Nutritional Deficiencies and Sarcopenia in Heart Failure: A Therapeutic Opportunity to Reduce Hospitalization and Death
Peter A. McCullough, MD, MPH, FACC, FACP, FAHA, FCCP, FNKF, FNLA, FCRSA,1-4 Mohammad Kazem Fallahzadeh, MD,1 Refaat M. Hegazi, MD, MPH, MS, PhD5
1Baylor University Medical Center, Dallas, TX; 2Baylor Heart and Vascular Institute, Dallas, TX; 3Baylor Jack and Jane Hamilton Heart and Vascular Hospital, Dallas, TX; 4The Heart Hospital Baylor Plano, Plano, TX; 5Abbott Nutrition, Inc, Columbus, OH
There is an expanding prevalence pool of heart failure (HF) due to the increasing prevalence of survivors of myocardial infarction, diabetes, hypertension, chronic kidney disease, and obesity. There is increasing interest in the role of nutrition in all forms of HF, given observations concerning micro- and macronutrient deficiencies, loss of lean body mass or sarcopenia, and their relationships with hospitalization and death. This review examines the relationships among loss of lean body mass, macro- and micronutrient intake, and the natural history of HF, particularly in the elderly, in whom the risks for all-cause rehospitalization, infection, falls, and mortality are increased. These risks are potentially modifiable through strategies that improve nutrition in this vulnerable population.
[Rev Cardiovasc Med. 2016;17(suppl 1): S30-S39 doi: 10.3909/ricm17S1S004]
© 2016 MedReviews®, LLC
Nutritional Deficiencies and Sarcopenia in Heart Failure: A Therapeutic Opportunity to Reduce Hospitalization and Death
Peter A. McCullough, MD, MPH, FACC, FACP, FAHA, FCCP, FNKF, FNLA, FCRSA,1-4 Mohammad Kazem Fallahzadeh, MD,1 Refaat M. Hegazi, MD, MPH, MS, PhD5
1Baylor University Medical Center, Dallas, TX; 2Baylor Heart and Vascular Institute, Dallas, TX; 3Baylor Jack and Jane Hamilton Heart and Vascular Hospital, Dallas, TX; 4The Heart Hospital Baylor Plano, Plano, TX; 5Abbott Nutrition, Inc, Columbus, OH
There is an expanding prevalence pool of heart failure (HF) due to the increasing prevalence of survivors of myocardial infarction, diabetes, hypertension, chronic kidney disease, and obesity. There is increasing interest in the role of nutrition in all forms of HF, given observations concerning micro- and macronutrient deficiencies, loss of lean body mass or sarcopenia, and their relationships with hospitalization and death. This review examines the relationships among loss of lean body mass, macro- and micronutrient intake, and the natural history of HF, particularly in the elderly, in whom the risks for all-cause rehospitalization, infection, falls, and mortality are increased. These risks are potentially modifiable through strategies that improve nutrition in this vulnerable population.
[Rev Cardiovasc Med. 2016;17(suppl 1): S30-S39 doi: 10.3909/ricm17S1S004]
© 2016 MedReviews®, LLC
Nutritional Deficiencies and Sarcopenia in Heart Failure: A Therapeutic Opportunity to Reduce Hospitalization and Death
Peter A. McCullough, MD, MPH, FACC, FACP, FAHA, FCCP, FNKF, FNLA, FCRSA,1-4 Mohammad Kazem Fallahzadeh, MD,1 Refaat M. Hegazi, MD, MPH, MS, PhD5
1Baylor University Medical Center, Dallas, TX; 2Baylor Heart and Vascular Institute, Dallas, TX; 3Baylor Jack and Jane Hamilton Heart and Vascular Hospital, Dallas, TX; 4The Heart Hospital Baylor Plano, Plano, TX; 5Abbott Nutrition, Inc, Columbus, OH
There is an expanding prevalence pool of heart failure (HF) due to the increasing prevalence of survivors of myocardial infarction, diabetes, hypertension, chronic kidney disease, and obesity. There is increasing interest in the role of nutrition in all forms of HF, given observations concerning micro- and macronutrient deficiencies, loss of lean body mass or sarcopenia, and their relationships with hospitalization and death. This review examines the relationships among loss of lean body mass, macro- and micronutrient intake, and the natural history of HF, particularly in the elderly, in whom the risks for all-cause rehospitalization, infection, falls, and mortality are increased. These risks are potentially modifiable through strategies that improve nutrition in this vulnerable population.
[Rev Cardiovasc Med. 2016;17(suppl 1): S30-S39 doi: 10.3909/ricm17S1S004]
© 2016 MedReviews®, LLC
KEY WORDS
Heart failure • Cardiomyopathy • Lean body mass • Macronutrient supplementation • Hospitalization • Mortality
KEY WORDS
Heart failure • Cardiomyopathy • Lean body mass • Macronutrient supplementation • Hospitalization • Mortality
Human nutrition and the intake of both micro- and macronutrients play a role in the provision of energy-containing substrate to the myocardium, as well as the entire body. Thus, oxygen extraction and utilization by skeletal muscles, as well as overall skeletal muscle mass, is considerably influenced by nutritional factors.
Deficiencies of taurine, carnitine, and thiamine are associated with reduced lean body mass and are known causes of IDCM in extreme cases. Each of these deficiencies is at least partially treatable through dietary supplementation…

Figure 1. Normal myocardial metabolism. ADP, adenosine diphosphate; ATP, adenosine triphosphate; acetyl-CoA, acetyl coenzyme A; PDH, pyruvate dehydrogenase; NADH, nicotinamide adenine dinucleotide; FADH2, flavin adenine dinucleotide; GTP, guanosine triphosphate; CK, creatine kinase; PCr, creatine phosphate; Pi, inorganic phosphate. Reproduced with permission from Weiss and Maslov.62
A prospective randomized trial of supplementation with carnitine, 2 g/d, resulted in an improved 3-year survival for patients with New York Heart Association class III-IV symptoms when compared with placebo.
IDCM patients most likely also have systemic inflammation, and the acute phase response of inflammation is known to affect serum levels of both copper and zinc, adding an additional layer of complexity to the functional relationships of these specific micronutrients in oxidative stress-dependent cardiovascular disease.
… lean mass is correlated with improved endothelial function, vasoreactivity, and the ability to modulate hemodynamic stress. Thus, it is not surprising that lean body mass has been related to improved outcomes in hospitalized patients.

Figure 2. Constellation of malnutrition, micronutrient deficiencies, and clinical outcomes in heart failure.

Figure 3. Effect of B-hydroxy-B-methylbutyrate (HMB) on lean body mass in elderly patients. Reproduced with permission from Deutz NE et al.63
… improved nutrition and inferred lean tissue mass, although not influencing the rate of intercurrent illness, is associated with improved chances of survival should another event occur. Hence, nutritional supplement therapy, particularly high in protein and HMB, should be considered in HF patients with low lean body mass who are at risk for malnutrition.

Figure 4. Results from the NOURISH Trial. AMI, acute myocardial infarction; COPD, chronic obstructive pulmonary disease; HF, heart failure; HP-HMB, high-protein β-hydroxy-β-methylbutyrate. HMB supplement given twice daily containing 20 g of protein, 1.5 g of HMB, and 40 IU of vitamin D. Reproduced with permission from Deutz NE et al.53
Main Points
• B vitamins (thiamine, riboflavin, and pyridoxine), L-carnitine, creatine, and taurine, may be important in myocardial energy production, and have been shown to be deficient in the heart failure population. These deficiencies could compromise antioxidant defenses, allowing for oxidative stress to impart free radical damage to cardiomyocytes. A small number of human trials have shown that dietary replacement of some of these nutritional factors can result in improvement in myocardial function and exercise capacity.
• Malnutrition and its associated loss of muscle mass exert negative effects on clinical outcome in patients with heart failure. The loss of muscle mass and function can be remedied with increased intake of dietary proteins, amino acids and their metabolites, and oral nutritional supplements.
• Specific metabolic intervention in patients with idiopathic dilated cardiomyopathy should be expanded to include thorough micronutrient and mineral testing prior to intervention, as this will identify additional therapeutic targets and individualize treatment.
Main Points
• B vitamins (thiamine, riboflavin, and pyridoxine), L-carnitine, creatine, and taurine, may be important in myocardial energy production, and have been shown to be deficient in the heart failure population. These deficiencies could compromise antioxidant defenses, allowing for oxidative stress to impart free radical damage to cardiomyocytes. A small number of human trials have shown that dietary replacement of some of these nutritional factors can result in improvement in myocardial function and exercise capacity.
• Malnutrition and its associated loss of muscle mass exert negative effects on clinical outcome in patients with heart failure. The loss of muscle mass and function can be remedied with increased intake of dietary proteins, amino acids and their metabolites, and oral nutritional supplements.
• Specific metabolic intervention in patients with idiopathic dilated cardiomyopathy should be expanded to include thorough micronutrient and mineral testing prior to intervention, as this will identify additional therapeutic targets and individualize treatment.
Heart failure (HF) represents a major healthcare concern worldwide. In the United States alone, it is estimated that approximately 6 million people have HF, and nearly 915,000 more are diagnosed annually.1 Survival is dependent on the age of onset and is approximately 50% at 5 years; the most common mechanisms of death are pump failure and sudden arrhythmic death.2 The risk of death or rehospitalization is similar for those with reduced or preserved systolic function, suggesting that, potentially, both cardiac and noncardiac determinants of survival could play a role in the epidemiology of HF.3
Despite the common perception that systole requires more energy than diastole, diastole is the more energy-dependent part of the cardiac cycle. The metabolic demands of the myocardium are higher in diastole, requiring more adenosine triphosphate (ATP) to stretch and fill the heart chambers.4,5 Invariably, as ATP concentrations decline in cardiomyocytes of the failing heart, dysfunction in diastole occurs irrespective of systolic function. Importantly, diastolic dysfunction is related to mortality in a graded manner according to its severity when evaluated in those with preserved or reduced left ventricular ejection fraction (LVEF).6 Therefore, regardless of its etiology or LVEF, metabolic demands in cardiomyopathy are potentially at risk and should be considered as a target for intervention.
Idiopathic dilated cardiomyopathy (IDCM) describes a group of myocardial diseases of unknown cause, whose common clinical presentation is HF with reduced LVEF. IDCM is probably the result of an underlying genetic predisposition and then a superimposed insult to the heart over the course of time (eg, hypertension, diabetes, chronic kidney disease, viral infection, alcohol).7 Although the prevalence of IDCM is only estimated to be between 7% and 13% of all patients with systolic dysfunction, the disease can provide helpful hints and insights into some of the biochemical and nutritional determinants involved in myocardial dysfunction and adverse morphologic changes over time. Human nutrition and the intake of both micro- and macronutrients play a role in the provision of energy-containing substrate to the myocardium, as well as the entire body. Thus, oxygen extraction and utilization by skeletal muscles, as well as overall skeletal muscle mass, is considerably influenced by nutritional factors. Sarcopenia is a condition characterized by loss of skeletal muscle mass and function. Although it is primarily a problem of the elderly, its development may be associated with conditions, including HF, that can occur in younger individuals.8 Low lean body mass or sarcopenia has been consistently associated with an increased risk of morbidity and mortality in HF.9 Thus, a chain of logic could be constructed that optimization of nutrition would not only improve cardiac energetics, but also increase lean tissue mass, improve respiratory mechanics and oxygen extraction and delivery, enhance functional capacity, improve immune function, reduce the risk of serious falls, and, in a summative fashion, be associated with a reduced risk of death.
Diagnostic and Therapeutic Implications
In the past four decades, much of the research in the field of HF has focused on pharmacotherapeutic management with agents that effect neurohormonal regulation, salt and water balance, hemodynamic abnormalities, and risk of ventricular arrhythmias. The field of cardiac resynchronization therapy and defibrillation has given relatively definitive control over the risk of sudden death. By comparison, the contribution of metabolic abnormalities to the progression of HF has received lesser degrees of attention.10,11 Micronutrients such as the B vitamins (thiamine, riboflavin, and pyridoxine), L-carnitine, creatine, and taurine, known to be important in myocardial energy production, have been shown to be deficient in HF and may play a role in myocyte energetics (Figure 1). Limited studies in humans have shown that dietary replacement of some of these nutritional factors can result in improvement in myocardial function and exercise capacity.12-14
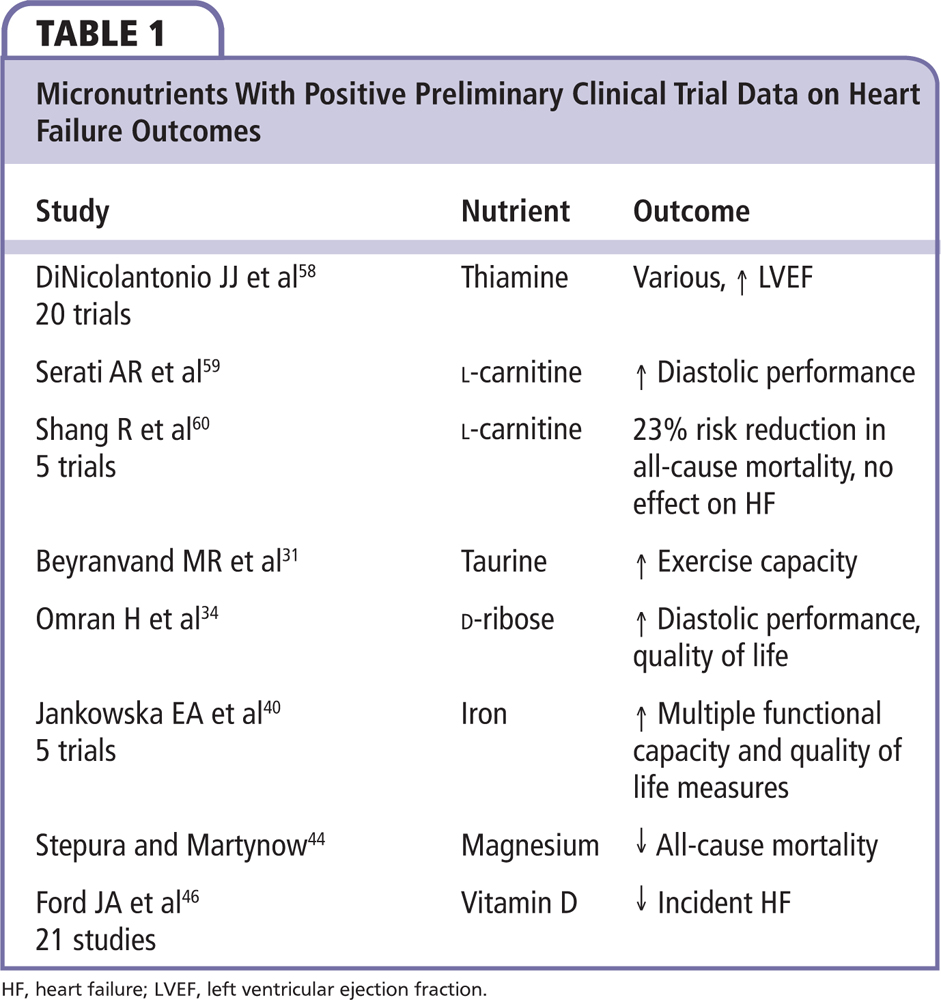
Deficiencies of taurine, carnitine, and thiamine are associated with reduced lean body mass and are known causes of IDCM in extreme cases. Each of these deficiencies is at least partially treatable through dietary supplementation with modest, preliminary supportive evidence (Table 1). For example, randomized trials of thiamine supplementation have demonstrated improvements in LVEF; however, they have not resulted in differences in functional outcomes or survival.14-16 Thus, micronutrient deficiencies are a potential cause and at times contributors to HF, and, as such, deserve careful consideration as potentially modifiable factors.
B Complex Vitamins
Referred to as vitamin B complex, the eight B vitamins—B1, B2, B3, B5, B6, B7, B9, B12—play important roles in protein and carbohydrate metabolism as well as many other cellular functions. One of the micronutrients most investigated in IDCM has been thiamine (vitamin B1). Although alcoholic dilated cardiomyopathy is a distinct clinical entity from IDCM, studies on this disease supported a link to thiamine deficiency as early as the 1960s.17 In this report, 20% of patients presented with cardiac beriberi and thiamine deficiency. The validity of a similar relation between thiamine deficiency and IDCM was highly suggested much later by a 10-year prospective study from New Zealand, which examined the demographic, hemodynamic, and prognostic features of IDCM.18 The study noted a 9.5% occurrence of an abnormal transketolase test result, which suggests thiamine deficiency is physiologically significant. Additionally, a cross-sectional study has reported that approximately one-third of hospitalized patients with HF had low thiamine tissue levels.19 It is important to recognize that thiamine is lost in the urine in response to the use of loop diuretics; thus, there is a need for physiologic replacement in patients with HF.20
Riboflavin (vitamin B2) and pyridoxine (vitamin B6) are two other water-soluble B vitamins whose depletions have been shown to play important roles in myocardial energy production. Like thiamine, these B vitamins are subject to limited tissue storage and are renally excreted. In 2009, Keith and colleagues21 reported that 27% of hospitalized patients with HF had evidence of vitamin B2 deficiency, whereas 38% had low concentrations of vitamin B6.21 In the same study, the use of B vitamin-containing supplements did not significantly reduce deficiency rates (vitamin B2 with P = .38 and B6 with P = .18), suggesting the need for supplementation may be greater than expected.
Amino Acids
Carnitine is an amino acid that plays an important role in myocardial energy production. l-carnitine plays a role in β-oxidation, transport of long-chain fatty acids from the cytoplasm into mitochondria, and shuttling of toxic acylcarnitines out of cells.22 l-carnitine availability has been linked to improved cardiac myocyte function.23 The myocardial concentration of carnitine is approximately 80- to 140-fold higher than the plasma concentration. Nakamura and coworkers24 demonstrated that serum free carnitine concentrations were significantly higher in patients with IDCM than in normal subjects (P = .0001); the ratios of acylcarnitine to carnitine were abnormal, suggesting impairment in carnitine cellular uptake, action, or catabolic fate. These findings are consistent with several studies performed on patients with ischemic cardiomyopathy. A prospective randomized trial of supplementation with carnitine, 2 g/d, resulted in an improved 3-year survival for patients with New York Heart Association class III-IV symptoms when compared with placebo.25 Therefore, l-carnitine could be considered a nutritional adjunct to other nutritional interventions in patients with HF.
Creatine is an amino acid that, via phosphate transfer, works to provide energy to all cells in the body, primarily muscle. This is achieved by increasing the formation of ATP. In one report of 39 patients, lower phosphocreatine-to-ATP levels were associated with reduced total and cardiovascular mortality.26 Creatine supplementation plays a role in reducing lactate production in skeletal muscle during intense exercise in patients with HF, suggesting a possible target for randomized trials.27 One trial in a mixed population (n = 70) of coronary artery disease and HF patients showed that creatine supplementation had no effect on functional or symptomatic outcomes after 3 months.28
Taurine, a nonessential amino acid in humans, is the most abundant free amino acid in the cardiac muscle, and is involved in calcium regulation in the cytosol and mitochondria.29 Taurine is thought to facilitate calcium release and affect calcium sensitivity of contractile proteins. It may attenuate the intracellular effects of neurohormonal antagonism of the renin-angiotensin and sympathetic nervous systems.30 In one small randomized trial (n = 29), taurine, 500 mg by mouth three times daily, increased exercise capacity in HF.31 As a caveat, taurine is a component of high-energy drinks and has been implicated in sudden deaths after large ingestions.32
Fatty Acids
There has been great interest in essential fatty acids and their role in cardiovascular disease. Omega-3 and -6 fatty acids have been the subject of many dietary and interventional trials mainly focussed on atherosclerosis or arrhythmias. A recent review by Poudyal and Brown33 concluded that n-3 polyunsaturated fatty acid trials have not demonstrated significant impact on secondary and other observed outcomes in patients with HF.
Sugars
D-Ribose is a simple sugar that supports the production of ATP. Omran and colleagues34 performed a double-blind, placebo-controlled, cross-over trial (n = 15) demonstrating that 3 weeks of ribose supplementation significantly enhanced indices of diastolic heart function and exercise tolerance. Given the more recent appreciation for HF due to or worsened by diastolic dysfunction, additional research on D-ribose and myocardial diastolic energetics appears to be warranted.
Minerals
Selenium is an essential nutrient that makes an integral part of the enzyme glutathione peroxide, which, in turn, plays an important protective role in metabolism of cardiomyocytes and all other somatic cells. Several studies have found an association between selenium deficiency and HF, immune deficiency, and diabetes.35 African-American patients with IDCM appear to have low serum selenium levels.36 The Selenium Treatment and Chagasic Cardiopathy trial is currently underway, testing the efficacy and safety of selenium supplementation in Chagas cardiomyopathy.37 There are no completed, definitive trials of selenium treatment in HF.
Both copper and zinc play roles as cofactors in biochemical reactions. IDCM patients most likely also have systemic inflammation, and the acute phase response of inflammation is known to affect serum levels of both copper and zinc, adding an additional layer of complexity to the functional relationships of these specific micronutrients in oxidative stress-dependent cardiovascular disease. The role of copper and zinc imbalances in IDCM remains controversial, as there are studies that found no association between copper or zinc deficiency and IDCM.38
The deleterious effects of iron-deficiency anemia on the heart must not be overlooked. According to current data, iron deficiency is the most common nutritional deficiency in the developed world; some 2% of US men and 9% of US women have it. One of the extreme results of iron deficiency is cardiomyopathy and HF.39 In a study by Jankowska and associates,40 37% of patients with HF and reduced LVEF had iron deficiency that was associated with worsened severity of heart failure and higher levels of natriuretic peptides. Iron is essential to the structure of both oxygen-carrying myoglobin and hemoglobin. Iron deficiency can result in anemia and cardiac myocyte oxygen deprivation, reduced oxygen storage (myoglobin) and energetic efficiency, increased anaerobic metabolism and mitochondrial dysfunction.41 A recent meta-analysis of five randomized trials of intravenous iron therapy showed that iron therapy was associated with a reduction in cardiovascular hospitalization (odds ratio [OR] = 0.44, 95% confidence interval [CI], 0.30-0.64; P < .0001), and the combined endpoint of cardiovascular death or hospitalization for worsening HF (OR = 0.39, 95% CI, 0.24-0.63; P = .0001).42 Intravenous iron treatment was associated with improvement in functional class, an increase in 6-minute walking test distance, and an improvement in quality of life.
Hypomagnesemia has been linked to HF and hereditary hypomagnesemia can cause a progressive dilated cardiomyopathy.43 One small trial (n = 790) demonstrated high-dose chelated oral magnesium was associated with improved survival; however, larger trials have not been planned or completed as of this writing.44
Fat-soluble Vitamins
In recent years, vitamin D deficiency has drawn attention in its association to bone and mineral, neurodegenerative, immune, metabolic, and cardiovascular disease. Vitamin D deficiency and secondary hyperparathyroidism are common in patients with HF.45 Ford and colleagues46 have published a synthesis of the interventional data to date suggesting benefit of vitamin D supplementation on HF outcomes and other cardiovascular events. In this report, the Randomised Evaluation of Calcium Or vitamin D (RECORD) trial (n = 5292) found a 25% risk reduction for the HF endpoint, and a meta-analysis of 21 studies found an 18% risk reduction with vitamin D supplementation. Thus, at the time of this writing, vitamin D may be one of the best-supported nutritional interventions for HF patients.
Vitamins A, E, and K are the other fat-soluble vitamins. It is beyond the scope of this review to cover the results of trials in cardiovascular disease with these micronutrients. Although there are important effects on reversal of anticoagulation and they may play a role in the pathophysiology of cardiomyopathy in some extreme malabsorption syndromes, in general these entities have not been implicated in HF.
Importance of Lean Body Mass
The body can be subdivided into lean (mainly muscle) and fat mass and could be assessed by a variety of methods, including whole-body densitometry.47 There is a consistent signal in the general medical literature, oncology, critical care, and now in cardiology that lean mass is associated with a variety of cellular and tissue functions that are associated with the ability to survive intercurrent illnesses and are protective against death in the in-hospital setting (Figure 2).48 Higher levels of muscle mass are associated with improved oxygen, glucose, and fatty acid metabolism in all tissues and organ systems. In addition, lean mass is correlated with improved endothelial function, vasoreactivity, and the ability to modulate hemodynamic stress. Thus, it is not surprising that lean body mass has been related to improved outcomes in hospitalized patients.
DiBello and associates9 studied 402 patients with HF from the National Health and Nutrition Examination Study and found that the 20th percentile of the sex-specific distribution of lean appendicular mass had a statistically significant association with reduced ability to perform household chores, walking one-fourth of a mile, and hospitalization. Narumi and coworkers49 measured fat-free or lean mass using densitometry in 267 patients with HF and found that lean mass was inversely associated with hospitalization and mortality.49 Related terms sarcopenia, sarcopenic obesity, and cachexia have been used in the recent literature in HF. The important concept is that lean mass and not fat or total body mass is associated with clinically meaningful outcomes. Sarcopenia refers to the age-associated loss of muscle mass and/or function. Importantly it is possible for an obese individual to have a low muscle mass. Therefore, sarcopenic obesity is a state that may be even worse than sarcopenia alone, because all the disadvantages of obesity are present, including insulin resistance, proclivity for thrombosis and venous stasis, and greater degrees of inactivity and musculoskeletal problems.
Nutritional Interventions to Address Low Muscle Mass
Malnutrition and its associated loss of muscle mass exert negative effects on clinical outcome in patients with HF.50 The loss of muscle mass and function could be addressed by increased intake of dietary proteins, amino acids, their metabolites, and oral nutritional supplements. Dietary protein induces muscle protein synthesis and decreases protein degradation. Interestingly, it was shown that the net protein accretion was significantly higher in elderly individuals consuming 1.5 g/kg/d of dietary protein rather than the dietary recommended dose of 0.8 g/kg/d. In the same study, protein accretion was not different whether dietary protein was distributed equally through the three daily meals or mainly administered at dinner. Dietary amino acids, especially the branched-chain amino acid leucine and its metabolite, β-hydroxy β-methylbutyrate (HMB), have been shown to stimulate muscle protein synthesis and inhibit protein degradation (Figure 3). Of particular interest to disease-associated low muscle mass, HMB had been shown to inhibit muscle protein degradation in patients with cancer and human immunodeficiency virus cachexia. We recently reviewed the effects of HMB and its potential role in HF-associated cachexia.51 Oral nutritional supplements providing a mix of macronutrients and micronutrients are effective therapy for malnutrition, especially in elderly malnourished patients. These supplements have been consistently shown to improve weight, muscle function, and clinical outcomes in patients with disease-associated malnutrition.52 The overall protein intake appears to be the most important variable in the maintenance or increase in lean tissue mass.52
Oral Nutritional Supplement in Older Hospitalized Patients: the NOURISH Trial
In the A Randomized Double Blinded Controlled Trial of an Oral Nutritional Supplement Containing AN 777 in Older Hospitalized Patients (NOURISH) trial, Deutz and colleagues53 randomized 652 hospitalized adults ≥ 65 years of age, evidence of malnutrition (subjective global assessment [SGA] class B or C) with diagnoses of HF, acute myocardial infarction, pneumonia, or chronic obstructive pulmonary disease. Patients were randomized to a high-protein oral nutritional supplement containing HMB (HP-HMB) or usual care. The primary composite endpoint of death or rehospitalization at 90 days was similar between HP-HMB (26.8%) and placebo (31.1%) groups; however, 90-day mortality was significantly lower with HP-HMB as compared with placebo (4.8% vs 9.7%; relative risk 0.49, 95% CI, 0.27-0.90; P = .018). The number-needed-to-treat to prevent one death was 20.3 (95% CI, 10.9, 121.4) (Figure 4). Compared with placebo, HP-HMB resulted in improved odds of better nutritional status (SGA class, OR, 2.04, 95% CI, 1.28, 3.25; P = .009) at day 90, and an increase in body weight at day 30 (P = .035). This trial suggested that improved nutrition and inferred lean tissue mass, although not influencing the rate of intercurrent illness, is associated with improved chances of survival should another event occur. Hence, nutritional supplement therapy, particularly high in protein and HMB, should be considered in HF patients with low lean body mass who are at risk for malnutrition.
Clinical Implications for Heart Failure Patients
Metabolic Demands of the Starved Myocardium
The metabolic demands of the myocyte require conversion of the chemical energy from the ATP bond into mechanical energy specific to the cells function (contraction, conduction of electrical charge) (Figure 1). In the case of the normal heart, large fluctuations in workload and energy demand rely on adequate ATP generation and/or recycling mechanisms in the mitochondria. These include oxidative phosphorylation, where fatty acid and carbohydrate metabolism takes place, and creatine kinase (CK) activity, where ATP and free creatine are generated from adenosine diphosphate and creatine phosphate. Conversely, it is well documented that in the failing heart the balance between energy supply and demand does not function adequately, resulting in a progressively decreasing ATP tissue content.4,54 In HF, these metabolic mechanisms are disrupted, leading to inefficient energy expenditure and the creation of the term starved myocardium. In essence, a chronic energy supply/demand mismatch is created.
Because of the similarities of the energy-starved myocardium with both IDCM and ischemic heart disease, an integrative approach in the treatment of congestive HF has been previously entertained.55 Relying on nutritional biochemical interventions that preserve and promote ATP production, metabolic cardiology was introduced as an indispensable therapeutic intervention in the context of HF. This approach includes therapeutic options that focus on preservation of energy substrates, such as ribose, or acceleration of ATP turnover, such as carnitine and coenzyme Q10. We argue that a metabolic intervention in IDCM patients should be expanded to include thorough micronutrient and mineral testing prior to intervention, as this would identify additional therapeutic targets and individualize treatment, most notably iron, magnesium, and vitamin D.
Traditional Heart Failure Therapy and Nutrient Depletion
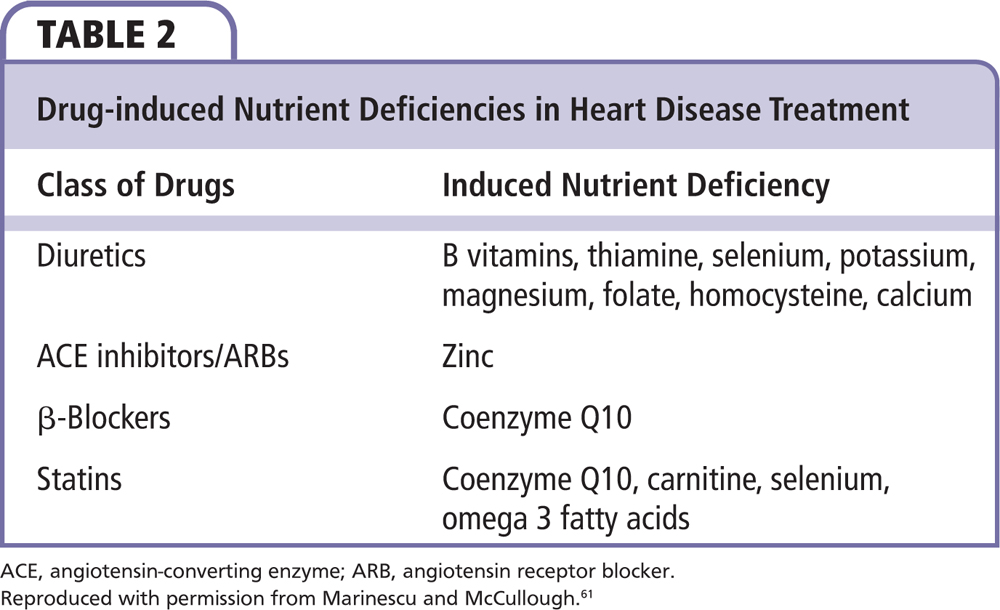
With the emergence of diuretics, angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) as major components of HF management, the balance of many of the nutrients needs to be monitored. More specifically, ACE inhibitors/ARBs are known to exert their function by binding zinc. Additionally, the use of diuretics has been reported to induce thiamine, selenium, zinc, and coenzyme Q10 deficiencies (Table 2).56
Recognition of Sarcopenic Obesity
Increasingly, patients with HF are obese, and thus sarcopenia may be more difficult to recognize. Trends in overall loss in lean body mass may be masked by fluid weight gain. It is important for the clinician to develop a sense of muscle bulk, strength, and functional status in the office and during the bedside examination. Advanced tools such as whole body densitometry, computed tomography of the iliopsoas, and bioimpedance can be used to assist in tracking lean tissue mass.57 Early intervention with increasing protein intake and HMB should be considered to attenuate the loss of lean body mass and to augment rebuilding of muscle after hospitalization.
Conclusions
There appear to be opportunities for future research and improvements in clinical practice for patients with HF with respect to micro- and macronutrient supplementation. Assessment for deficiency states among those with HF seems reasonable. Interventional studies suggest potential benefit with thiamine, L-carnitine, taurine, D-ribose, iron, magnesium, and vitamin D. In terms of macronutrients, protein malnutrition plays a key role in loss of lean body mass and cardiac cachexia. A possible future approach may include assessment of lean body mass, and macronutrient supplementation with high protein and HMB-containing nutritional formulations that appear to reduce the risk of death in elderly hospitalized patients, including those with HF. ![]()
Dr. Hegazi is an employee and stockholder of Abbott Laboratories (Abbott Park, IL).
References
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38-e360.
- McCullough PA, Philbin EF, Spertus JA, et al; Resource Utilization Among Congestive Heart Failure (REACH) Study. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol. 2002;39:60-69.
- McCullough PA, Khandelwal AK, McKinnon JE, et al. Outcomes and prognostic factors of systolic as compared with diastolic heart failure in urban America. Congest Heart Fail. 2005;11:6-11.
- Ingwall JS. On the hypothesis that the failing heart is energy starved: lessons learned from the metabolism of ATP and creatine. Curr Hypertens Rep. 2006;8:457-464.
- Pouleur H. Diastolic dysfunction and myocardial energetics. Eur Heart J. 1990;11(suppl C):30-34.
- Halley CM, Houghtaling PL, Khalil MK, et al. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171:1082-1087.
- Japp AG, Gulati A, Cook SA, et al. The diagnosis and evaluation of dilated cardiomyopathy. J Am Coll Cardiol. 2016;67:2996-3010.
- Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177-180.
- DiBello JR, Miller R, Khandker R, et al. Association between low muscle mass, functional limitations and hospitalisation in heart failure: NHANES 1999-2004. Age Ageing. 2015;44:948-954.
- Witte KK, Clark AL, Cleland JG. Chronic heart failure and micronutrients. J Am Coll Cardiol. 2001;37: 1765-1774.
- Weber KT, Weglicki WB, Simpson RU. Macro- and micronutrient dyshomeostasis in the adverse structural remodelling of myocardium. Cardiovasc Res. 2009;81:500-508.
- Anand I, Chandrashekhan Y, De Giuli F, et al. Acute and chronic effects of propionyl-L-carnitine on the hemodynamics, exercise capacity, and hormones in patients with congestive heart failure. Cardiovasc Drugs Ther. 1998;12:291-299.
- Pfitzenmeyer P, Guilland JC, d’Athis P, et al. Thiamine status of elderly patients with cardiac failure including the effects of supplementation. Int J Vitam Nutr Res. 1994;64:113-118.
- Shimon I, Almog S, Vered Z, et al. Improved left ventricular function after thiamine supplementation in patients with congestive heart failure receiving long-term furosemide therapy. Am J Med. 1995;98:485-490.
- Witte KK, Nikitin NP, Parker AC, et al. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur Heart J. 2005;26:2238-2244.
- Schoenenberger AW, Schoenenberger-Berzins R, der Maur CA, et al. Thiamine supplementation in symptomatic chronic heart failure: a randomized, double-blind, placebo-controlled, cross-over pilot study. Clin Res Cardiol. 2012;101:159-164.
- Alexander CS. Idiopathic heart disease. I. Analysis of 100 cases, with special reference to chronic alcoholism. Am J Med. 1966;41:213-228.
- Ikram H, Williamson HG, Won M, et al. The course of idiopathic dilated cardiomyopathy in New Zealand. Br Heart J. 1987;57:521-527.
- Hanninen SA, Darling PB, Sole MJ, et al.. The prevalence of thiamine deficiency in hospitalized patients with congestive heart failure. J Am Coll Cardiol. 2006;47:354-361.
- Katta N, Balla S, Alpert MA. Does long-term furosemide therapy cause thiamine deficiency in patients with heart failure? A focused review. Am J Med. 2016;129:753.e7-753.e11.
- Keith ME, Walsh NA, Darling PB, et al. B-vitamin deficiency in hospitalized patients with heart failure. J Am Diet Assoc. 2009;109:1406-1410.
- Arsenian MA. Carnitine and its derivatives in cardiovascular disease. Prog Cardiovasc Dis. 1997;40:265-286.
- Schonekess BO, Allard MF, Lopaschuk GD. Propionyl L-carnitine improvement of hypertrophied rat heart function is associated with an increase in cardiac efficiency. Eur J Pharmacol. 1995;286:155-166.
- Nakamura T, Sugihara H, Kinoshita N, et al. Serum carnitine concentrations in patients with idiopathic hypertrophic cardiomyopathy: relationship with impaired myocardial fatty acid metabolism. Clin Sci (Lond). 1999;97:493-501.
- Rizos I. Three-year survival of patients with heart failure caused by dilated cardiomyopathy and L-carnitine administration. Am Heart J. 2000;139(2 Pt 3):S120-S123.
- Neubauer S, Horn M, Cramer M, et al. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190-2196.
- Gordon A, Hultman E, Kaijser L, et al. Creatine supplementation in chronic heart failure increases skeletal muscle creatine phosphate and muscle performance. Cardiovasc Res. 1995;30:413-418.
- Cornelissen VA, Defoor JG, Stevens A, et al. Effect of creatine supplementation as a potential adjuvant therapy to exercise training in cardiac patients: a randomized controlled trial. Clin Rehabil. 2010;24:988-999.
- Kohlmeier M. Nutrient Metabolism. London, UK: Academic Press; 2003.
- Ito T, Schaffer S, Azuma J. The effect of taurine on chronic heart failure: actions of taurine against catecholamine and angiotensin II. Amino Acids. 2014;46:111-119.
- Beyranvand MR, Khalafi MK, Roshan VD, et al. Effect of taurine supplementation on exercise capacity of patients with heart failure. J Cardiol. 2011;57:33-337.
- Avcı S, Sarıkaya R, Büyükcam F. Death of a young man after overuse of energy drink. Am J Emerg Med. 2013;31:1624.e3-1624.e4.
- Poudyal H, Brown L. The role of n-3 polyunsaturated fatty acids in human heart failure. Endocr Metab Immune Disord Drug Targets. 2013;13:105-117.
- Omran H, Illien S, MacCarter D, et al. D-Ribose improves diastolic function and quality of life in congestive heart failure patients: a prospective feasibility study. Eur J Heart Fail. 2003;5:615-619.
- Benstoem C, Goetzenich A, Kraemer S, et al. Selenium and its supplementation in cardiovascular disease--what do we know? Nutrients. 2015;7:3094-3118.
- Arroyo M, Laguardia SP, Bhattacharya SK, et al. Micronutrients in African-Americans with decompensated and compensated heart failure. Transl Res. 2006;148:301-308.
- Alvarenga Americano do Brasil PE, Pereira de Souza A, Hasslocher-Moreno AM, et al. Selenium Treatment and Chagasic Cardiopathy (STCC): study protocol for a double-blind randomized controlled trial. Trials. 2014;15:388.
- McKeag NA, McKinley MC, Woodside JV, et al. The role of micronutrients in heart failure. J Acad Nutr Diet. 2012;112:870-886.
- Hegde N, Rich MW, Gayomali C. The cardiomyopathy of iron deficiency. Tex Heart Inst J. 2006;33:340-344.
- Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31:1872-1880.
- van Veldhuisen DJ, Anker SD, Ponikowski P, Macdougall IC. Anemia and iron deficiency in heart failure: mechanisms and therapeutic approaches. Nat Rev Cardiol. 2011;8:485-493.
- Jankowska EA, Tkaczyszyn M, Suchocki T, et al. Effects of intravenous iron therapy in iron-deficient patients with systolic heart failure: a meta-analysis of randomized controlled trials. Eur J Heart Fail. 2016;18:786-795.
- Riggs JE, Klingberg WG, Flink EB, et al. Cardioskeletal mitochondrial myopathy associated with chronic magnesium deficiency. Neurology. 1992;42:128-130.
- Stepura OB, Martynow AI. Magnesium orotate in severe congestive heart failure (MACH). Int J Cardiol. 2009;134:145-147.
- Zittermann A, Schleithoff SS, Tenderich G, et al. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41:105-112.
- Ford JA, MacLennan GS, Avenell A, et al; RECORD Trial Group. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746-755.
- Goodman MJ, Ghate SR, Mavros P, et al. Development of a practical screening tool to predict low muscle mass using NHANES 1999-2004. J Cachexia Sarcopenia Muscle. 2013;4:187-197.
- Franklin BA. Survival of the fittest: evidence for high-risk and cardioprotective fitness levels. Curr Sports Med Rep. 2002;1:257-259.
- Narumi T, Watanabe T, Kadowaki S, et al. Sarcopenia evaluated by fat-free mass index is an important prognostic factor in patients with chronic heart failure. Eur J Intern Med. 2015;26:118-122.
- Adejumo OL, Koelling TM, Hummel SL. Nutritional Risk Index predicts mortality in hospitalized advanced heart failure patients. J Heart Lung Transplant. 2015;34:1385-1389.
- McCullough PA, Berberich CB, Alish C, Hegazi RA. The potential role of β− hydroxy− β− methylbutyrate (HMB) in the management of lean body mass loss in older adults with heart failure and cardiac cachexia. Cardiovasc Pharm Open Access. 2015;4:161.
- Kim IY, Schutzler S, Schrader A, et al. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am J Physiol Endocrinol Metab. 2015;308:E21-E28.
- Deutz NE, Matheson EM, Matarese LE, et al; NOURISH Study Group. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: a randomized clinical trial. Clin Nutr. 2016;35:18-26.
- Harinstein ME, Berliner JI, Shah SJ, et al. Normalization of ejection fraction and resolution of symptoms in chronic severe heart failure is possible with modern medical therapy: clinical observations in 11 patients. Am J Ther. 2008;15:206-213.
- Katz AM. Is the failing heart an energy-starved organ? J Card Fail. 1996;2:267-272.
- Dunn SP, Bleske B, Dorsch M, et al. Nutrition and heart failure: impact of drug therapies and management strategies. Nutr Clin Pract. 2009;24:60-75.
- Devriese J, Beels L, Maes A, et al. Review of clinically accessible methods to determine lean body mass for normalization of standardized uptake values. Q J Nucl Med Mol Imaging. 2016;60:1-11.
- DiNicolantonio JJ, Niazi AK, Lavie CJ, et al. Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail. 2013;19:214-222.
- Serati AR, Motamedi MR, Emami S, et al. L-carnitine treatment in patients with mild diastolic heart failure is associated with improvement in diastolic function and symptoms. Cardiology. 2010;116:178-182.
- Shang R, Sun Z, Li H. Effective dosing of L-carnitine in the secondary prevention of cardiovascular disease: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2014;14:88.
- Marinescu V, McCullough PA. Nutritional and micronutrient determinants of idiopathic dilated cardiomyopathy: diagnostic and therapeutic implications. Exp Rev Cardiovasc Ther. 2011;9:1161-1170.
- Weiss RG, Maslov M. Normal myocardial metabolism fueling cardiac contraction. Advanced Studies in Medicine. 2004;4(6B):S457-S463.
- Deutz NE, Pereira SL, Hays NP, et al. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr. 2013;32:704-712.
Portions of this article have been reproduced from Marinescu V, McCullough PA, Nutritional and micronutrient determinants of idiopathic cardiomyopathy: diagnostic and therapeutic implications, Expert Rev Cardiovasc Ther. 2011;9(9):1161-1170, with permission of Taylor and Francis (www.tandfonline.com).