A Treatment Approach for Patients With Chronic Systolic Heart Failure
Barry H. Greenberg, MD
Department of Medicine, Cardiology Division, University of California-San Diego, La Jolla, CA
The treatment of heart failure with reduced ejection (HFrEF) is changing rapidly. Advances over the past several decades have focused on blocking the adverse effects of neurohormonal activation. This approach has resulted in marked improvement in outcomes in the HFrEF population. Despite these advances, however, mortality and morbidity remain high and HFrEF patients have poor quality of life. New approaches to therapy now offer additional benefits. Combined neprilysin inhibition and angiotensin receptor blockade using sacubitril-valsartan (LCZ696) has been shown to be superior to an angiotensin-converting enzyme inhibitor in HFrEF patients. Compared with enalapril, treatment with LCZ696 was associated with significant reductions in the composite of cardiovascular mortality and heart failure hospitalization, both components of this composite endpoint and all-cause mortality. Another approach that has been shown to be effective is the use of ivabradine, an agent that blocks If channels in the sinus node to reduce heart rate. When added to standard therapy (that included a b-blocker in 89% of patients) in symptomatic HFrEF patients who were in sinus rhythm, ivabradine significantly reduced combined cardiovascular mortality and heart failure hospitalizations. Death from heart failure, all-cause hospitalization, and heart failure hospitalization were also significantly reduced when ivabradine was added to the medical regimen. Thus, both LCZ696 and ivabradine represent significant advances in the therapy of HFrEF. Utilization of these drugs in the growing HFrEF population will benefit millions of patients around the world.
[Rev Cardiovasc Med. 2016;17(suppl 1):S22-S29 doi:10.3909/ricm17S1S0003]
© 2016 MedReviews®, LLC
A Treatment Approach for Patients With Chronic Systolic Heart Failure
Barry H. Greenberg, MD
Department of Medicine, Cardiology Division, University of California-San Diego, La Jolla, CA
The treatment of heart failure with reduced ejection (HFrEF) is changing rapidly. Advances over the past several decades have focused on blocking the adverse effects of neurohormonal activation. This approach has resulted in marked improvement in outcomes in the HFrEF population. Despite these advances, however, mortality and morbidity remain high and HFrEF patients have poor quality of life. New approaches to therapy now offer additional benefits. Combined neprilysin inhibition and angiotensin receptor blockade using sacubitril-valsartan (LCZ696) has been shown to be superior to an angiotensin-converting enzyme inhibitor in HFrEF patients. Compared with enalapril, treatment with LCZ696 was associated with significant reductions in the composite of cardiovascular mortality and heart failure hospitalization, both components of this composite endpoint and all-cause mortality. Another approach that has been shown to be effective is the use of ivabradine, an agent that blocks If channels in the sinus node to reduce heart rate. When added to standard therapy (that included a b-blocker in 89% of patients) in symptomatic HFrEF patients who were in sinus rhythm, ivabradine significantly reduced combined cardiovascular mortality and heart failure hospitalizations. Death from heart failure, all-cause hospitalization, and heart failure hospitalization were also significantly reduced when ivabradine was added to the medical regimen. Thus, both LCZ696 and ivabradine represent significant advances in the therapy of HFrEF. Utilization of these drugs in the growing HFrEF population will benefit millions of patients around the world.
[Rev Cardiovasc Med. 2016;17(suppl 1):S22-S29 doi:10.3909/ricm17S1S0003]
© 2016 MedReviews®, LLC
A Treatment Approach for Patients With Chronic Systolic Heart Failure
Barry H. Greenberg, MD
Department of Medicine, Cardiology Division, University of California-San Diego, La Jolla, CA
The treatment of heart failure with reduced ejection (HFrEF) is changing rapidly. Advances over the past several decades have focused on blocking the adverse effects of neurohormonal activation. This approach has resulted in marked improvement in outcomes in the HFrEF population. Despite these advances, however, mortality and morbidity remain high and HFrEF patients have poor quality of life. New approaches to therapy now offer additional benefits. Combined neprilysin inhibition and angiotensin receptor blockade using sacubitril-valsartan (LCZ696) has been shown to be superior to an angiotensin-converting enzyme inhibitor in HFrEF patients. Compared with enalapril, treatment with LCZ696 was associated with significant reductions in the composite of cardiovascular mortality and heart failure hospitalization, both components of this composite endpoint and all-cause mortality. Another approach that has been shown to be effective is the use of ivabradine, an agent that blocks If channels in the sinus node to reduce heart rate. When added to standard therapy (that included a b-blocker in 89% of patients) in symptomatic HFrEF patients who were in sinus rhythm, ivabradine significantly reduced combined cardiovascular mortality and heart failure hospitalizations. Death from heart failure, all-cause hospitalization, and heart failure hospitalization were also significantly reduced when ivabradine was added to the medical regimen. Thus, both LCZ696 and ivabradine represent significant advances in the therapy of HFrEF. Utilization of these drugs in the growing HFrEF population will benefit millions of patients around the world.
[Rev Cardiovasc Med. 2016;17(suppl 1):S22-S29 doi:10.3909/ricm17S1S0003]
© 2016 MedReviews®, LLC
KEY WORDS
Heart failure • Neurohormones • LCZ696 • Ivabradine
KEY WORDS
Heart failure • Neurohormones • LCZ696 • Ivabradine
… neurohormonal blocking agents have become the cornerstone of therapy for HFrEF.

Figure 1. Summary of Class I recommendations for medical therapy of patients with HFrEF from the 2013 American College of Cardiology Foundation/American Heart Association guidelines. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; HFrEF, heart failure with reduced ejection fraction; LOE, level of evidence; NYHA, New York Heart Association. Reprinted with permission from Yancy CW et al.20
The rationale for the use of neprilysin inhibition is that this strategy benefits heart failure patients by enhancing levels of these counter-regulatory vasoactive peptides.

Figure 2. Kaplan-Meier curves for the primary composite outcome in the Prospective Comparison of ARNI with ACE-I to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) study, its components, and all-cause mortality. ACE-I, angiotensin-converting enzyme inhibitor; ARNI, angiotensin receptor-neprilysin inhibitor; CI, confidence interval. Reprinted with permission from McMurray JJ et al.12
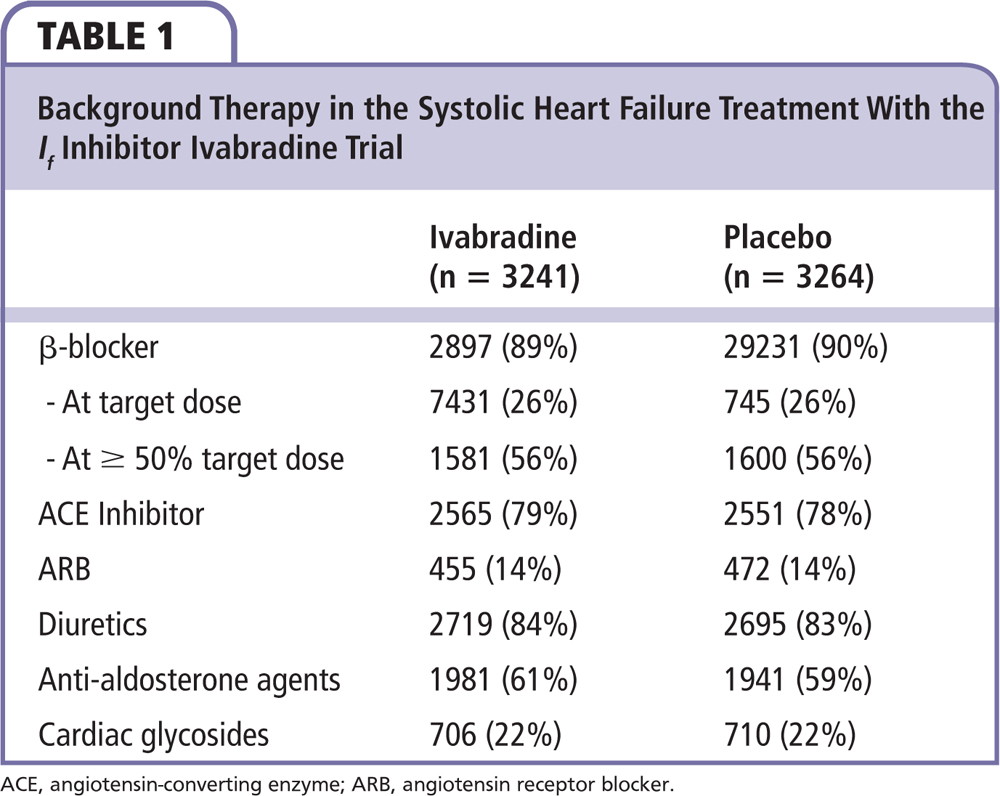

Figure 3. Kaplan-Meier curves for the primary composite outcome in the Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial (SHIFT) and its two components. CI, confidence interval; HR, hazard ratio. Reprinted with permission from Swedberg K et al.13
… ivabradine has been approved in the United States for treating symptomatic HFrEF patients who are receiving optimal medical therapy, including b-blockers (as tolerated), and are in sinus rhythm with a heart rate of 70 beats/min or above.
Main Points
• Patients who have heart failure with reduced ejection fraction (HFrEF) comprise approximately 50% of the overall heart failure population in the United States. Advances in treatment have focused on blocking the adverse effects of neurohormonal activation, which has resulted in marked improvement in outcomes in the HFrEF population.
• Neurohormonal blocking agents have become the cornerstone of therapy for HFrEF. The use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), mineralocorticoid receptor antagonists, and b-blockers have been shown to improve clinical outcomes, including reduced hospitalization rate and increased survival, in HFrEF patients. These drugs have a variety of beneficial effects, including vasodilation, natriuresis, and diuresis. They also inhibit, and in some cases even reverse, pathologic cardiac remodeling.
• Patients with HFrEF who develop atrial fibrillation usually require anticoagulation. The non-vitamin K antagonist oral anticoagulants appear to be as—or even more—effective than warfarin in protecting against thromboembolic complications.
• LCZ696 is a molecule that contains drugs that can inhibit neprilysin and also block angiotensin receptors. It is likely to replace ACE inhibitors and ARBs in the treatment of a large number of HFrEF patients.
• Ivabradine is a novel therapeutic agent that blocks the hyperpolarization-activated cyclic nucleotide-gated channels. The effects of ivabradine are almost solely confined to heart rate slowing, as opposed to the b-blockers, which have a variety of other effects on cardiovascular and other tissues throughout the body.
Main Points
• Patients who have heart failure with reduced ejection fraction (HFrEF) comprise approximately 50% of the overall heart failure population in the United States. Advances in treatment have focused on blocking the adverse effects of neurohormonal activation, which has resulted in marked improvement in outcomes in the HFrEF population.
• Neurohormonal blocking agents have become the cornerstone of therapy for HFrEF. The use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), mineralocorticoid receptor antagonists, and b-blockers have been shown to improve clinical outcomes, including reduced hospitalization rate and increased survival, in HFrEF patients. These drugs have a variety of beneficial effects, including vasodilation, natriuresis, and diuresis. They also inhibit, and in some cases even reverse, pathologic cardiac remodeling.
• Patients with HFrEF who develop atrial fibrillation usually require anticoagulation. The non-vitamin K antagonist oral anticoagulants appear to be as—or even more—effective than warfarin in protecting against thromboembolic complications.
• LCZ696 is a molecule that contains drugs that can inhibit neprilysin and also block angiotensin receptors. It is likely to replace ACE inhibitors and ARBs in the treatment of a large number of HFrEF patients.
• Ivabradine is a novel therapeutic agent that blocks the hyperpolarization-activated cyclic nucleotide-gated channels. The effects of ivabradine are almost solely confined to heart rate slowing, as opposed to the b-blockers, which have a variety of other effects on cardiovascular and other tissues throughout the body.
Heart failure is a common disease and its prevalence is increasing around the world.1,2 In the United States alone, nearly 6 million people have this condition.3 Moreover, the number of heart failure patients is expected to increase substantially over the next several decades. In the United States and other developed countries, the expected increase in prevalence is related to better survival of patients with coronary artery disease and myocardial infarction, as well as the overall aging of the population. In developing nations, the reason is more complex; substantial increases in longevity, reduction in infectious disease mortality, and an increase in cardiovascular risk factors all play a role. Once manifest, heart failure is associated with increased likelihood of hospitalization, reduced survival, and one of the lowest quality of life measurements for patients with any chronic disease. The burden on patients and their families, and on health care systems, is considerable.
Patients who develop heart failure with reduced ejection fraction (HFrEF) comprise approximately 50% of the overall heart failure population in the United States, although there is some indication that number may be shrinking, as the prevalence of heart failure with preserved ejection fraction (HFpEF) appears to be growing.4 Over the past several decades there has been considerable progress in treating HFrEF patients.5-11 In 2015 alone, the US Food and Drug Administration (FDA) approved two additional new agents that improve heart failure outcomes.12,13 Although there are several promising agents in development for treating HFpEF patients, current therapies are focused on controlling symptoms, because no available drug has been shown to improve either morbidity or mortality outcomes.14,15 This article reviews currently available therapies for treating chronic HFrEF with a focus on newer agents that are being incorporated into the treatment armamentarium.
Cornerstones of Therapy
Recognition of the adverse effects of prolonged neurohormonal activation in the setting of heart failure led to the development, testing, and eventual incorporation of drugs that blocked this maladaptive response into the treatment strategy for HFrEF.16 This approach has been so effective that neurohormonal blocking agents have become the cornerstone of therapy for HFrEF. The use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), mineralocorticoid receptor antagonists (MRAs), and β-blockers has been shown to improve clinical outcomes, including reduced hospitalization rate and increased survival, in HFrEF patients.5-11 These drugs have a variety of beneficial effects, including vasodilation, natriuresis, and diuresis. They also inhibit, and in some cases even reverse, pathologic cardiac remodeling.17,18 Progressive remodeling of the heart involving dilatation, hypertrophy, and increases in interstitial fibrosis of the left ventricle and other chambers has been shown to play a key role in the pathogenesis of HFrEF.19 Consequently, the use of ACE inhibitors or ARBs and β-blockers has been given strong recommendations (Class I, Level of Evidence A) in the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines for heart failure management (Figure 1).20
MRAs are given a similar endorsement in ACCF/AHA heart failure guidelines for patients who remain symptomatic.20 Notably, this latter class of drugs is also of benefit in maintaining serum potassium levels, which are often reduced by diuretic therapy. As a result of their propensity for increasing potassium levels above the upper limit of normal, there are caveats in the guidelines cautioning clinicians to avoid their use in patients who have more severe renal dysfunction (serum creatinine level > 2.0 Meq/L for women and 2.5 Meq/L for men), in patients who already have evidence of hyperkalemia, and in patients in whom follow-up with repeat measurement of serum electrolytes may be problematic. Although the exact incidence of hyperkalemia that limits therapy with MRAs (and also ACE inhibitors or ARBs) is not precisely known, clinical experience suggests that it is substantial. The recent development of safe and effective drugs for treating hyperkalemia offers a potential solution that may allow continuation of drugs that target the renin-angiotensin-aldosterone system (RAAS) activation when hyperkalemia develops. One of these agents, patiromer, has already been approved; a second, zirconium silicate, is currently under FDA review.21-24 Preliminary data suggest that their use in patients with underlying chronic kidney disease increases the percentage of patients who can tolerate RAAS blockers.
In addition to the neurohormonal blocking agents just described, patients who remain congested should be treated with diuretics (Figure 1). Loop diuretics are usually selected for this purpose due to their greater potency than thiazide diuretics. On occasion, a combination of loop and thiazide diuretics is used to treat patients who are refractory to a single diuretic alone. This approach, however, requires careful attention to electrolyte abnormalities (involving both potassium and sodium) that occur commonly with combined diuretic therapy. The ACCF/AHA guidelines also recommend the use of a combination of hydralazine and long-acting nitrate in African-American patients,20 as there is good evidence that this combination, in patients on good background neurohormonal therapy, provides considerable benefit (including a reduction in mortality).25
Other Drugs to Consider
Through the 1970s, digoxin was used in most HFrEF patients. Since that time, there has been a steady erosion in the number of patients treated with this agent; recent clinical trial data suggest that between 20% and 30% of the HFrEF population is currently being treated with digoxin. There are several reasons for the lower utilization rate of digoxin in HFrEF patients. Widespread utilization of the neurohormonal blocking agents described here that favorably affect survival has led some clinicians to conclude that digoxin no longer has any value in the HFrEF population. Studies using data from administrative databases reporting worsening outcomes with digoxin have further called the value of this agent into question. Although digoxin does not appear to improve survival in HFrEF patients, clinical trial results show that it does reduce heart failure hospitalizations.26 Use of digoxin at doses designed to achieve lower serum levels than in the past is an important way of maximizing benefits while minimizing risk.27 Patients who remain symptomatic with more severe heart failure are the most likely to benefit from use of this drug.28
Patients with HFrEF who develop atrial fibrillation will almost always require anticoagulants to protect against the risk of thromboembolic complications.29 Whereas warfarin was the standard therapy employed for this purpose due to its greater efficacy compared with antiplatelet agents, the availability of non-vitamin K antagonist oral anticoagulants (NOACs) has considerably altered the therapeutic options. These drugs appear to be as—or even more—effective than warfarin in protecting against thromboembolic complications, and they have an overall safety profile that is at least comparable with that of warfarin.30 The fact that the NOACs do not require regular monitoring of anticoagulation is an important benefit. Although the absence of agents to reverse the anticoagulant effects has been a concern, the availability of agents that can reverse the effects of at least some of the NOACs should help reduce the likelihood of serious bleeding events.
New Strategies for Treating HFrEF Patients
Combined Neprilysin Inhibition and Angiotensin Receptor Blockade
Evidence of widespread neurohormonal activation in heart failure patients led to the recognition that not all of the systems that were upregulated had deleterious effects. Although the adverse effects of peptides such as angiotensin II, aldosterone, and the catecholamines have been well described, other peptides appear to have counter-regulatory effects that would be expected to modulate the adverse effects of the vasoconstricting peptides.31,32 The counter-regulatory peptides promote vasodilation, and salt and water excretion in the urine. In addition, they act to inhibit maladaptive cardiac remodeling. The most carefully studied of these peptides in heart failure patients are the natriuretic peptides, including atrial natriuretic peptide, B-type natriuretic peptide, and C-type natriuretic peptide.33 Others include bradykinin, adrenomedullin, and apelin. Neprilysin, an ectoenzyme released into the circulation, is involved in the breakdown of the natriuretic peptides and other vasoactive peptides.34 The rationale for the use of neprilysin inhibition is that this strategy benefits heart failure patients by enhancing levels of these counter-regulatory vasoactive peptides. However, neprilysin also breaks down angiotensin II; inhibition of this enzyme without inhibiting the effects of this main effector molecule of the RAAS is problematic.
LCZ696 is a molecule that contains drugs that can inhibit neprilysin and also block angiotensin receptors.31 Once ingested, the molecule separates into two distinct drugs: sacubitril, a prodrug that is quickly converted to a neprilysin inhibitor, and valsartan, an ARB. The efficacy and safety of LCZ696 was assessed in the Prospective Comparison of ARNI with ACE-I to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) study.12 The primary hypothesis of this clinical trial, which included over 8400 patients, was that LCZ696 was superior to enalapril in reducing the composite endpoint of first occurrence of cardiovascular death or heart failure hospitalization. The study was stopped prematurely when it became apparent that patients randomized to the LCZ696 arm were having fewer events than were the patients who were randomized to the enalapril arm. The overall risk reduction for the primary endpoint was 20%, as shown in Figure 2. LCZ696 proved superior to enalapril in reducing both of the components of the primary endpoint and in reducing all-cause mortality. Subgroup analysis for the primary endpoint showed remarkable consistency of the benefits of LCZ696 in virtually all subgroups of patients. Overall, the side-effect profile of LCZ696 compared with enalapril demonstrated that the new agent had an acceptable safety and tolerability profile. Symptomatic hypotension, however, was more common with LCZ696, as was the risk of angioedema, particularly in black patients. Based on these results, LCZ696 is likely to replace ACE inhibitors and ARBs in the treatment of a large number of HFrEF patients.
Selective If Channel Inhibitors to Slow Heart Rate
Resting heart rate has been identified as a potent risk factor for both cardiovascular and all-cause mortality in heart failure patients. In an analysis from the Framingham study, each standard deviation of increase in heart rate above baseline increased mortality risk by 17% over a follow-up period that extended for nearly two decades.35,36 This relationship was independent of comorbidities and activity level. Support for the concept that lowering heart rate favorably affects the clinical course of heart failure patients comes from β-blocker trials that show that the greater the heart rate reduction, the greater the reduction in mortality. A meta-analysis of 23 β-blocker trials reported that for every reduction of 5 beats/min in heart rate there was a corresponding 18% risk reduction in mortality.37 However, whether the beneficial effects of β-blockers on the clinical course of heart failure patients was due to a slowing in heart rate per se, or to some effect of these agents that resulted in a secondary heart rate reduction, was uncertain.
Ivabradine is a novel therapeutic agent that blocks the hyperpolarization-activated cyclic nucleotide-gated channels that are responsible for the current through the If “funny” channels.38,39 The net effect is slowing of phase 4 depolarization in sinoatrial node pacemaker cells, which results in a reduction in heart rate if the patient is in sinus rhythm. Based on its distinct mechanism of action, the effects of ivabradine are almost solely confined to heart rate slowing, whereas β-blockers have a variety of other effects on cardiovascular and other tissues throughout the body. Evidence that the heart rate–slowing effect of ivabradine might benefit HFrEF patients came from the Morbidity-Mortality Evaluation of the If Inhibitor Ivabradine in Patients with Coronary Disease and Left Ventricular Dysfunction (BEAUTIFUL) trial. This trial randomized 10,917 coronary artery disease patients with an ejection fraction of ≤ 0.40% and a resting heart rate > 60 beats/min to ivabradine or placebo in addition to standard therapy (which included β-blockers in 84% of the patients).40 Although the results showed no significant effect on all-cause mortality, heart failure hospitalization, or hospitalization for myocardial infarction or unstable angina, predefined subgroup analysis in the patients whose resting heart rate was > 70 beats/min found that ivabradine was associated with decreases in hospitalizations for unstable angina or myocardial infarction and for coronary revascularization. Neither heart failure admissions, all-cause mortality, nor cardiovascular mortality, however, were significantly affected.
The Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial (SHIFT) tested whether heart rate reduction by the selective sinus node inhibitor ivabradine favorably affected outcomes in symptomatic HFrEF patients with an ejection fraction ≤ 0.35 who were in sinus rhythm with a resting heart rate ≥ 70 beats/min.13 Patients were on stable background therapy, including a β-blocker, if tolerated. The study randomized 6558 patients to either placebo or ivabradine, which was uptitrated to a maximum dose of 7.5 mg twice daily. The primary endpoint of SHIFT was the composite of cardiovascular death or hospitalization for worsening heart failure. As shown in Table 1, background therapy was excellent in the SHIFT population. In particular, β-blockers were being used in 89% of the patients randomized to ivabradine and 90% of the patients in the placebo group. In both study groups, 56% were at ≥ 50% of their target dose and 26% were on target β-blocker dose.
The mean dose of ivabradine in SHIFT was 6.4 ± 1.6 mg twice daily at 28 days and 6.5 ± 1.6 mg twice daily at 1 year. Placebo-corrected reductions in heart rate were 10.9 beats/min and 9.1 beats/min on the average at 28 days and 1 year, respectively. The primary endpoint of cardiovascular death or hospital admission for worsening heart failure occurred in 793 (24%) of ivabradine-treated patients and 937 (29%) of placebo-treated patients, a reduction of 18% (P < .0001) (Figure 3). Although ivabradine did not significantly reduce all-cause mortality, which occurred in 552 (17%) placebo-treated patients and 503 (16%) ivabradine-treated patients (P = .092), death from heart failure, which occurred in 151 placebo-treated patients compared with 113 ivabradine-treated patients, was significantly reduced by 26% (P = .014) with ivabradine. All-cause hospital admission, which occurred in 1356 (42%) placebo-treated patients as compared with 1231 (38%) ivabradinetreated patients, was reduced by 11% (P = .003), whereas hospital admission for worsening heart failure, which went from 672 in the placebo group (21%) to 514 in the ivabradine group (16%), was reduced by 26% (P < .0001). Subgroup analysis including age, sex, use of β-blockers, cause of heart failure (ischemic vs nonischemic), New York Heart Association class, diabetes history, hypertension, and baseline heart rate demonstrated no significant interaction between the efficacy of ivabradine and these subgroups for all the variables studied except for heart rate. Patients with a heart rate under 77 beats/min demonstrated only a 7% reduction in the primary composite endpoint compared with a 25% reduction in patients whose baseline heart rate exceeded 77 beats/min (P = .029 for the interaction). Overall, tolerability of ivabradine was good with bradycardia recorded in 10% of the population. Excessive slowing of the heart resulted in withdrawal from the drug in only 1% (despite background therapy with β-blockers in 89% of patients). There was a slight excess of atrial fibrillation (9% vs 8%; P = .012) in the ivabradine group compared with the placebo group. Phosphenes, defined as transient enhanced brightness in a restricted area of the visual field, occurred in 3% of ivabradine-treated patients compared with 1% of placebo-treated patients (P < .0001), but the drug was withdrawn in only seven drug-treated patients as compared with three placebo-treated patients. Based on these findings, ivabradine has been approved in the United States for treating symptomatic HFrEF patients who are receiving optimal medical therapy, including β-blockers (as tolerated), and are in sinus rhythm with a heart rate of 70 beats/min or above.
Conclusions
HFrEF is a growing clinical problem throughout the world. Despite advances in treatment over the past several decades, outcomes for this population are not favorable. However, recently available agents added to guideline-recommended therapy have been shown to further improve outcomes in HFrEF. Both LCZ696 and ivabradine were shown in large-scale clinical trials to reduce combined morbidity and mortality. Use of these agents in appropriate patients is recommended. ![]()
References
- Roger VL. Cardiovascular diseases in populations: secular trends and contemporary challenges-Geoffrey Rose lecture, European Society of Cardiology meeting 2014. Eur Heart J. 2015;36:2142-2146.
- Sato N. Epidemiology of heart failure in Asia. Heart Fail Clin. 2015;11:573-579.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6-e245.
- Gerber Y, Weston SA, Redfield MM, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996-1004.
- Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol. 1988;62:60A-66A.
- Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325:293-302.
- Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigators. N Engl J Med. 1992;327:685-691.
- Hjalmarson A, Goldstein S, Fagerberg B, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA. 2000;283:1295-1302.
- Krum H, Roecker EB, Mohacsi P, et al; Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effects of initiating carvedilol in patients with severe chronic heart failure: results from the COPERNICUS Study. JAMA. 2003;289: 712-718.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709-717.
- Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362: 772-776.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004.
- Swedberg K, Komajda M, Böhm M, et al; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875-885.
- Yusuf S, Pfeffer MA, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777-781.
- Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383-1392.
- Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341: 577-585.
- Greenberg B, Quinones MA, Koilpillai C, et al. Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction. Results of the SOLVD echocardiography substudy. Circulation. 1995;91:2573-2581.
- Colucci WS, Kolias TJ, Adams KF, et al; REVERT Study Group. Metoprolol reverses left ventricular remodeling in patients with asymptomatic systolic dysfunction: the REversal of VEntricular Remodeling with Toprol-XL (REVERT) trial. Circulation. 2007;116:49-56.
- Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128:388-400.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810-1852.
- Bakris GL, Pitt B, Weir MR, et al; AMETHYST-DN Investigators. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314:151-161.
- Pitt B, Bakris GL, Bushinsky DA, et al. Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalaemia in patients with heart failure and chronic kidney disease on RAAS inhibitors. Eur J Heart Fail. 2015;17:1057-1065.
- Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312:2223-2233.
- Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372:222-231.
- Taylor AL, Ziesche S, Yancy C, et al; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049-2057.
- Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525-533.
- Adams KF Jr, Patterson JH, Gattis WA, et al. Relationship of serum digoxin concentration to mortality and morbidity in women in the digitalis investigation group trial: a retrospective analysis. J Am Coll Cardiol. 2005;46:497-504.
- Gheorghiade M, Patel K, Filippatos G, et al. Effect of oral digoxin in high-risk heart failure patients: a pre-specified subgroup analysis of the DIG trial. Eur J Heart Fail. 2013;15:551-559.
- Lip GY, Potpara T, Boriani G, et al. A tailored treatment strategy: a modern approach for stroke prevention in patients with atrial fibrillation. J Intern Med. 2016;279:467-476.
- Lip GY, Lane DA. Matching the NOAC to the patient: remember the modifiable bleeding risk factors. J Am Coll Cardiol. 2015;66:2282-2284.
- Buggey J, Mentz RJ, DeVore AD, Velazquez EJ. Angiotensin receptor neprilysin inhibition in heart failure: mechanistic action and clinical impact. J Card Fail. 2015;21:741-750.
- Booz GW. Putting the brakes on cardiac hypertrophy: exploiting the NO-cGMP counter-regulatory system. Hypertension. 2005;45:341-346.
- Volpe M, Rubattu S, Burnett J Jr. Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J. 2014;35:419-425.
- Turner AJ, Tanzawa K. Mammalian membrane metallopeptidases: NEP, ECE, KELL, and PEX. FASEB J. 1997;11:355-364.
- Barbuti A, DiFrancesco D. Control of cardiac rate by “funny” channels in health and disease. Ann N Y Acad Sci. 2008;1123:213-223.
- Ho JE, Larson MG, Ghorbani A, et al. Long-term cardiovascular risks associated with an elevated heart rate: the Framingham Heart Study. J Am Heart Assoc. 2014;3:e000668.
- McAlister FA, Wiebe N, Ezekowitz JA, et al. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150:784-794.
- Du XJ, Feng X, Gao XM, et al. I(f) channel inhibitor ivabradine lowers heart rate in mice with enhanced sympathoadrenergic activities. Br J Pharmacol. 2004;142:107-112.
- Mulder P, Barbier S, Chagraoui A, et al. Long-term heart rate reduction induced by the selective I(f) current inhibitor ivabradine improves left ventricular function and intrinsic myocardial structure in congestive heart failure. Circulation. 2004;109:1674-1679.
- Fox K, Ford I, Steg PG, et al; SIGNIFY Investigators. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807-816.