Intracavitary Immunotherapy and Chemotherapy for Upper Urinary Tract Cancer: Current Evidence
Luca Carmignani, MD,1 Roberto Bianchi, MD,2 Gabriele Cozzi, MD,3 Angelica Grasso, MD,3
Nicola Macchione, MD,4 Carlo Marenghi, MD,1 Sara Melegari, MD,2 Marco Rosso, MD,3
Elena Tondelli, MD,5 Augusto Maggioni, MD3
1Università degli Studi di Milano, Urology Department, IRCCS Policlinico San Donato, San Donato Milanese, Italy; 2Università; degli Studi di Milano, Urology Department, European Institute of Oncology, Milan, Italy; 3Università; degli Studi di Milano, Urology Department, Fondazione IRCCS Caí GrandaóOspedale Maggiore Policlinico, Milan, Italy; 4Università; degli Studi di Milano, Urology Department, S. Paolo Hospital, Milan, Italy; 5Università; degli Studi di Milano, Urology Department, IRCCS S. Giuseppe Hospital, Milan, Italy
A review of the literature was performed to summarize current evidence regarding the efficacy of topical immunotherapy and chemotherapy for upper urinary tract urothelial cell carcinoma (UUT-UCC) in terms of post-treatment recurrence rates.A Medline database literature search was performed in March 2012 using the terms upper urinary tract, urothelial cancer, bacillus Calmette-Guérin (BCG), and mitomycin C. A total of 22 full-text articles were assessed for eligibility, and 19 studies reporting the outcomes of patients who underwent immunotherapy or chemotherapy with curative or adjuvant intent for UUT-UCC were chosen for quantitative analysis. Overall, the role of immunotherapy and chemotherapy for UUT-UCC is not firmly established. The most established practice is the treatment of carcinoma in situ (CIS) with BCG, even if a significant advantage has not yet been proven. The use of BCG as adjuvant therapy after complete resection of papillary UUT-UCC has been studied less extensively, even if recurrence rates are not significantly different than after the treatment of CIS. Only a few reports describe the use of mitomycin C, making it difficult to obtain significant evidence.
[ Rev Urol. 2013;15(4):145-153 doi: 10.3909/riu0579]
© 2014 MedReviews®, LLC
Key words
Upper urinary tract • Urothelial cell carcinoma • Bacillus Calmette-Guérin • Mitomycin C • Chemotherapy • Immunotherapy
Conservative surgery for low-risk UUT-UCC allows for preservation of the upper urinary renal unit; conservative management can be considered in imperative cases (renal insufficiency, solitary functional kidney) or in elective cases (ie, when the contralateral kidney is functional) for low-grade, low-stage tumors.
In our cumulative analysis, we found 165 UUT CIS that were administered BCG. There were 53 recurrences (32.12%).
Patients with solitary kidney, bilateral disease, poor renal function, small tumor burden, or low-grade disease force the clinician to consider other treatment options, such as segmental ureterectomy and ureteroscopic or percutaneous resection.
Patients with solitary kidney, bilateral disease, poor renal function, small tumor burden, or low-grade disease force the clinician to consider other treatment options, such as segmental ureterectomy and ureteroscopic or percutaneous resection.
In our cumulative analysis, BCG showed the same efficacy when used with curative intent for UUT CIS as when used with adjuvant intent after endoscopic resection for papillary UUT UCC: recurrences were 32.15% versus 30.15%, respectively (P 5 .71).
In our cumulative analysis, BCG showed the same efficacy when used with curative intent for UUT CIS as when used with adjuvant intent after endoscopic resection for papillary UUT UCC: recurrences were 32.15% versus 30.15%, respectively (P = .71).
Main Points
• Although immunotherapy and chemotherapy for upper urinary tract urothelial cell carcinoma (UUT-UCC) has been described since the 1980s, their role has not been firmly established. The low prevalence of the disease makes it difficult to undertake randomized controlled trials, and only two comparative studies were found in the literature search.
• The gold standard for treatment of UUT-UCC for patients with a normal contralateral kidney and no evidence of metastatic disease is nephroureterectomy with excision of the bladder cuff. This therapeutic option offers 5-year survival rates of 91% for stages Ta, T1, and CIS, 43% for T2, and 23% for T3 and T4 disease.
• The most established practice is the treatment of carcinoma in situ (CIS) with bacillus Calmette-Guérin (BCG), even if a significant advantage has not yet been proven. The use of BCG as adjuvant therapy after complete resection of papillary UUT-UCC has been studied less extensively; according to our cumulative analysis, recurrence rates were not significantly different than that after the treatment of CIS.
• Only a few reports described the use of mitomycin C, making it difficult to obtain significant evidence.
According to the 2011 update of the European Guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinoma (UUT-UCC),1 urothelial carcinomas are the fourth most common tumors after prostate and breast cancer, lung cancer, and colorectal cancer. Bladder tumors account for 90% to 95% of urothelial carcinomas; UUT-UCC are relatively uncommon and account for only 5% to 10% of urothelial carcinomas. The annual incidence of UUT-UCC in Western countries is approximately one or two new cases per 100,000 inhabitants. Pyelocaliceal tumors are approximately twice as common as ureteral tumors. In 8% to 13% of cases, concurrent bladder cancer is present, and 60% of UUT-UCC are invasive at diagnosis, compared with only 15% of bladder tumors. This kind of carcinoma has a peak incidence in people in their 70s and 80s, with a higher prevalence in men.
Radical nephroureterectomy (RNU) with excision of the bladder cuff represents the gold standard treatment for UUT-UCC, regardless of the location of the tumor in the upper urinary tract.1 Lymph node dissection associated with RNU is of therapeutic interest and allows for optimal staging of the disease.
Conservative surgery for low-risk UUT-UCC allows for preservation of the upper urinary renal unit; conservative management can be considered in imperative cases (renal insufficiency, solitary functional kidney) or in elective cases (ie, when the contralateral kidney is functional) for low-grade, low-stage tumors. Endoscopic ablation can be considered if a flexible ureteroscope, laser generator, and pliers (pluck) for biopsies are available, if the patient is informed of the need for closer follow-up, and if a complete resection is advocated.
Segmental ureteral resection with wide margins provides adequate pathologic specimens for definitive staging and grade analysis while also preserving the ipsilateral kidney. Segmental resection is possible for the treatment of low- and high-risk tumors of the distal ureter, whereas segmental resection of the iliac and lumbar ureter is associated with a greater failure rate. Open resection of tumors of the renal pelvis or calices has almost disappeared.
Percutaneous management can be considered for low-grade or noninvasive UUT-UCC that are inaccessible or difficult to manage by ureteroscopy, even if a theoretical risk of seeding exits in the puncture tract and if perforations occur during the procedure.
After conservative treatment of UUT-UCC or for the treatment of carcinoma in situ (CIS), the instillation of bacillus Calmette-Guérin (BCG) or mitomycin C (MMC) is technically feasible by means of a percutaneous nephrostomy or even through a ureteric stent.
Different agents have been used for topical therapy, including BCG, MMC, epirubicine, and thiotepa. Topical chemotherapeutic agents can be administered after endoscopic management, whereas instillations of BCG need to be postponed until the urothelium heals to avoid systemic side effects.
According to a recent review,2 topical therapy appears to be safe, although its efficacy is debatable. Complications from the administration of topical immunotherapy or chemotherapy can be avoided by maintaining low intracavitary pressures during administration. Renal function does not seem to be impaired after instillation of BCG or MMC.3 No systemic side effects result from perfusion with MMC, and persistent fever was reported in 5% of patients in combined major series after BCG administration; therefore, this side effect was resolved with appropriate antimicrobial therapy in all cases. Furthermore, up to 25% of patients may have granulomatous involvement of the urinary tract after BCG.
This review summarizes current evidence about the efficacy of topical immunotherapy and chemotherapy in terms of post-treatment recurrence rates.
Evidence Acquisition
A literature search was performed in March 2012 using the Medline database. We searched using the terms upper urinary tract, urothelial cancer, bacillus Calmette-Guérin, and mitomycin C across the “Title” and “Abstract” fields with the following limits: humans and language (English).
After we screened the results, 22 full-text articles were assessed for eligibility. We selected for quantitative analysis 19 studies reporting the outcomes of cohorts of patients who underwent immunotherapy or chemotherapy with curative or adjuvant intent for UUT-UCC.
An article by Elliott and colleagues4 was excluded from this review because data about recurrences were provided without distinguishing between chemotherapy and immunotherapy. Two articles, one by Palou and associates5 and one by Goel and colleagues,6 were excluded because outcomes of patients who received immunotherapy or chemotherapy were not selectively reported.
The included articles were distinguished according to the grade of evidence for therapy/prevention/etiology/harm studies, as stated by Phillips and colleagues.7 Two reportswere retrospective studies comparing contemporary series of patients (level of evidence: 3b)8,9; 17 studies were observational studies without control (level of evidence: 4).10-26
We analyzed the use of BCG with curative intent for CIS or as adjuvant therapy after endoscopic resection of papillary tumor and the use of MMC as adjuvant therapy after endoscopic resection of papillary tumor. One report dealt with the use of BCG as salvage therapy in patients not eligible for surgery.24
The main outcome evaluated was recurrence of the urothelial disease, defined as a positive biopsy result, imaging evidence, or a positive selective cytology after intracavitary immunotherapy/chemotherapy.
Evidence Synthesis
Statistical Analyses
The data available in the selected articles were collected and entered into an electronic database. Cumulative analysis was conducted using Review Manager Version 5.1 (Cochrane Collaboration, Oxford, United Kingdom), designed for composing Cochrane Reviews.
Statistical heterogeneity was tested using the χ2 test. A P value < .05 was used to indicate heterogeneity. Random effects models were used in case of heterogeneity.
BCG With Curative Intent for CIS
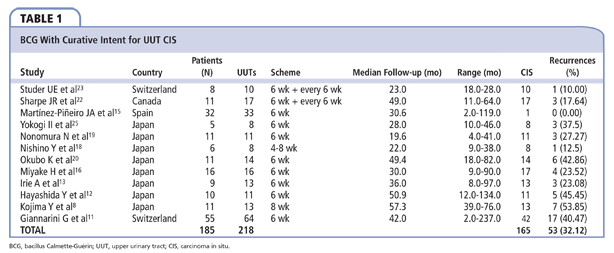
A total of 11 articles reported outcomes of patients who received BCG with curative intent for UUT CIS (Table 1).
In 1989, Studer and colleagues23 reported the treatment of 10 UUT CIS in 8 patients. Diagnosis of CIS was made by selective ureteral catheterization. BCG was administered weekly by percutaneous nephrostomy for 6 weeks. If cytology remained positive 6 weeks later, a further treatment course was started. Median follow-up was 23 months (range, 18-28), and one recurrence (10%) after 24 months was reported.
Sharpe and colleagues22 treated 11 patients with BCG 17 UUT CIS. CIS was defined as the presence of positive selective urinary cytology with no radiographic evidence of papillary transitional cell carcinoma (TCC). Treatment was repeated weekly for 6 weeks by medians of a 4 Fr ureteral catheter, and repeated if cytology remained positive. Median follow-up was 49 months (range, 11-64), and three recurrences (17.65%) were reported.
Martínez-Piñeiro and colleagues15 reported a large case study dealing with the endourological treatment of UUT-UCC. Only one UUT CIS was treated with BCG (weekly for 6 weeks), showing no recurrence.
Yokogi and associates25 treated 8 UUT patients (3 by nephrostomy, 2 with a ureteral catheter). CIS diagnosis was made in the presence of positive selective upper tract cytology in urine collected directly and by washing with normal saline after ureteric catheterization with no abnormalities on retrograde pyelography. BCG was administered weekly for 6 weeks. Median follow-up was 28 months (range, 10-46), and three recurrences were observed (37.5%).
Nonomura and coauthors19 stated the following diagnostic criteria for UUT CIS: positive urinary cytology, negative multiple random biopsy of the bladder and prostatic urethra, negative radiographic findings in the UUT, and two serial positive cytologies in selective ipsilateral urine sampling from the pyeloureteral system. A total of 11 UUT CIS were treated in 11 patients. After placing a 6 Fr double-J (DJ), BCG was administered into the bladder weekly for 6 weeks. Median follow-up was 19.6 months (range, 4-41), and three recurrences were reported (27.27%).
The study conducted by Nishino and colleagues18 included 6 men (8 UUT CIS, defined as positive selective upper urinary cytology results with no radiographic evidence of papillary TCC on either intravenous or retrograde pyelography). BCG was administered weekly for 4 to 8 weeks by means of a ureteral catheter. Median follow-up was 22 months (range, 9-38), and only a ureteral recurrence (12.5%) at 22 months was reported.
Okubo and coauthors20 studied 11 patients (14 UUT CIS). Diagnostic criteria included positive voided urinary cytology and negative multiple random biopsies of the bladder and prostatic urethra; negative radiographic studies including computed tomography (CT), and two serial positive cytology results in selective ipsilateral urine samples. Twelve renal patients were treated by selective retrograde catheterization, and two units were treated using the percutaneous antegrade approach. BCG was administered weekly for 6 weeks. Median follow-up was 49.4 months (range, 18-82), and six recurrences were recorded (42.86%).
A total of 16 cases of UUT CIS were treated by Miyake and colleagues.16 The diagnostic criteria were the same as for Okubo and coauthors.20 BCG was administered weekly for 6 weeks through a nephrostomy tube, a single-J (SJ) catheter, or urethral catheter for patients carrying a DJ stent. Median follow-up was 30 months (range, 9-90), and four recurrences were reported (25%).
Irie and colleagues13 treated 13 UUT CIS using the vesicoureteral reflux created by a DJ catheter. The diagnosis of UUT CIS was made when the irrigated fluid of the UUT collected by retrograde ureteral catheterization revealed class 4 or 5 at least two times. BCG was administered weekly for 6 weeks. Median follow-up was 36 months (range, 8-97), and recurrences were found in three UUT (23.08%).
Hayashida and associates12 studied 10 patients (11 UUT CIS). The diagnostic criteria were positive voided urinary cytology (more than class 4), positive cytology in selective urine samples, negative radiographic findings of UUT, and negative multiple random biopsies of the bladder and prostatic urethra. BCG was administered weekly for 6 weeks by means of a percutaneous nephrostomy, an SJ stent or using the vesicoureteral reflux created by a DJ stent. Median follow-up was 50.9 months (range, 12-134), and five recurrences (45.45%) were found.
Kojima and colleagues8 conducted a comparative study. UUT CIS was diagnosed in cases of positive voided urinary cytology; negative multiple random biopsy of the bladder and prostatic urethra; no abnormality on radiographic examinations including excretory urogram, retrograde pyelography, and CT; three serial positive cytologies in selective UUT urine sample. A total of 6 patients were treated with RNU, whereas 11 patients (13 UUT CIS) were administered BCG weekly for 8 weeks. Median follow-up was 57.3 months (range, 39-76). In the RNU group, two patients had contralateral recurrence, whereas in the BCG group, seven UUTs showed a recurrence (53.84%). There was no significant difference in 5-year recurrence-free survival between the two groups (67% vs 78%; P = .55); the 5-year cancer-specific survival was also not significantly different (80% vs 91%; P = .62).
The latest study was conducted by Giannarini and associates11 who described a large cohort of patients treated for UUT-UCC. A total of 42 UUT CIS were administered BCG for 6 weeks through a 10 Fr nephrostomy. Median follow-up was 42 months (range, 2-237), and 17 recurrences (40.47%) were observed.
In our cumulative analysis, we found 165 UUT CIS that were administered BCG. There were 53 recurrences (32.12%).
BCG as Adjuvant Therapy After Resection of Papillary UUT-UCC
Six of the included reports described the use of BCG as adjuvant therapy after resection of papillary UUT-UCC (Table 2) .
In 1995, Jarrett and colleagues14 described 36 patients (64 UUT) who underwent percutaneous resection of papillary UUT-UCC. Nineteen renal units received BCG weekly for 6 weeks. Median follow-up was 55 months, and only 1 recurrence (5.26%) was reported.
In the previously reported study by Martínez-Piñeiro and colleagues,15 eight UUT were administered BCG after endoscopic ablation of papillary UUT-UCC. The schedule was the same as for UUT CIS (weekly for 6 weeks). Mean follow-up was 30.6 months (range, 2-119), and one recurrence (12.5%) was observed.
Patel and Fuchs21 conducted a study on 13 patients who underwent ureteroscopic tumor ablation for 17 papillary UUT-UCC. Sixteen renal units were administered BCG weekly for 6 weeks. Median follow-up was 14.6 months (range, 6-36), and two recurrences (12.5%) were observed.
From July 1985 to January 1998, Clark and associates10 attempted definitive percutaneous management of 18 papillary UUT-UCC in 17 patients. All 18 UUT were administered BCG weekly for 6 weeks. Median follow-up was 20.5 months (range, 1.7-75.5); six recurrences (33.33%) were observed.
Between 1997 and 2005, Montanari and coauthors17 treated four patients with papillary UUT-UCC by means of percutaneous resection. After the resection, all the UUT were perfused with MMC. Second-look nephroscopy with multiple biopsies was performed in all cases after 7 days and an 8 Fr nephrostomy was inserted. Second-look biopsy was negative in all cases; therefore, BCG was administered weekly through the nephrostomy for 6 weeks. Median follow-up was 71 months (range, 10-97), and no recurrences were reported.

Rastinehad and colleagues9 performed a retrospective analysis of 133 patients (89 renal units) who underwent percutaneous resection of papillary UUT-UCC. Thirty-nine renal units received no adjuvant therapy after resection, whereas 50 were administered BCG weekly for 6 weeks. Median follow-up was 61 months (range, 6-115.9). Recurrences were 12 (30.77%) versus 18 (36%), with no statistically significant differences (P > .05).
In the report by Giannarini and coauthors,11 22 UUT were administered BCG weekly for 6 weeks after ablation of papillary UUT-UCC. Median follow-up was 42 months (range, 2-237), and 13 recurrences (59.09%) were observed.
In our cumulative analysis, we found 136 papillary UUT-UCC that were administered BCG. Recurrences were 41 (30.15%).
BCG as Salvage Therapy in Patients Not Eligible for Surgery
In 2002, Thalmann and colleagues24 described their experience with BCG therapy of UUT-UCC in patients not eligible for surgery. Twenty-two patients (25 UUTs) presented UUT CIS, whereas 15 patients (16 UUTs) had papillary disease (TaG1 in 2, TaG2 in 6, TaG3 in 2, T1G3 in 2, and Tx in 4). Recurrence was observed in 8 UUT in the CIS group (32%), and 13 recurrences occurred in the papillary group (81.25%).
MMC as Adjuvant Therapy After Resection of Papillary UUT-UCC
Three of the included reports described the use of MMC as adjuvant therapy after resection of papillary UUT-UCC (Table 3).
In the previously cited report by Martínez-Piñeiro and colleagues,15 14 UUT were administered MMC (3-6 times) after endoscopic ablation of papillary UUT-UCC. Mean follow-up was 30.6 months (range, 2-119), and two recurrences (14.28%) were observed.
In 1997, Keeley and Bagley26 reported on 19 patients (19 renal units) who underwent ureteroscopic treatment of upper tract TCC, followed by the weekly administration of topical MMC by means of a ureteral catheter for 3 weeks. Seven UUT-UCC were G1, 8 were G2, and 4 were G3. Median follow-up was 30 months. There were two recurrences (28.57%) in the G1 group, and four (50%) in the G2 group. Two patients with G3 TCC in three renal units died of unrelated causes, and one had a marked decrease in tumor burden.
Patel and Fuchs21 described one renal unit who was treated with MMC, with no recurrence of the disease in 13 patients who underwent ureteroscopic tumor ablation for 17 papillary UUT-UCC.
In our cumulative analysis, we found 34 papillary UUT-UCC that were administered MMC. A total of nine recurrences were observed (26.47%).
Discussion
The gold standard for treatment of UUT-UCC for patients with a normal contralateral kidney and no evidence of metastatic disease is nephroureterectomy with excision of the bladder cuff. This therapeutic option offers 5-year survival rates of 91% for stages Ta, T1, and CIS, 43% for T2, and 23% for T3 and T4 disease.27 This choice is also supported by the presence of polychronotropism, the low risk of contralateral TCC (3%), and the high recurrence rate associated with open nephron-sparing surgery.28
Patients with solitary kidney, bilateral disease, poor renal function, small tumor burden, or low-grade disease force the clinician to consider other treatment options, such as segmental ureterectomy and ureteroscopic or percutaneous resection.26
Whereas the role of intravesical immunotherapy/chemotherapy in nonmuscle-invasive urothelial cancer of the bladder has been firmly established, the topical treatment of UUT-UCC remains problematic because agents must be delivered to renal pelvis and ureter to be effective,29 and the literature dealing with this topic is scarce. No randomized studies have been completed to evaluate the true value of such therapy, and most studies have set the number of instillations empirically.27
In 1985, the first case of adjuvant topical BCG immunotherapy for a UUT-UCC was described by Herr: a patient with a solitary kidney and T2 TCC underwent excision of the renal pelvis and ureter, followed by a pyelovesical anastomosis.30 The margins of the renal pelvis and infundibola were positive for CIS, so the patient underwent weekly vesical administrations of BCG for 6 weeks. Urinary cytology became normal and remained negative for more than 13 months.
Afterward, BCG perfusion has been proposed with either curative intent for CIS or adjuvant intent after ablation of papillary UUT-UCC.11
Most available evidence deals with the treatment of CIS, and most are case series. No randomized, controlled trials (RCT) were conducted. Furthermore, in our review, we encountered various definitions of CIS, as we reported in the evidence synthesis. With our search criteria, we found 12 studies reporting outcomes of the treatment of CIS with BCG (185 patients, 218 UUT, 165 CIS). Except for the case series by Nishino and colleagues,18 who proposed administration schemes of 4 to 8 weeks, all other series adopted a 6-week scheme. Studer and colleagues23 and Sharpe and associates22 started another 6 weeks of treatment if urinary cytology remained positive after the first attempt. In our cumulative analysis, we found a recurrence rate of 32.12%.
Seven of the included studies described patients who underwent endoscopic resection of a papillary UUT-UCC and then underwent intracavitary treatment with BCG (190 patients, 244 UUTs, 136 papillary tumors). All studies applied a 6-week scheme. Montanari and colleagues first performed a one-shot MMC perfusion right after resection. Cumulative analysis showed a recurrence rate of 30.15%.17
In our cumulative analysis, BCG showed the same efficacy when used with curative intent for UUT CIS as when used with adjuvant intent after endoscopic resection for papillary UUT UCC: recurrences were 32.15% versus 30.15%, respectively (P = .71).
The administration of MMC after percutaneous resection of a UUT-UCC in a solitary kidney was first described in 1986 by Streem and Pontes.31
Our literature search found only three studies (64 patients, 64 UUTs, 34 papillary UUT-UCC) providing outcomes of a small series of patients treated with MMC after endoscopic resection of papillary UUT-UUCs. According to our cumulative analysis, 3 UUT-UUCs were Gx/G0, 14 were G1, 12 were G2, and 5 were G3. Recurrence rates were 0%, 21.43%, 41.66%, and 20%, respectively.
Conclusions
Although immunotherapy and chemotherapy for UUT-UCC has been described since the 1980s, their role has not been firmly established. The low prevalence of the disease makes it difficult to undertake RCTs, and only two comparative studies were found in the literature search. The most established practice is the treatment of CIS with BCG, even if a significant advantage has not yet been proven. The use of BCG as adjuvant therapy after complete resection of papillary UUT-UCC has been studied less extensively; according to our cumulative analysis, recurrence rates were not significantly different than that after the treatment of CIS. Only a few reports described the use of MMC, making it difficult to obtain significant evidence. 
References
- Rouprêt M, Zigeuner R, Palou J, et al. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol. 2011;59:584-594.
- Pfister C. Role of chemotherapy and immunotherapy for urinary upper tract cancer. Eur Urol Suppl. 2010;9:446-449.
- Jarrett TW, Sweetser PM, Weiss GH, Smith AD. Percutaneous management of transitional cell carcinoma of the renal collecting system: 9-year experience. J Urol. 1995;154:1629-1635.
- Elliott DS, Blute ML, Patterson DE, et al. Long-term follow-up of endoscopically treated upper urinary tract transitional cell carcinoma. Urology. 1996;47(6):819-825.
- Palou J, Piovesan LF, Huguet J, et al. Percutaneous nephroscopic management of upper urinary tract transitional cell carcinoma: recurrence and long-term follow-up. J Urol. 2004;172:66-69.
- Goel MC, Mahendra V, Roberts JG. Percutaneous management of renal pelvic urothelial tumors: long-term follow-up. J Urol. 2003;169:925-929; discussion 929-930.
- Phillips B, Ball C, Sackett D, et al. Levels of evidence and grades of recommendation. Oxford Centre for Evidence-Based Medicine Web site. http://www.cebm.net/index.aspx?=o1025. Accessed March 3, 2013.
- Kojima Y, Tozawa K, Kawai N, et al. Long-term outcome of upper urinary tract carcinoma in situ: effectiveness of nephroureterectomy versus bacillus Calmette-Guérin therapy. Int J Urol. 2006;13:340-344.
- Rastinehad AR, Ost MC, Vanderbrink BA, et al. A 20-year experience with percutaneous resection of upper tract transitional carcinoma: is there an oncologic benefit with adjuvant bacillus Calmette Guérin therapy? Urology. 2009;73:27-31.
- Clark PE, Streem SB, Geisinger MA. 13-year experience with percutaneous management of upper tract transitional cell carcinoma. J Urol. 1999;161:772-775.
- Giannarini G, Kessler TM, Birkhäuser FD, et al. Antegrade perfusion with bacillus Calmette-Guérin in patients with non-muscle-invasive urothelial carcinoma of the upper urinary tract: who may benefit? Eur Urol. 2011;60:955-960.
- Hayashida Y, Nomata K, Noguchi M, et al. Long-term effects of bacille Calmette-Guérin perfusion therapy for treatment of transitional cell carcinoma in situ of upper urinary tract. Urology. 2004;63:1084-1088.
- Irie A, Iwamura M, Kadowaki K, et al. Intravesical instillation of bacille Calmette-Guérin for carcinoma in situ of the urothelium involving the upper urinary tract using vesicoureteral reflux created by a double-pigtail catheter. Urology. 2002;59:53-57.
- Jarrett TW, Sweetser PM, Weiss GH, Smith AD. Percutaneous management of transitional cell carcinoma of the renal collecting system: 9-year experience. J Urol. 1995;154:1629-1635.
- Martínez-Piñeiro JA, García Matres MJ, Martínez-Piñeiro L. Endourological treatment of upper tract urothelial carcinomas: analysis of a series of 59 tumors. J Urol. 1996;156(2 Pt 1):377-385.
- Miyake H, Eto H, Hara S, et al. Clinical outcome of bacillus Calmette-Guérin perfusion therapy for carcinoma in situ of the upper urinary tract. . 2002;9(12):677-680.
- Montanari E, Del Nero A, Bernardini P, et al. Percutaneous therapy of low stage and grade urothelial neoplasia: long-term follow-up. Arch Ital Urol Androl. 2005;77:211-214.
- Nishino Y, Yamamoto N, Komeda H, et al. Bacillus Calmette-Guérin instillation treatment for carcinoma in situ of the upper urinary tract. BJU Int. 2000;85:799-801.
- Nonomura N, Ono Y, Nozawa M, et al. Bacillus Calmette-Guérin perfusion therapy for the treatment of transitional cell carcinoma in situ of the upper urinary tract. Eur Urol. 2000;38:701-704; discussion 705.
- Okubo K, Ichioka K, Terada N, et al. Intrarenal bacillus Calmette-Guérin therapy for carcinoma in situ of the upper urinary tract: long-term follow-up and natural course in cases of failure. BJU Int. 2001;88:343-347.
- Patel A, Fuchs GJ. New techniques for the administration of topical adjuvant therapy after endoscopic ablation of upper urinary tract transitional cell carcinoma. J Urol. 1998;159:71-75.
- Sharpe JR, Duffy G, Chin JL. Intrarenal bacillus Calmette-Guerin therapy for upper urinary tract carcinoma in situ. J Urol. 1993;149:457-459.
- Studer UE, Casanova G, Kraft R, Zingg EJ. Percutaneous bacillus Calmette-Guerin perfusion of the upper urinary tract for carcinoma in situ. J Urol. 1989;142:975-977.
- Thalmann GN, Markwalder R, Walter B, Studer UE. Long-term experience with bacillus Calmette-Guerin therapy of upper urinary tract transitional cell carcinoma in patients not eligible for surgery. J Urol. 2002;168(4 Pt 1):1381-1385.
- Yokogi H, Wada Y, Mizutani M, et al. Bacillus Calmette-Guérin perfusion therapy for carcinoma in situ of the upper urinary tract. Br J Urol. 1996;77:676-679.
- Keeley FX Jr, Bagley DH. Adjuvant mitomycin C following endoscopic treatment of upper tract transitional cell carcinoma. J Urol. 1997;158:2074-2077.
- Joudi FN, Crane CN, O’Donnell MA. Minimally invasive management of upper tract urothelial carcinoma. Curr Urol Rep. 2006;7:23-30.
- Lee BR, Jabbour ME, Marshall FF, et al. 13-year survival comparison of percutaneous and open nephroureterectomy approaches for management of transitional cell carcinoma of renal collecting system: equivalent outcomes. J Endourol. 1993;13:289-294.
- Nepple KG, Joudi FN, O’Donnell MA. Review of topical treatment of upper tract urothelial carcinoma. Adv Urol. 2009:472831.
- Herr HW. Durable response of a carcinoma in situ of the renal pelvis to topical bacillus Calmette-Guerin. J Urol. 1985;134:531-532.
- Streem SB, Pontes EJ. Percutaneous management of upper tract transitional cell carcinoma. J Urol. 1986;135:773-775.