Heart Failure University 2015: Presentation Summaries
[Rev Cardiovasc Med. 2016;17(suppl 1):S1-S8 doi:10.3909/ricm17S1S0001]
© 2016 MedReviews®, LLC
Heart Failure University 2015: Presentation Summaries
[Rev Cardiovasc Med. 2016;17(suppl 1):S1-S8 doi:10.3909/ricm17S1S0001]
© 2016 MedReviews®, LLC
Heart Failure University 2015: Presentation Summaries
[Rev Cardiovasc Med. 2016;17(suppl 1):S1-S8 doi:10.3909/ricm17S1S0001]
© 2016 MedReviews®, LLC
Heart Failure University convened December 11-13, 2015, in Los Angeles, CA. The conference presented, discussed, and debated all available preventative, diagnostic, therapeutic, and management options for heart failure in a highly interactive format. This program included didactic lectures, case-based discussions, and faculty panels. Presentation summaries are provided below; video clips of the presentations can be found online at http://read.nxtbook.com/medreviews/
reviewscardiovascularmedicine/volume17suppl1/.
Heart Failure: Scope of the Problem, Pathophysiology, and Risk
Presented by Gregg C. Fonarow, MD, FACC, FAHA
Dr. Fonarow began his presentation with clarification of what comprises heart failure: it is a complex clinical syndrome resulting from any structural or functional cardiac disorder impairing the ability of the ventricle to either fill with or eject blood. As not all patients have volume overload at the time of initial or subsequent evaluation, the term heart failure is preferred over the older term congestive heart failure. It falls into four New York Heart Association (NYHA) categories: Class I, normal exercise tolerance; Class II, symptoms with ordinary exertion; Class III, symptoms with only mild exertion; and Class IV, symptoms at rest.
A significant impact exists with respect to hospitalizations and the burden of disease: the prevalence is estimated to be 6 million individuals presently and 8 million individuals by 2030. In addition, heart failure is the leading cause for adults to be hospitalized in the United States. Most recent estimates show 1 million hospitalizations annually and a 50% readmission rate within 6 months; the cost of the disease is $21 billion annually, with the estimated costs increasing to $53 billion by 2030. Heart failure is presently one of the costliest diseases as measured by Medicare and Medicaid reimbursements: the reasons for this are largely driven by hospitalizations and readmission of patients. Hence, primary goals are the prevention of hospitalization, which repeatedly occurs due to symptom progression, and reducing mortality. As heart failure can progress even with multiple treatment interventions, a primary challenge is to discover therapies and medical management that will reduce hospitalizations , reduce mortality, and prevent heart failure in the first place.
Diagnosis, Evaluation, and Role of Biomarkers in Heart Failure
Presented by Alan S. Maisel, MD
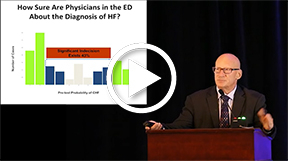
This presentation focused on the role of biomarkers in diagnosing and evaluating heart failure. Dr. Maisel emphasized that history and physical examination findings (eg, rales, tachycardia, S3 gallop and/or murmur suggesting obstructive/regurgitant valvular disease, S4 gallop, pedal edema, jugular venous distention or other evidence of increased left ventricular [LV] filling pressure [hepatojugular reflex, ascites, hepatomegaly], cold extremities) often are not enough to correctly diagnose acute heart failure patients. He cited studies showing significant numbers of patients incorrectly diagnosed for heart failure in both primary care and emergency room settings.
Specifically, he said biomarkers are surrogates for many underlying pathophysiologic processes associated with diagnosis and management of heart failure. They can help to establish or refute a diagnosis and assess underlying pathophysiologic processes, as well as risk stratify or screen to determine presence and/or severity of disease. In addition, they can guide selection of appropriate therapeutic intervention for guiding treatment. Several relevant biomarkers are natriuretic peptides (B-type natriuretic peptide [BNP] or N-terminal pro B-type natriuretic peptide) for assessing congestion; procalcitonin for assessing infection/sepsis; troponin for assessing cardiac muscle contraction; and ST2 for assessing biomechanical strain. The utility of biomarkers has been applied in several clinical studies. The Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT) applied and integrated BNP use to assess patient prognosis. The Guiding Evidence Based Therapy Using Biomarker Intensified Treatment (GUIDE-IT) study evaluated safety, efficacy, and cost-effectiveness of BNP-guided therapy for chronic systolic heart failure. The Biomarkers in Acute Heart Failure (BACH) and IMPACT trials assessed procalcitonin levels for pneumonia risk. The Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) study evaluated troponin to assess cardiac ischemia. sST2 was also discussed as the “new kid on the block.” It is a robust biomarker of fibrosis and, when levels are above 35 ng/mL, there is significant risk of mortality. Treatment that lowers sST2 levels clearly improve survival and, hence, guided heart failure treatment is possible. Because there is less variation in levels than NPs, sST2 levels may be a better option to monitor in patients. Early data suggests that sST2 levels may predict response to sacubitril/valsarten.
Pharmacologic Treatment of Heart Failure With Reduced Ejection Fraction: New Innovations and Implications for Guidelines
Presented by Clyde Yancy, MD
This presentation discussed three paradigms of heart failure treatment guidelines: prevention, treatment for reduced ejection fraction (EF) heart failure, and regenerative therapies. Dr. Yancy mentioned the physiologic process from normal cardiac function toward heart failure due to neurohormonal activation of the renin-angiotensin system (RAS). He discussed the implications of the Systolic Blood Pressure Intervention Trial (SPRINT), in which medically treating high-risk hypertensive adults aged > 50 years to the target of 120 mm Hg significantly reduced cardiovascular events by 30% and reduced all-cause mortality by nearly 25% compared with patients treated to a target of 140 mm Hg. These results were significant, showing heart failure can be prevented.
He also noted treatments benefiting specific groups, such as loop diuretics for patients with volume overload, hydralazine-nitrates for African American patients (often diagnosed with hypertension), and aldosterone antagonists for patients with elevated creatinine/potassium. Likewise, newer treatments such as ivabradine, which decreases cardiac muscle contraction, may benefit stable heart failure patients with a resting heart rate of at least 70 beats/min who are taking the highest tolerable β-blocker dose. This premise was seen in the Systolic Heart Failure Treatment with the If Inhibitor Ivabradine Trial (SHIFT), which showed that ivabradine reduces hospitalization for heart failure in specialized populations. In patients with more chronic heart failure, the PARADIGM-HF trial showed that angiotensin receptor-neprilysin inhibitors (ARNIs) such as sacubitril/valsartan can work better than angiotensin-converting enzyme (ACE) inhibitors. Regenerative therapy focusing on stem cells, organ regeneration, and gene transfer also shows promise. The presentation concluded with a suggestion for heart failure management to focus on prevention and treatment with biomarkers, diagnostic imaging, and early introduction of RAS inhibitors.
Pathophysiology, Diagnosis, and Management of Chronic Heart Failure With Preserved Ejection Fraction
Presented by Michael B. Fowler, MD, MB, FRCP
This presentation started by emphasizing the poor prognosis associated with heart failure with preserved EF. Dr. Fowler noted that another name for this condition is diastolic heart failure with signs and symptoms of heart failure. It is believed that this condition is caused by pathophysiologic alterations in vascular and inflammatory components. It is heterogeneous in etiology and pathophysiology (LV hypertrophy and arterial stiffness). Compared with heart failure patients with reduced EF, these patients have a higher rate of diabetes, obesity, and previously diagnosed hypertension. They are generally older, and more likely to be women. The most common cause of death in these patients is cardiovascular; among this group of diseases, the most frequent cause of death is cardiac arrhythmia, occurring in the outpatient rather than inpatient setting.
Clinical trials have assessed specific medical treatments. For example, the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial showed no clear benefit for using aldosterone antagonists compared with a placebo group based on a composite of cardiovascular death, aborted cardiac arrest, or hospitalization for heart failure. No treatment has yet been shown convincingly to reduce both morbidity and mortality in patients with diastolic heart failure with normal EF. Hence, the search continues for additional treatments for this disease. Medications such as diuretics and sodium restriction manage fluid status and control hypertension and prevent progression to diastolic heart failure. In addition to aldosterone antagonists, clinical trials have assessed ARNIs, statins, and advanced glycation end products as future management options. Prevention remains the optimal option.
The Role of Device Therapy in the Monitoring and Treatment of Heart Failure
Presented by William T. Abraham, MD, FACP, FACC, FAHA, FESC
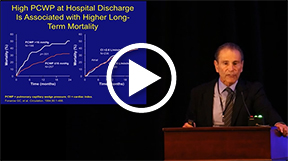
This presentation focused on implantable hemodynamic monitoring with a brief update on cardiac resynchronization therapy (CRT). Dr. Abraham also discussed implantable pulmonary artery hemodynamic monitoring and concluded with a brief discussion of investigational electrical and structural disease therapies. In discussing CRT, he mentioned that clinical studies showed it delivered a consistent improvement in quality of life, functional status, and exercise capacity. Based on clinical data, the latest American Heart Association clinical guidelines strongly recommend CRT for patients with both a reduced LVEF (≤ 35%) and a wide QRS complex (> 120 ms), with left branch bundle block. It improves clinical status and prolongs survival in NYHA Class II, III, and IV patients.
The Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure (COMPASS-HF) trial was the first trial of an implantable hemodynamic monitor to determine if monitoring intracardiac pressures could decrease patient mortality, important as optimal intracardiac pressure is based on normal fluid volume (optivolemia). Heart failure patients are at great risk of acute decompensation: they may be hypervolemic (“wet”) with an increased risk of arrhythmia, or hypovolemic (“dry”) with an increased risk of hypotension and renal impairment. Results of the COMPASS-HF trial prompted the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) trial, which evaluated wireless pulmonary artery pressure measurement/monitoring for heart failure. It showed a 33% decreased annual risk of heart failure hospitalization compared with control patients along with reduction in the combined risk of heart failure and all-cause mortality. These findings showed implantable monitoring reduced the risk of heart failure regardless of EF. Newer mechanical and structural devices have shown promise in treating heart failure through utilizing cardiac contractility modulation and neuromodulation.
Catheterization Laboratory-based Interventions for Acute and Chronic Heart Failure
Presented by Raj Makkar, MD
Dr. Makkar discussed valvular therapies, transcatheter techniques, and hemodynamic devices for high-risk patients, and presented several high-risk patient cases who had structural heart disease (mitral and aortic insufficiency) that resulted in acute and chronic heart failure. They were treated with transcatheter procedures to replace their valves which improved their prognosis. He followed with a discussion of the Placement of Aortic Transcatheter Valves (PARTNER) trial which demonstrated that transcatheter aortic valve replacement (TAVR) with the SAPIEN valve (Edwards Lifesciences, Irvine, CA) was superior to medical therapy in patients with aortic stenosis and equivalent to surgical aortic valve replacement. Those not receiving a valve replacement died within 5 years of diagnosis; even those who had valve replacements had a high mortality rate of 70%, indicating the fragile health of the study cohort. Thus, the PARTNER II trial followed to determine TAVR safety and efficacy, showing it to be both a safe and effective technique.
Mitral regurgitation is another valvular disorder that may lead to acute and chronic heart failure. Many individuals with mitral regurgitation are high-risk patients; they are poor surgical candidates and refractive to surgical therapy. Hence, interventional procedures including leaflet repair, coronary sinus annuloplasty, direct annuloplasty, coronary replacement, transcatheter mitral valve replacement, and devices (MitraClip; Abbott Vascular, Abbott Park, IL) can benefit this population. Studies with MitraClip showed it was safer and had equivalent clinical benefits when compared with surgery. The Endovascular Valve Edge-to-Edge Repair Study (EVEREST) and Clinical Outcomes Assessment of the MitraClip Percutaneous Therapy for High Surgical Risk Patients (COAPT) studies will further assess transcatheter mitral valve replacement along with the interventional Carillon (Cardiac Dimensions, Kirkland, WA) and Fortis (Edwards Lifesciences) devices. Overall, transcatheter procedures for patients with aortic insufficiency and mitral regurgitation have shown promising results.
Treatment of Acute Heart Failure
Presented by Barry H. Greenberg, MD
Dr. Greenberg covered the treatments available for acute heart failure, and noted that three types of drugs exist for treating these patients: diuretics to reduce volume overload, vasodilators to decrease vascular pressure, and inotropic agents to strengthen myocardial contractility. These drugs are utilized according to patient hemodynamic profiles: an elevating filling pressure indicates a hypervolemic patient and a reduced cardiac output indicates a hypovolemic patient. Most volume overloaded patients will be diuresed, first by using a loop diuretic, followed by a thiazide or metolazone. Clinical literature, as per the Diuretic Optimization Strategies Evaluation (DOSE) study, has shown a high dose of intravenous diuretic can be more effective than a low dose. Although vasodilatory drugs such as low-dose dopamine (< 5 µg/k/ min) nitroglycerin, nitroprusside, or nesiritide may be considered for use with diuretics, the Renal Optimization Strategies Evaluation (ROSE) study showed they do not always improve patient congestion. Inotropic agents such as dobutamine or milrinone increase cardiac output and stroke volume and are used in individuals who present with tissue hypoperfusion.
Clinical studies have shown significant decreases in inpatient mortality and mean length of stay for acute heart failure patients. However, high readmission rates and overall mortality persist, most likely due to myocardial and/or renal damage occurring after each hospitalization. Hence, investigational therapies are being developed to treat residual congestion and reduce repeat admissions. One such agent, serelaxin, is the recombinant form of relaxin, which rises substantially in women during pregnancy and is believed to be responsible for some of the favorable cardiorenal effects seen in pregnancy.
Management of Special Populations and Comorbid Conditions: Sex, Race-Ethnicity, Age, Diabetes, Obesity, and Hyponatremia
Presented by Tamara Horwich, MD, MS
This presentation noted heart failure treatment requires specialized assessment according to sex, race, ethnicity, and age, as well as for comorbid conditions such as diabetes, obesity, and hyponatremia. Dr. Horwich mentioned that women are more likely than men (50% vs 30%) to have heart failure with preserved EF (HFPEF). Women have differing cardiopulmonary testing ranges and higher oxygen consumption levels than men. They have higher rates of diabetes, hypertension, and depression, but lower rates of coronary disease. A sex-specific model predicted HFPEF mortality based on the heart failure patient database: BNP level, peak oxygen consumption, being on ACE inhibitor/angiotensin receptor blocker (ARB) therapy, and NYHA class. The African-American Heart Failure Trial (A-HEFT) showed hydralazine and isosorbide dinitrate treatment reduced morbidity and mortality in African-American heart failure patients. Heart failure prevalence increases with age, most likely due to physiologic factors and increased comorbidity rates.
Diabetes is prevalent in heart failure patients. A comparison of clinical trials showed roughly 25% of outpatients and almost 50% of inpatients were diagnosed with diabetes. This finding may result from cardiometabolic pathogenetic mechanisms (increased inflammation, neurohormonal activation, myocardial muscle remodeling) leading to LV dysfunction and heart failure. The Framingham Heart study showed an elevated body mass index in men and women (> 25 kg/m2) increases the risk of developing heart failure. An “obesity paradox” exists, with greater survival rates among overweight rather than underweight patients, most likely due to the catabolic process of HF. Hyponatremia in HF primarily results from inability to excrete free water; its presence indicates a poor prognosis.
Controversies in Heart Failure With Panel Discussion: Cast Your Vote
Moderated by Gregg C. Fonarow, MD, FACC, FAHA
This panel included Drs. Fonarow, Greenberg, Horwich, and Yancy, who asked a series of questions regarding management of a heart failure patient:
- Would a Class III heart failure patient with reduced EF (< 35%) taking an ACE inhibitor and β-blocker require additional therapy?
- Most participants and the panel believed an aldosterone antagonist was appropriate. If the patient improved to class II, he or she could receive an ARNI compound, drawing on PARADIGM-HF trial data.
- What additional therapy is needed in a Class II patient with an EF of 35% who is taking an ACE inhibitor and β-blocker?
- Most participants suggested adding an aldosterone antagonist, drawing on the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) trial. However, one on the panel thought no additional therapy was needed.
- Should the patient’s β-blocker metoprolol tartrate (100 mg twice daily) be increased?
- Most participants and the panel agreed on increasing therapy to metoprolol succinate, 200 mg, a long-acting β-blocker, based on clinical guidelines.
- What steps should be taken after a patient develops acute decompensation on metoprolol succinate, 200 mg?
- Most participants wanted to continue the metoprolol, believing abrupt withdrawal causes adverse effects.
- What medication is needed in a patient with an EF of 30%, systolic blood pressure of 90 mm Hg, normal sinus rhythm, and a heart rate of 82 beats/min despite metoprolol succinate, 200 mg/d?
- Most participants thought the patient would be a good candidate for ivabradine, 5 mg twice daily, as an LVEF under 35% was inadequately controlled by β-blockers alone and the patient’s heart rate was > 70 beats/min.
- The patient has an EF of 65% and Class III symptoms. What medication is needed?
- Most participants suggested starting the patient on a loop diuretic and an aldosterone antagonist, drawing on TOPCAT trial data. The patient should maintain adequate potassium levels while removing excess fluid.
- What additional monitoring should be considered in a patient with an EF of 65%, Class III symptoms, and multiple hospitalizations?
- Most participants believed in CardioMEMS™ (St. Jude Medical, St. Paul, MN) hemodynamic monitoring system along with a heart disease management program. However, some of the panel thought management alone was best, as the CardioMEMS data are not fully resolved.
- What additional medication, aside from ACE inhibitors, should be given to this Class III African-American patient with reduced EF?
- Most participants agreed to stepwise add carvedilol, hydralazine isosorbide dinitrate, and aldosterone antagonist, and switch the ACE inhibitor to a ARNI. This patient’s health status is precarious: an RAS inhibitor regimen plus an evidence-based β-blocker would be life-saving.
Mitigating the Risk of Sudden Cardiac Death in Heart Failure
Presented by Kalyanam Shivkumar MD, PhD
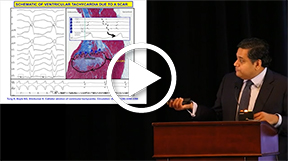
This presentation covered the subject of sudden cardiac death in heart failure and also discussed strategies to reduce the risk of death. Dr. Shivkumar noted that approximately 5 to 7 million deaths worldwide occur annually from heart failure. Even though atrial fibrillation and heart failure are chronic debilitating conditions, improved management strategies do exist, such as neurocardiac signaling. The neurocardiac process addresses the very deleterious process of neural signaling sensed by the neurons that send impulses to the brain. The center is in the rostral ventrolateral medulla, which helps activate the central sympathetic autonomic centers and the RAS. The electrophysiologic process results in arrhythmias, often tachyarrhythmic in nature.
Heart failure progresses from an asymptomatic to severe dysfunction. This can be symptomatic or asymptomatic and lead to death. There is symptomatic worsening of status, leading to hospitalization and sudden death, which can occur at any stage of the disease. The process can be treated with various medications: antithrombotic/anticoagulant agents, statins, or RAS-blocking drugs. Secondary prevention (implantable cardioverter defibrillators [ICDs]) can prevent cardiac arrest and ventricular tachycardia; it is the treatment of choice for high-risk patients. Numerically, a higher incidence of high-risk ischemic and nonischemic cardiomyopathy patients exist with ventricular function < 35% and EF < 35%. LV dysfunction can significantly impact survival outcome, affecting mild ventricular dysfunction patients to patients with serious systolic heart failure and dysfunction. The ICD has shown a clear survival benefit, especially in those patients at risk for sudden cardiac death.
Case Presentation With Panel Discussion
Moderated by Gregg C. Fonarow, MD, FACC, FAHA (Panel Chair)
Drs. Fonarow, Yancy, Horwich, Peter A. McCullough, MD, MPH, FACC, FAHA, FACP, FCCP, FNKF, and Norman E. Lepor, MD, FACC, FAHA, FSCAI, led a panel discussion of a 65-year-old patient being discharged from the hospital after a 4-day admission for heart failure. He had type 2 diabetes, an LVEF of 20%, and atrial fibrillation. Physical examination prior to discharge showed only a trace of edema. The panel followed with several questions:
- Is the patient ready for discharge?
- Most participants agreed the patient should have a BNP to rule out uncertainty. He had a trace of edema, and BNP can assess readmission risk.
- Should he receive an ACE inhibitor or ARB and β-blocker at discharge?
- Most participants agreed with giving the patient both of these medications, as they can help treat the patient with chronic heart failure.
- Which drug should be added before discharge?
- Most agreed an aldosterone antagonist was the best choice, matching guidelines. The patient can be over-diuresed and at risk for hyperkalemia.
- Would you switch from an ACE inhibitor (lisinopril) to ARNI (valsartan/sacubitril) prior to discharge?
- Most agreed to defer switching medication prior to discharge, as it increases risk for hypotensive-related problems after hospitalization.
- Which is the best approach to stroke prevention in this patient?
- Most participants agreed on warfarin, checking that the International Normalized Ratio remains between 2.0 and 3.0. The panel also recommended newer oral anticoagulants such as dabigatran, which have an even lower rate of hemorrhage.
One week later, the patient presented with dyspnea on climbing stairs and ambulation without shortness of breath, rest orthopnea, or chest pain. His weight had decreased by 2 pounds. His blood pressure was 105/80 mm Hg, his heart rate was 76 beats/min, his jugular venous pressure was 7, and his lungs were clear. A soft S3 sound was heard and his edema was measured as 11. His creatinine level increased to 1.9 mg/dL and his BNP to 452 pg/mL.
- What is the patient’s volume status?
- Most participants believed the patient was hypervolemic (“wet”), given his physical examination findings and elevated creatinine and BNP levels.
- What drug dose should be changed?
- Most participants believed in increasing the loop diuretic dose.
The patient returned 2 weeks later. His weight had decreased further and his blood pressure decreased slightly. The S3 sound had disappeared, and both creatinine and BNP levels were increased.
- What is the patient’s volume status?
- The elevated creatinine and BNP status, along with the disappeared S3 sound, point to a hypovolemic state.
- Should he be admitted to the hospital?
- Most participants believed he should be kept as an outpatient. His diuretics and ACE inhibitors were withheld, resulting in decreased creatinine and BNP levels.
Two months later, he returned presenting with class III heart failure; his systolic blood pressure was 90 mm Hg and his heart rate was 78 beats/min.
- What should be done?
- Participants were divided between changing the ACE inhibitor to an ARNI and adding ivabradine to reduce the heart rate. The panel agreed that both were valid choices.
- Should he have a new echocardiogram?
- A number of participants thought it could assist with decision making.
- Should he have a CRT device inserted?
- Most participants believed he was an ideal participant for CRT.
- Should he continue his spironolactone, carvedilol, and atorvastatin?
- Most participants wanted him to continue his medications.
He underwent CRT placement and improved to NYHA Class II, with an EF of 45%, and he continued his medical therapy.
Preventing and Treating Heart Failure in Special Populations
Presented by Karol E. Watson, MD, PhD
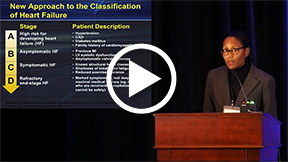
This presentation focused on preventing heart failure in specific populations. Dr. Watson also covered specific heart failure prevention strategies in comorbid conditions such as hypertension, diabetes, coronary artery disease (CAD), and obesity. Heart failure mortality rates have increased with the aging population, indicating a need for preventive strategies. By the year 2020, 50% of the American population will be over age 65 years. Despite dramatic improvements in heart failure treatments, survival rates have not changed significantly. Mortality rates remain poor; in the most recent cohort, it is 70% per year.
A new preventive approach is demonstrated in the American College of Cardiology/AHA classification of heart failure, placing a greater emphasis on risk factors and disease processes (hypertension, diabetes, CAD, obesity) rather than symptoms alone. Thus, an increased focus is on drugs treating comorbid conditions. CAD is the cause of heart failure in approximately two thirds of patients with LV dysfunction. Hypertension is the primary cardiovascular risk factor for heart failure, and patients receive optimal benefit with drugs treating both conditions such as diuretics, ACE inhibitors, and β-blockers. This was evident in the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), focusing on hypertension treatments, and the SPRINT trial, which focused on optimizing blood pressure levels. Diabetic patients have greater benefit with gliflozins (sodium-glucose cotransporter 2 inhibitors) than other oral hypoglycemic agents, such as metformin, which can worsen heart failure. Statins also can decrease heart failure in CAD patients. Interestingly, diet and exercise have not been shown to reduce heart failure. Thus, treating risk factors can prevent heart failure.
Gene Therapy, Cell-based Therapies, and Myocardial Regeneration for Heart Failure
Presented by Ali Nsair, MD
Dr. Nsair discussed regenerative gene therapy, cell rejuvenation, and myocardial replacement therapies for heart failure. He noted the significance of cardiac muscle repair, given the increased numbers of end-stage acute heart failure patients in the United States. Initial research has focused on the potential use of autologous adult stem cells and cardiac myocytes as cell-based transplant therapy. The stem cells may be from bone marrow, hematopoietic, endothelial, or cardiac myocyte sources. Numerous studies have shown cell transplantation improves LVEF after myocardial infarction. Regenerative therapies aim to have long-term engraftment and regeneration of functional tissue from adult and induced pluripotent stem cells.
The LV assist device (LVAD) and drug therapy have been used for reversing heart failure. A new paradigm has adapted use of both stem cell therapy and ventricular device placement. The combined gene therapy and LV device paradigm has been assessed in the Calcium Upregulation by Percutaneous Administration of Gene Therapy in Patients With Cardiac Disease (CUPID-2) trial, focusing on individuals with advanced heart failure. However, the CUPID-2 trial failed to support the hypothesis that gene therapy has clinical benefits in patients with moderate to severe heart failure and reduced EF. Research continues with the transdifferentiation of cardiac fibroblasts into cardiomyocytes through epigenetic modification with promising results. The in vivo delivery of cardiac reprogramming factors also has improved cardiac function after myocardial infarction. Clinicians have become more familiar with the “heart in the box concept,” combined with strategies to regenerate, rejuvenate, and replace damaged cardiac tissue until EF normalizes in patients. The potential for positive future outcomes is increasing annually.
Left Ventricular Assist Device Therapy as Bridge to Transplant and Destination Therapy
Presented by Jaime D. Moriguchi, MD
This presentation focused on LVADs, particularly as a destination and bridging therapy. Mechanical circulatory support (MCS) is a bridge to transplant, serving to improve blood pressure and flow to organs until a suitable donor is available. MCS also stabilizes cardiogenic shock patients while improving their nutrition, functional status (mobilization), and candidacy for transplantation. Individuals with sepsis, active bleeding, unresolved malignancy, and/or active drug use are contraindicated for MCS. The focus is on Class IIIB and Class IV patients, a group of 400,000 patients who have a poor quality of life and a horrible survival prognosis. Despite maximum medical therapy and interventions, only 100,000 are potential transplant (destination therapy) candidates with 2100 to 2300 hearts available each year. Thus, more than 97,000 patients require bridge therapy. The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) scale breaks down the NYHA Class IIIB and IV patients into seven different categories.
Several devices can be used for this patient group. Some complications may occur such as thrombosis, sepsis, or organ failure. The extracorporeal membrane oxygenator is a short-term bridge device emergently placed at the bedside for the patient. The continuous flow device is silent and portable, suitable for most candidates. The heart ventricular assist system is a small implantable bridge device. For destination therapy, the HeartMate II (Thoratec, Pleasanton, CA) is the only US Food and Drug Administration–approved device, but other promising devices are in development. MCS represents a highly effective and durable means of circulatory support in cardiogenic shock and Class IIIb-IV patients, depending on patient prognosis, device selection, and quick referral.
Heart Transplantation 2015: Evaluation, Management, and Novel Strategies to Evaluate for and Prevent Post-transplant Rejection
Presented by Jon A. Kobashigawa, MD
This presentation focused on medical indications for heart transplantation, which include severe heart disease despite adequate medical therapy, poor quality of life due to disabling symptoms, unacceptable cardiac death risk, and lack of surgical options (high-risk bypass and valve surgery). Individuals on the heart transplant waiting list fall into three risk categories (Status 1A, 1B, and 2). Survival has improved dramatically and the number of transplants has grown to 2400 per year in North America. Heart transplantation does not indicate a normal lifespan. Although the 1-year survival is now approaching 90%, the half-life is approximately 14 years. Post-transplant complications can limit patient survival in the first year; they most commonly include rejection and infection. Lesser causes of mortality include cardiac allograph vasculopathy, hypertension, nephropathy, and malignancy.
Thus, heart transplantation is the therapy of choice for select patients with end-stage heart disease. In addition, there have been decreases in rejection-associated infection as the leading cause of death in the first year after transplant. Pravastatin has immunomodulatory properties which are accentuated with cyclosporine and tacrolimus to reduce severe rejection and cardiac vasculopathies. Immune monitoring reflects the immunosuppressed state and can help avoid infectious complications when used to guide immunosuppression. The AlloMap blood test (CareDx, Brisbane, CA) is a noninvasive alternative to heart biopsy in low-risk patients performed on a blood sample to evaluate probability of acute cellular rejection. Although heart transplantation has been subject to multiple postprocedure complications, these continue to decrease annually. Many individuals have benefited from this procedure.
The Role of Antithrombotics and Antiplatelet Agents in Chronic Heart Failure
Presented by Uri Elkayam, MD
This presentation focused on antithrombotic and antiplatelet agents in a patient with chronic heart failure. Dr. Elkayam started with several questions for the group:
- Is deep vein thrombosis (DVT) prophylaxis necessary during hospitalization?
- If DVT prophylaxis is necessary, what kind?
- Should patients be sent home on chronic antiplatelet or anticoagulation therapy?
He mentioned clinical studies showing that thrombotic events can impact patient morbidity and mortality in heart failure patients, as pulmonary embolisms can present with similar symptoms. Thus, depending on the patient, anticoagulant/antithrombotic treatment can be beneficial. A treatment benefit exists for patients older than age 60 years with ischemic stroke, intracranial hemorrhage, or mortality. However, no treatment benefit exists in patients under age 60 years with those factors.
The Warfarin and Antiplatelet Therapy in Chronic Heart Failure Trial (WATCH) assessed antithrombotic therapy for heart failure patients with reduced EF. Patients with a history of large symptomatic/asymptomatic myocardial infarction and/or documented LV thrombus should be treated. Aspirin is recommended for heart failure patients with cardiovascular disease, although not those with heart failure alone. AHA and ACC guidelines do not recommend anticoagulation in patients with chronic heart failure and reduced EFs, without atrial fibrillation, and a prior thromboembolic/cardioembolic event. Heart Failure Society for America guidelines recommend anticoagulation in patients with nonischemic cardiomyopathy and LVEF < 35%, even without additional risk factors. Heart failure patients with atrial fibrillation may receive chronic anticoagulation depending on thrombus characteristics (size, mobility, degree of calcification). Antithrombotic/anticoagulant therapy is both an underassessed and important treatment worthy of further study for heart failure patients. ![]()