Utilization Trends and Positive Biopsy Rates for Prostate Biopsies in the United States: 2005 to 2011
Deepak A. Kapoor, MD,1 David G. Bostwick, MD, MBA,2 Savvas E. Mendrinos, MD,1 Ann E. Anderson, MD,1 Carl A. Olsson, MD1
1Integrated Medical Professionals, PLLC, Melville, NY; 2Bostwick Laboratories, Inc., Uniondale, NY
This article assesses the positive biopsy rate and core sampling pattern in patients undergoing needle biopsy of the prostate in the United States at a national reference laboratory (NRL) and anatomic pathology laboratories integrated into urology group practices, and analyzes the relationship between positive biopsy rates and the number of specimen vials per biopsy. For the years 2005 to 2011 we collected pathology data from an NRL, including number of urologists and urology practices referring samples, total specimen vials submitted for prostate biopsies, and final pathologic diagnosis for each case. The diagnoses were categorized as benign, malignant, prostatic intraepithelial neoplasia, or atypical small acinar proliferation. Over the same period, similar data were gathered from urology practices with in-house laboratories performing global pathology services (urology practice laboratories; UPLs) as identified by a survey of members of the Large Urology Group Practice Association. For each year studied, positive biopsy rate and number of specimen vials per biopsy were calculated in aggregate and separately for each site of service. From 2005 to 2011, 437,937 biopsies were submitted in > 4.23 million vials (9.4 specimen vials/biopsy); overall positive biopsy rate was 40.3%—this was identical at both the NRL and UPL (P = .97). Nationally, the number of specimen vials per biopsy increased sharply from a mean of 8.8 during 2005 to 2008 to a mean of 10.3 from 2009 to 2011 (difference, 1.5 specimen vials/biopsy; P = .03). For the most recent 3-year period (2009-2011), the difference of 0.6 specimen vials per biopsy between the NRL (10.0) and UPL (10.6) was not significant (P = 0.08). Positive biopsy rate correlated strongly (P < .01) with number of specimen vials per biopsy. The positive prostate biopsy rate is 40.3% and is identical across sites of service. Although there was a national trend toward increased specimen vials per biopsy from 2005 to 2011, from 2009 to 2011 there was no significant difference in specimen vials per biopsy across sites of service. Increased cancer detection rate correlated significantly with increased number of specimens examined. Segregation of prostate biopsy cores into 10 to 12 unique specimen vials has been widely adopted by urologists across sites of service.
[ Rev Urol. 2013;15(4)137-144 doi: 10.3909/riu0600]
© 2014 MedReviews®, LLC
This article includes Supplementary Material that is available online at www.medreviews.com
Key words
Prostate cancer • Prostate biopsy • Utilization trends • National reference laboratory • Urology practice laboratories
. . . calculation of prostate cancer incidence has been identified as particularly susceptible to error when determined by analysis of outpatient claims data alone.
From 2005 to 2011, the number of specimen vials per biopsy submitted nationally increased 34.4%, from 7.9 to 10.7 (mean, 9.4 specimen vials/biopsy).
Main Points
• Data demonstrate that the positive biopsy rate for prostate needle biopsy (40.3%) at pathology laboratories operated by both integrated urology group practices and a prominent national reference laboratory is mathematically and statistically identical.
• From 2005 to 2011, there was a national trend towards submitting prostate biopsy specimens in 10 to 12 segregated vials; although urology group practices with integrated pathology laboratories adopted this standard sooner that urologists referring specimens outside their practices, after 2008 there was no significant difference in specimen submission based on site of service.
• In our sample of 437,937 total prostate biopsies submitted in . 4.23 million discrete specimen vials there was a significant correlation between increased number of specimen vials per biopsy and a higher positive biopsy rate.
• Utilization patterns and outcomes for prostate biopsy represent national trends and are similar across sites of service—data do not support the notion that urology ownership of anatomic pathology laboratories leads to increased utilization.
Published data over the past decade suggesting that prostate cancer detection rates are enhanced with additional sampling of the prostate have resulted in modifications to the traditional 6-core (sextant) biopsy regimen1,2 such that recent clinical guidelines recommend that extended biopsy schemes with 10 to 12 specimens be obtained.3-5 There are also data that suggest that segregation of prostate biopsy tissue specimens into individual vials improves specimen handling, enhances tissue representation, and improves diagnostic accuracy.6-8 Furthermore, focal prostate cancer treatment strategies gaining recent popularity are dependent on more precise tumor mapping, requiring even greater tissue sampling.9
Over approximately the same time frame, there has been an increase in consolidation of medical practices into larger single- or multispecialty group practices. By incorporating efficiencies of scale, these groups afford physicians the opportunity to retain the characteristics of traditional medical practices while improving their ability to adapt to changing health care circumstances.10 These groups often integrate additional capabilities beyond professional services, including anatomic and clinical pathology, diagnostic imaging, and radiation therapy. Proponents of these arrangements argue that integration of medical services facilitates the development of coordinated clinical pathways, improves communication between specialists, offers better quality control of ancillary services, and enhances data collection—all of which can improve patient care and lead to lower costs.11-13 Specifically with regard to anatomic pathology, recent data suggest that certain specimen handling errors are significantly lower (P = .018) at urology practices with integrated in-house pathology laboratories (urology practice laboratories [UPLs]) than at other sites of service14; however, some contend that group practice integration creates conflicts of interest and self-referral issues, which ultimately leads to increased utilization of services.15-19 A recent study based on analysis of Medicare claims data purported that positive prostate biopsy rates and the number of samples submitted per biopsy are significantly different across sites of service20; however, this study has been criticized as both methodologically flawed and scientifically inaccurate.21 Also problematic is the fact that calculation of prostate cancer incidence has been identified as particularly susceptible to error when determined by analysis of outpatient claims data alone.22
We sought to determine positive biopsy rates and utilization trends in the United States via direct analysis of laboratory records from both a national reference laboratory (NRL) and UPLs, and to determine if there was a correlation between positive biopsy rates and number of specimen vials submitted.
Materials and Methods
From 2005 to 2011, inclusive, we obtained information pertaining to prostate biopsies from the largest independent urologic NRL in the United States (Bostwick Laboratories, Glen Allen, VA). Laboratory records were reviewed to determine, by year of service, the number of patients biopsied, a number count of discrete specimen vials analyzed for prostate histology, and the number of urologists and urology groups submitting prostate biopsy specimens for analysis. Analysis was confined to those procedures using standard prostate needle biopsy (Current Procedural Terminology [CPT® American Medical Association, Chicago, IL] 55700) and anatomic pathology (CPT 88305) technique; saturation biopsies were not included in this analysis. For the NRL, only urology specimens originating from groups with no in-house pathology services were included; data from any practices that performed either professional or technical services with reference laboratory support were not reviewed. Member groups of the Large Urology Group Practice Association (LUGPA) were surveyed to determine which of the groups had integrated anatomic pathology services into their practices for the same time period. Groups identified as providing global pathology services (performed both technical and professional components) were asked to conduct a similar review of their pathology records after commencing in-house anatomic pathology services as described above, including the number of urologists that were practicing in the group during this interval.
Each group classified biopsy results according to four criteria: benign, malignant, prostatic intraepithelial neoplasia (PIN), or atypical small acinar proliferation (ASAP). For the purpose of this review, PIN and ASAP were grouped into a single category.
Statistical analysis was performed using Student’s pooled two-tailed t-test with unequal variances; linear regression analysis was performed using the least squares method. The slopes of linear regression trend lines were compared using an analysis of covariance,23 and the regression transition point was determined using grid analysis.24 Correlation was performed using Pearson product-moment analysis. Where applicable, statistical analysis was performed using GraphPad Prism 6 software (GraphPad Software, La Jolla, CA).
Results
Of 95 LUGPA group practices surveyed, 39 (41.1%) were identified as performing both technical and professional components of prostate histology. Of these 39 practices, 29 (74.4%) were able to gather the requested information and submitted data for analysis.
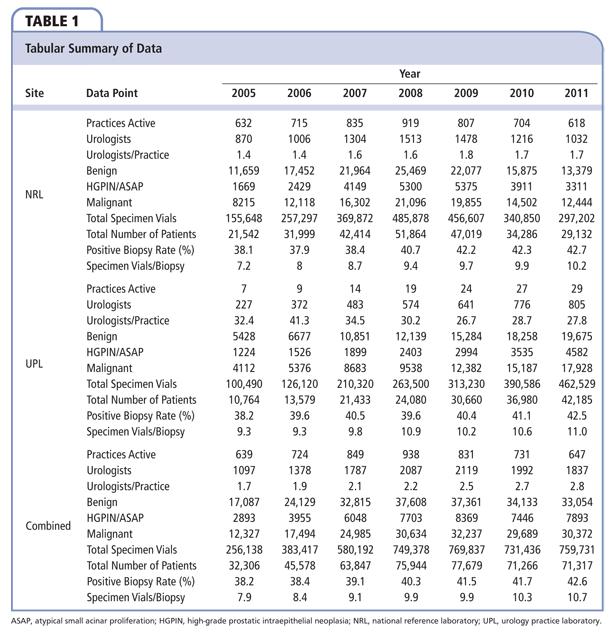
Data are summarized in Table 1. Over the study period we collected data from an annual mean of 1756.7 urologists in 765.6 practices in the United States, for an average of 2.3 urologists per group. From 2005 to 2011, the NRL performed anatomic pathology services for an annual mean of 747.1 practices representing a mean of 1202.7 urologists, averaging 1.6 urologists per group. During this interval, the number of groups utilizing UPL increased from 7 to 29; by 2011 these practices represented 805 urologists (mean, 31.7 urologists/practice).
From 2005 to 2011, the mean national positive biopsy rate was 40.3%; this was identical at both the NRL and the UPL (P = .97). From 2005 to 2011, the mean national rate of benign diagnoses was 49.9%, whereas that for PIN/ASAP was 9.9%. At the NRL these figures were 49.8% and 9.9% for benign and PIN/ASAP, respectively. Similarly, a mean of 49.5% and 10.3% rates of benign histology and PIN/ASAP, respectively, were seen at UPLs. Between the NRL and UPLs, neither the differences between rates of benign nor PIN/ASAP interpretations were significant (P = .83 and .66, respectively).
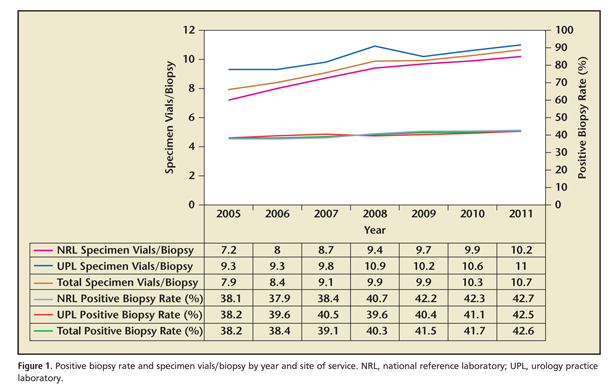
The relationship between positive biopsy rate and specimen vials per biopsy over time in total and by site of service is illustrated in Figure 1. From 2005 to 2011, the number of specimen vials per biopsy submitted nationally increased 34.4%, from 7.9 to 10.7 (mean, 9.4 specimen vials/biopsy). There was a mean of 9.0 specimen vials per biopsy submitted to the NRL, whereas during this period a mean 10.2 specimen vials per biopsy were submitted to UPLs (difference, 1.2 specimen vials/biopsy; P = .04). During this interval, there was an increase of 41.7% in the number of specimen vials per biopsy submitted to the NRL; conversely, the increase in the number of specimen vials per biopsy submitted to UPLs was only 18.3%.
To correct for variances in sample size in each year we compared simple and weighted averages of both positive biopsy rates and specimen vials per biopsy. The overall simple and weighted average for positive biopsy rate was 40.3% and 40.6%, respectively. By subgroup, the simple and weighted average for positive biopsy rate for the NRL was 40.3% versus 40.4%, respectively, and 40.3% versus 40.7%, respectively, for UPLs. For specimen vials per biopsy, the overall simple and weighted average was 9.4 versus 9.7 specimen vials per biopsy. By group, the simple and weighted average for UPLs was 10.2 versus 10.4 specimen vials per biopsy, respectively, and 9.0 versus 9.2 specimen vials per biopsy, respectively, for the NRL. Due to the large sample size, there was no material effect on statistical significance when these results were analyzed using t-test for weighted means.25
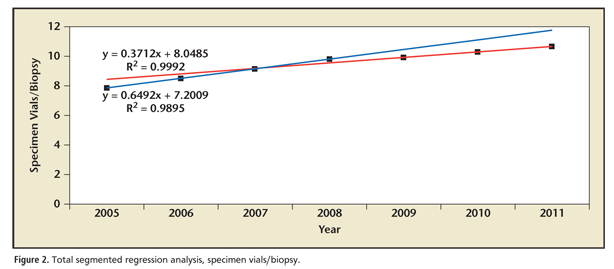
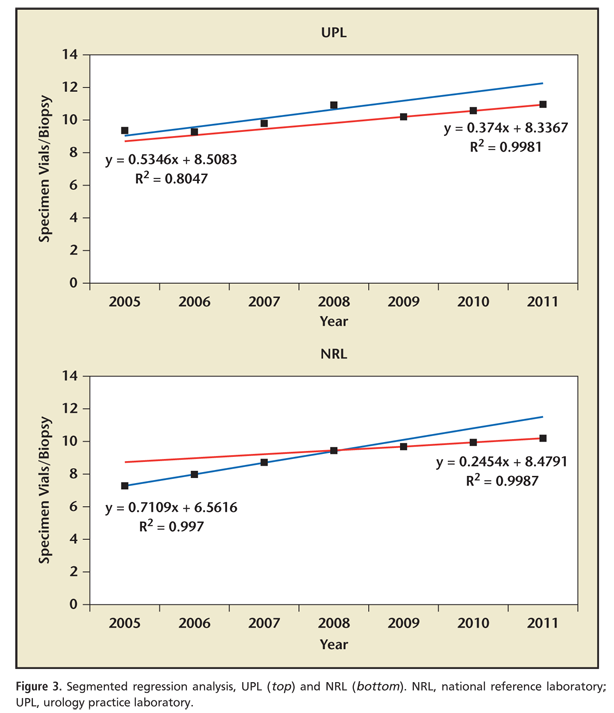
As updated clinical guidelines regarding the most appropriate number of specimen vials per biopsy were released during the study period, we performed segmented regression analysis to determine if any transition point existed in utilization patterns for this parameter; these are presented in Figures 2 and 3. Grid search revealed that such a transition point existed in 2008. There was a sharp change in the trend for overall specimen vials per biopsy for the period of 2005 to 2008 and 2009 to 2011 (m = 0.649 and 0.371, respectively; P = .03); a similar trend was observed in the NRL group (2005-2008, m = 0.711 vs 2009-2011, m = 0.245; P <01). Interestingly, there was no significant change in utilization trends for specimen vials per biopsy between the periods 2005 to 208 and 2009 to 2011 (m = 0.535 vs 0.374 respectively; P = .61) noted in the UPL group. Nationally, the number of specimen vials per biopsy before and after the 2008 transition point was 8.8 versus 10.3, respectively (difference, 1.5 specimen vials/biopsy; P = .03). Between subgroups, the number of specimen vials per biopsy from 2005 to 2008 at UPL was 9.8 compared with 8.3 for the NRL (difference, 1.5 specimen vials/biopsy; P = .05); conversely, this difference decreased to only 0.6 specimen vials per biopsy (10.6 and 10.0 in the UPL and NRL groups, respectively) after the 2008 transition point (P = .08).
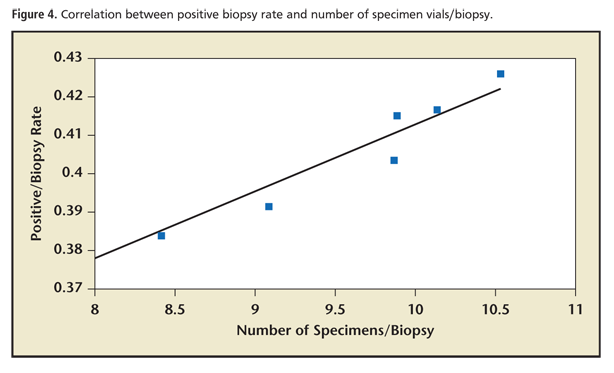
Correlation analysis reveals that for our 437,937 total prostate biopsies submitted in > 4.23 million discrete specimen vials there was a significant (Pearson’s r = 0.9665; P < .01) correlation between increased number of specimen vials per biopsy and an increased positive biopsy rate. A scatter plot of this finding is presented in Figure 4. Detailed statistical results can be found in the Supplementary Material (available at www.medreviews.com).
Discussion
This study, which in 2011 included the work product of over 1800 urologists in nearly 650 practices nationwide, reviewed biopsy results for 437,937 patients and > 4.23 million specimens. This represents by far the largest analysis of positive biopsy rates and utilization trends for prostate biopsies in the literature to date; the elegant meta-analysis of 87 studies performed by Eichler and colleagues1 comprised < 21,000 patients in total. In our large cohort of patients we found that the rate of positive prostate biopsy nationwide was 40.3%; this was mathematically and statistically identical for UPLs and the NRL. Overall, from 2005 to 2011, there was a national trend toward increased numbers of specimen vials per biopsy. For the entire study period there was a small (albeit significant) difference of 1.2 specimen vials per biopsy between UPLs and the NRL; however, for the most recent 3-year period (2009-2011) the difference of 0.6 specimen vials per biopsy between UPLs and the NRL was not significant. Furthermore, although it is technically feasible to place multiple biopsy cores in a single vial, these data demonstrate that by 2011 urologists nationwide had adopted the 10 to 12 specimen vials per biopsy for prostate biopsies; that this trend was seen at both UPLs and the NRL demonstrates that this is independent of site of service and represents changing national clinical standards.
Although the central focus of the study was to assess positive biopsy rate and trends in number of specimen vials per biopsy, given the size of the sample we also sought to verify findings that extended sextant biopsies improve cancer detection rate relative to historical schemes; our data confirm that of multiple smaller studies that the detection of prostate cancer via needle biopsy correlates strongly with the number of specimens submitted.
Segmented regression analysis demonstrates that nationally, there was a clear statistical transition point that occurred in or near 2008 for specimen vials per biopsy. Prior to this there was a mean of 8.8 specimen vials per biopsy; this subsequently increased to an annual average of 10.3 specimen vials per biopsy. This trend was mirrored in number of specimen vials per biopsy submitted to an NRL; conversely, no such change in utilization trends was seen for UPLs. Prior to this transition point, there were significantly greater numbers of specimen vials per biopsy in UPLs compared with the NRL; subsequent to the transition, the difference in specimen vials per biopsy was both de minimus and statistically insignificant.
Our data illustrate that it may be misleading to assess utilization data during a period of changing clinical patterns. This seems to be particularly true when comparing the behavior of large group practices with those of individual practitioners. We noted that physicians utilizing a UPL adopted 10 to 12 specimen vials per biopsy protocols sooner than those that submitting specimens to the NRL, although, ultimately, the number of specimen vials per biopsy was similar in both cohorts studied. The rationale for this is simple: the hallmark of group practice integration is centralized control of administrative functions, which generally includes standardization of clinical pathways. When these group practices adopt such pathways, practice patterns change for large numbers of physicians simultaneously; conversely, in the absence of central medical leadership, changes in practice patterns for individual physicians or small medical groups are dependent on decision making by multiple practitioners. Our data show that the average number of urologists in groups submitting specimens to a UPL was nearly 20 times greater (31.7 vs 1.6) than in groups that utilized an NRL; it is a practical reality that a single practice with strong central control can adopt changes more readily than 20 individual groups without such control.
Despite the large sample, we recognize that there are limitations to this study. Although our findings mirror previously published studies correlating positive biopsy rate with number of cores, we acknowledge that, as we were unable to determine the number of cores submitted per specimen vial, direct comparison between our findings and historical literature on this subject is difficult. As our control group was a single NRL, it is theoretically possible that the positive biopsy rate and/or specimen vials per biopsy are different at other reference or commercial laboratories; given the size of the data sample as well as the broad national penetration of the NRL reviewed, it is likely that our dataset represents a statistically valid sample. We understand that this analysis would have been even more comprehensive had we stratified data according to patient demographic (eg, age and race) as well as pathologic findings (eg, Gleason grade and stage); that analysis was beyond the scope of this manuscript but is presently being contemplated. Although we did capture data on nearly 75% of LUGPA practices identified as operating UPLs, it is also possible that smaller groups or large groups that are not LUGPA members operate such laboratories; however, it is unlikely that the former would have the resources to build and sustain such an undertaking and there are few such groups in the latter category. Analysis was confined to those urology practices with full-service pathology laboratories; those laboratories that perform either only technical or professional components of the anatomic pathology for prostate biopsies were not included in this review. Given the number and national scope of the sample it is unlikely that addition of smaller groups would materially alter our results. Confining the results to full-service UPLs served an additional purpose: if there was a discrepancy in utilization between sites of service, it is likely that review of those groups with the largest investment in personnel and infrastructure (ie, those that had constructed full-service pathology laboratories) would be most likely to show a discrepancy in utilization if one existed.
Conclusions
Our data demonstrate that the positive biopsy rate for prostate needle biopsy at pathology laboratories operated by integrated urology group practices is mathematically and statistically identical to that seen at a prominent NRL. Utilization trends for specimen vials per biopsy were stable for urology in-house pathology laboratories throughout the study period; however, the trend in the number of specimen vials per biopsy submitted to the NRL studied increased significantly more from 2005 to 2008 than during 2009 to 2011. During this more recent period there was no significant difference in specimen vials per biopsy submitted to an NRL and urology practices with in-house pathology laboratories. Our data also confirm that higher positive biopsy rates correlate strongly with increasing number of samples submitted. Most recent data suggest that biopsy schemes segregating specimens into 10 to 12 unique vials have been widely adopted; utilization patterns and outcomes for prostate biopsy likely represent national trends and are similar across sites of service. 
The authors thank Dr. Kiseop Lee, Associate Professor, Department of Mathematics, University of Louisville, for his invaluable assistance in statistical analysis.
References
- Eichler K, Hempel S, Wilby J, et al. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol. 2006;175:1605-1612.
- Bostwick DG, Meiers I. Prostate biopsy and optimization of cancer yield. Eur Urol. 2006;49:415-417.
- Kawachi MH, Bahnson RR, Barry M, et al. NCCN clinical practice guidelines in oncology: prostate cancer early detection. J Natl Compr Canc Netw. 2010;8:240-262.
- Greene KL, Albertsen PC, Babaian RJ, et al. Prostate specific antigen best practice statement: 2009 update. J Urol. 2009;182:2232-2241.
- El‐Hakim A, Moussa S. CUA guidelines on prostate biopsy methodology. Can Urol Assoc J. 2010;4:89-94.
- Gupta C, Ren JZ, Wojno KJ. Individual submission and embedding of prostate biopsies decreases rates of equivocal pathology reports. Urology. 2004;63:83-86.
- Kao J, Upton M, Zhang P, Rosen S. Individual prostate biopsy core embedding facilitates maximal tissue representation. J Urol. 2002;168:496-499.
- Boccon‐Gibod L, van der Kwast TH, Montironi R, et al; European Society of Pathology Uropathology Working Group. Handling and pathology reporting of prostate biopsies. Eur Urol. 2004;46:177-181.
- Barzell WE, Melamed MR. Appropriate patient selection in the focal treatment of prostate cancer: the role of transperineal 3-dimensional pathologic mapping of the prostate—a 4-year experience. Urology. 2007;70(suppl 1):S27-S35.
- Greaney TL. Managed competition, integrated delivery systems and antitrust. Cornell Law Rev. 1994;79:1507-1545.
- Uzych L. Physician ownership-referral arrangements in the United States. Med Law. 1990;9:701-706.
- McDowell TN Jr. Physician self referral arrangements: legitimate business or unethical “entrepreneurialism”. Am J Law Med. 1989;15:61-109.
- Todd JS, Horan JK. Physician referral—the AMA view. JAMA. 1989;262:395-396.
- Pfeifer JD, Liu J. Rate of occult specimen provenance complications in routine clinical practice. Am J Clin Pathol. 2013;139:93-100.
- Hillman BJ, Olson GT, Griffith PE, et al. Physicians’ utilization and charges for outpatient diagnostic imaging in a Medicare population. JAMA. 1992;268:2050-2054.
- Hillman BJ, Joseph CA, Mabry MR, et al. Frequency and costs of diagnostic imaging in office practice—comparison of self-referring and radiologists-referring physicians. N Engl J Med. 1990 323:1604-1608.
- Kouri BE, Parsons RG, Alpert HR. Physician self-referral for diagnostic imaging: review of the empiric literature. AJRAm J Roentgenol. 2002;179:843-850.
- Levin DC, Rao VM. Turf wars in radiology: updated evidence on the relationship between self-referral and the overutilization of imaging. J Am Coll Radiol. 2008;5:806-810.
- Thrall JH. Some facts on rapid imaging growth. Health Aff (Millwood). 2009;28:599.
- Mitchell JM . Urologists’ self-referral for pathology of biopsy specimens linked to increased use and lower prostate cancer detection. Health Aff (Millwood). 2012;31:741-749.
- Kapoor DA, Penson D. Adhering to the standard of care for prostate cancer. Health Aff (Millwood). 2012;31:1367.
- Cooper GS, Yuan Z, Stange KC, et al. The sensitivity of Medicare claims data for case ascertainment of six common cancers. Med Care. 1999;37:436-444.
- Zar J. Comparing simple linear regression equations. In: Zar J, ed. Biostatistical Analysis. 2nd ed. New York, NY: Prentice-Hall;1984:363-379.
- Gill R, Lee K, Song S. Computation of estimates in segmented regression and a liquidity effect model. Computational Statistics & Data Analysis. 2007;51:6459-6475.
- Goldberg LR, Kercheval AN, Lee K. T-statistics for weighted means in credit risk modeling. Journal of Risk Finance. 2005;6:349-365.