 2006;50:563-568.
- Gilling PJ, Aho TF, Frampton CM, et al. Holmium laser enucleation of the prostate: results at 6 years. Eur Urol. 2008;53:744-749.
- Gupta N, Sivaramakrishna, Kumar R, et al. Comparison of standard transurethral resection, transurethral vapour resection and holmium laser enucleation of the prostate for managing benign prostatic hyperplasia of >40 g. BJU Int. 2006;97:85-89.
- Kuo RL, Paterson RF, Siqueira TM Jr, et al. Holmium laser enucleation of the prostate: morbidity in a series of 206 patients. Urology. 2003;62:59-63.
- Ruszat R, Seitz M, Wyler SF, et al. GreenLight laser vaporization of the prostate: single-center experience and long-term results after 500 procedures. Eur Urol. 2008;54:893-901.
- Te AE, Malloy TR, Stein BS, et al. Impact of prostate-specific antigen level and prostate volume as predictors of efficacy in photoselective vaporization prostatectomy: analysis and results of an ongoing prospective multicentre study at 3 years. BJU Int. 2006;97:1229-1233.
- Heinrich E, Schiefelbein F, Schoen G. Technique and short-term outcome of green light laser (KTP, 80W) vaporisation of the prostate. Eur Urol. 2007;52:1632-1637.
- Erickson BA, Meeks JJ, Roehl KA, et al. Bladder neck contracture after retropubic radical prostatectomy: incidence and risk factors from a large single-surgeon experience. BJU Int. 2009;104:1615-1619.