Cardiovascular Complications of Radiation Exposure
William Finch, MD, Kamran Shamsa, MD, Michael S. Lee, MD
Division of Cardiology, The David Geffen School of Medicine at UCLA, Los Angeles, CA
The cardiovascular sequelae of radiation exposure are an important cause of morbidity and mortality following radiation therapy for cancer, as well as after exposure to radiation after atomic bombs or nuclear accidents. In the United States, most of the data on radiation-induced heart disease (RIHD) come from patients treated with radiation therapy for Hodgkin disease and breast cancer. Additionally, people exposed to radiation from the atomic bombs in Hiroshima and Nagasaki, Japan, and the Chernobyl, Ukraine, nuclear accident have an increased risk of cardiovascular disease. The total dose of radiation, as well as the fractionation of the dose, plays an important role in the development of RIHD. All parts of the heart are affected, including the pericardium, vasculature, myocardium, valves, and conduction system. The mechanism of injury is complex, but one major mechanism is injury to endothelium in both the microvasculature and coronary arteries. This likely also contributes to damage and fibrosis within the myocardium. Additionally, various inflammatory and profibrotic cytokines contribute to injury. Diagnosis and treatment are not significantly different from those for conventional cardiovascular disease; however, screening for heart disease and lifelong cardiology follow-up is essential in patients with past radiation exposure.
[Rev Cardiovasc Med. 2014;15(3):232-244 doi: 10.3909/ricm0689]
© 2014 MedReviews®, LLC
Cardiovascular Complications of Radiation Exposure
William Finch, MD, Kamran Shamsa, MD, Michael S. Lee, MD
Division of Cardiology, The David Geffen School of Medicine at UCLA, Los Angeles, CA
The cardiovascular sequelae of radiation exposure are an important cause of morbidity and mortality following radiation therapy for cancer, as well as after exposure to radiation after atomic bombs or nuclear accidents. In the United States, most of the data on radiation-induced heart disease (RIHD) come from patients treated with radiation therapy for Hodgkin disease and breast cancer. Additionally, people exposed to radiation from the atomic bombs in Hiroshima and Nagasaki, Japan, and the Chernobyl, Ukraine, nuclear accident have an increased risk of cardiovascular disease. The total dose of radiation, as well as the fractionation of the dose, plays an important role in the development of RIHD. All parts of the heart are affected, including the pericardium, vasculature, myocardium, valves, and conduction system. The mechanism of injury is complex, but one major mechanism is injury to endothelium in both the microvasculature and coronary arteries. This likely also contributes to damage and fibrosis within the myocardium. Additionally, various inflammatory and profibrotic cytokines contribute to injury. Diagnosis and treatment are not significantly different from those for conventional cardiovascular disease; however, screening for heart disease and lifelong cardiology follow-up is essential in patients with past radiation exposure.
[Rev Cardiovasc Med. 2014;15(3):232-244 doi: 10.3909/ricm0689]
© 2014 MedReviews®, LLC
Cardiovascular Complications of Radiation Exposure
William Finch, MD, Kamran Shamsa, MD, Michael S. Lee, MD
Division of Cardiology, The David Geffen School of Medicine at UCLA, Los Angeles, CA
The cardiovascular sequelae of radiation exposure are an important cause of morbidity and mortality following radiation therapy for cancer, as well as after exposure to radiation after atomic bombs or nuclear accidents. In the United States, most of the data on radiation-induced heart disease (RIHD) come from patients treated with radiation therapy for Hodgkin disease and breast cancer. Additionally, people exposed to radiation from the atomic bombs in Hiroshima and Nagasaki, Japan, and the Chernobyl, Ukraine, nuclear accident have an increased risk of cardiovascular disease. The total dose of radiation, as well as the fractionation of the dose, plays an important role in the development of RIHD. All parts of the heart are affected, including the pericardium, vasculature, myocardium, valves, and conduction system. The mechanism of injury is complex, but one major mechanism is injury to endothelium in both the microvasculature and coronary arteries. This likely also contributes to damage and fibrosis within the myocardium. Additionally, various inflammatory and profibrotic cytokines contribute to injury. Diagnosis and treatment are not significantly different from those for conventional cardiovascular disease; however, screening for heart disease and lifelong cardiology follow-up is essential in patients with past radiation exposure.
[Rev Cardiovasc Med. 2014;15(3):232-244 doi: 10.3909/ricm0689]
© 2014 MedReviews®, LLC
KEY WORDS
Radiation-induced heart disease • Cardiotoxicity • Myocardial fibrosis • Coronary artery stenoses • Pericardial disease • Valvular injury
KEY WORDS
Radiation-induced heart disease • Cardiotoxicity • Myocardial fibrosis • Coronary artery stenoses • Pericardial disease • Valvular injury


It is likely that cardiac disease develops with single radiation doses of < 10 Gy.
RT has previously been used to treat peptic ulcer disease and, in these patients, an average total cardiac dose of 2.8 Gy was the lowest dose that resulted in a statistically significant increase in CAD.
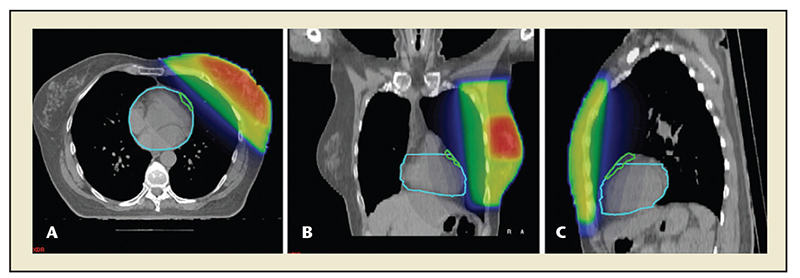
Figure 1. Planning computed tomography scan prior to radiation of the left breast (A, B, and C are transverse, coronal, and sagittal views, respectively). The light blue line outlines the heart, the green line outlines the left coronary artery. Radiation doses are a color-coded overlay over the left breast: red is 74 Gy, blue is 0 Gy. Treatment delivery may also be restricted to inspiration to maximize the distance of the heart to the radiation field. Reprinted with permission from Topolnjak R et al.144
Studies have demonstrated that greater fractionation of the total dose decreases acute pericarditis and myocardial necrosis.
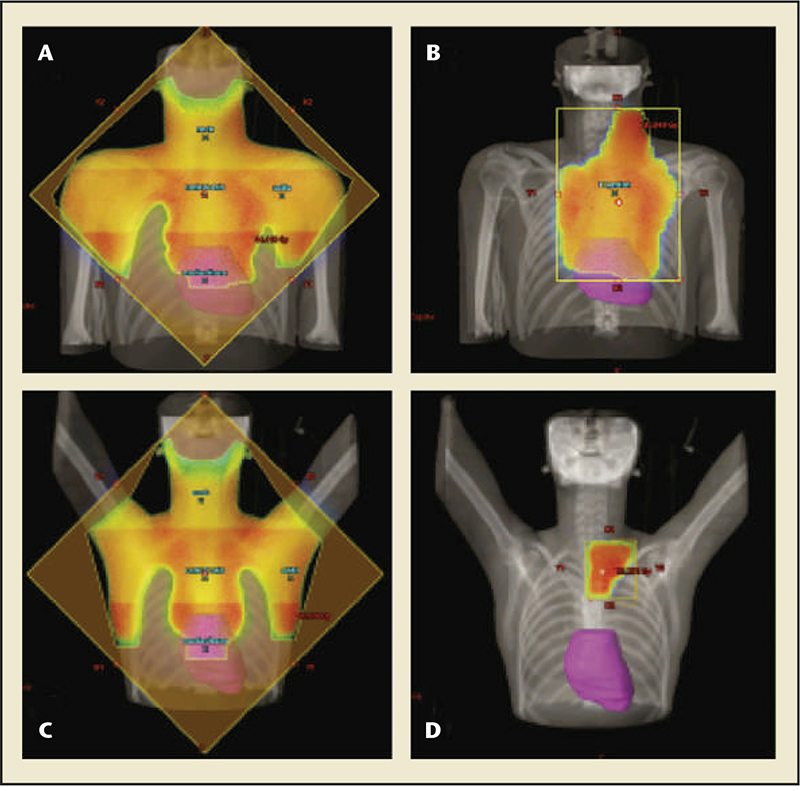
Figure 2. Mantle field radiation versus involved node radiation therapy for Hodgkin disease. (A) Mantle field radiation for large mediastinal tumor, (B) Involved node radiation for large mediastinal tumor, (C)Mantle field radiation for small mediastinal tumor, (D)Involved node radiation for small mediastinal tumor. Involved node radiotherapy reduces the amount of the cardiac volume within the treatment field. Reprinted with permission from Maraldo MV et al.23

Figure 2. Mantle field radiation versus involved node radiation therapy for Hodgkin disease. (A) Mantle field radiation for large mediastinal tumor, (B) Involved node radiation for large mediastinal tumor, (C)Mantle field radiation for small mediastinal tumor, (D)Involved node radiation for small mediastinal tumor. Involved node radiotherapy reduces the amount of the cardiac volume within the treatment field. Reprinted with permission from Maraldo MV et al.23
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are protective against radiation-induced fibrosis in multiple tissue types, including heart.
Because valvular tissue lacks vasculature, ischemia secondary to atherosclerosis is unlikely to cause radiation-induced valvular disease.
As with CHF due to other causes, orthotopic heart transplantation is the final option in cases that are refractory to medical therapy. This may be preferable to CABG or pericardiectomy, given that the severe fibrosis found in the mediastinum or diffuse CAD may impair operative success.
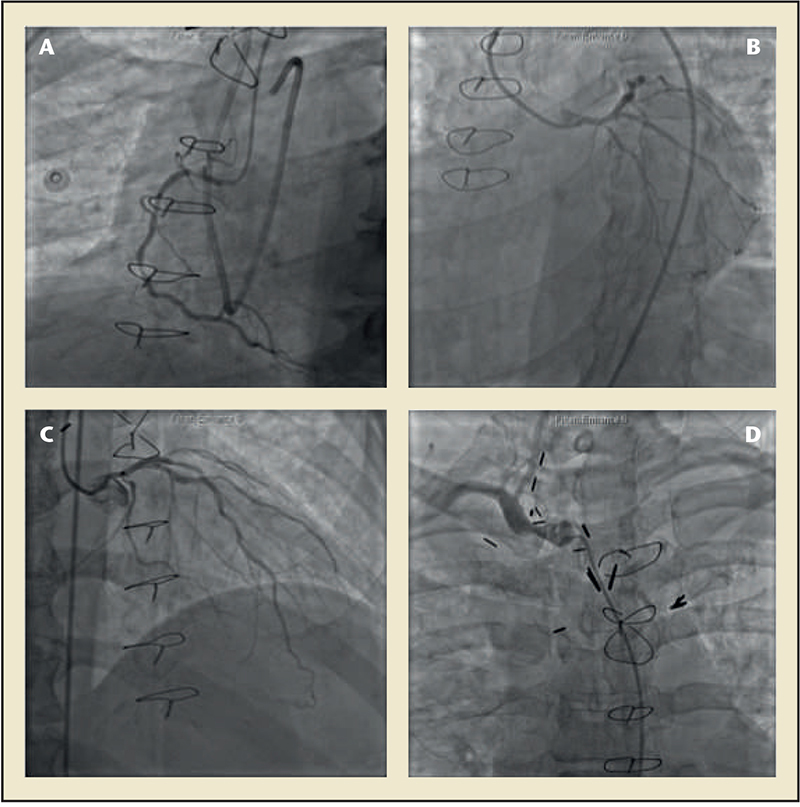
Figure 3. Coronary angiography images of a patient with coronary artery disease secondary to radiation exposure. (A)There is diffuse atherosclerosis of the right coronary artery with a fractional flow reserve of 0.69. (B)The left circumflex artery had a stenosis in the ostium. (C) The left anterior descending artery had diffuse atherosclerotic disease with a fractional flow reserve of 0.78. (D)Angiography of the right brachiocephalic artery reveals a moderate stenosis.

Figure 3. Coronary angiography images of a patient with coronary artery disease secondary to radiation exposure. (A)There is diffuse atherosclerosis of the right coronary artery with a fractional flow reserve of 0.69. (B)The left circumflex artery had a stenosis in the ostium. (C) The left anterior descending artery had diffuse atherosclerotic disease with a fractional flow reserve of 0.78. (D)Angiography of the right brachiocephalic artery reveals a moderate stenosis.

The most important method to prevent RIHD is preventing or reducing radiation exposure to the heart. This is accomplished with radiation protection blocks covering the heart, decreasing the radiation field, and altering total dose or dose fraction.
Main Points
• The cardiovascular sequelae of radiation exposure are an important cause of morbidity and mortality following radiation therapy for cancer, as well as after exposure to radiation after atomic bombs or nuclear accidents. Involvement of the pericardium, myocardium, coronary arteries, valves, and conduction system has been observed.
• The total dose of radiation received by the heart, and fractionation (the division of the total dose into smaller fractions separated by time), have both been studied with respect to risk of subsequent radiation-induced heart disease (RIHD). Early animal studies provided insight into the dose-response relationship of cardiac irradiation. Studies have demonstrated that greater fractionation of the total dose decreases acute pericarditis and myocardial necrosis.
• A complex set of causes contributes to the development of RIHD. There is an early finding of increased vascular permeability and fluid extravasation, as well as inflammatory cell infiltration. This finding correlates with the development of pericardial fibrin exudates and effusions. Coagulation necrosis is seen near arteries occluded by fibrointimal proliferation, indicating focal infarctions rather than direct radiation damage to myocardium. Myocardial fibrosis after radiation exposure is mediated by multiple cytokines and inflammatory cells. The level of gene expression is independent of radiation dose, indicating that this initial change in gene expression may not be the primary insult leading to fibrosis.
• The most important method to prevent RIHD is preventing or reducing radiation exposure to the heart. This is accomplished with radiation protection blocks covering the heart, decreasing the radiation field, and altering total dose or dose fraction. There is a paucity of literature regarding the prevention of RIHD post-exposure. Several preclinical studies have found that angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers mitigate injury to noncardiac tissues, and another study found that patients who were incidentally on ACE inhibitors had a decreased risk of radiation pneumonitis.
Main Points
• The cardiovascular sequelae of radiation exposure are an important cause of morbidity and mortality following radiation therapy for cancer, as well as after exposure to radiation after atomic bombs or nuclear accidents. Involvement of the pericardium, myocardium, coronary arteries, valves, and conduction system has been observed.
• The total dose of radiation received by the heart, and fractionation (the division of the total dose into smaller fractions separated by time), have both been studied with respect to risk of subsequent radiation-induced heart disease (RIHD). Early animal studies provided insight into the dose-response relationship of cardiac irradiation. Studies have demonstrated that greater fractionation of the total dose decreases acute pericarditis and myocardial necrosis.
• A complex set of causes contributes to the development of RIHD. There is an early finding of increased vascular permeability and fluid extravasation, as well as inflammatory cell infiltration. This finding correlates with the development of pericardial fibrin exudates and effusions. Coagulation necrosis is seen near arteries occluded by fibrointimal proliferation, indicating focal infarctions rather than direct radiation damage to myocardium. Myocardial fibrosis after radiation exposure is mediated by multiple cytokines and inflammatory cells. The level of gene expression is independent of radiation dose, indicating that this initial change in gene expression may not be the primary insult leading to fibrosis.
• The most important method to prevent RIHD is preventing or reducing radiation exposure to the heart. This is accomplished with radiation protection blocks covering the heart, decreasing the radiation field, and altering total dose or dose fraction. There is a paucity of literature regarding the prevention of RIHD post-exposure. Several preclinical studies have found that angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers mitigate injury to noncardiac tissues, and another study found that patients who were incidentally on ACE inhibitors had a decreased risk of radiation pneumonitis.
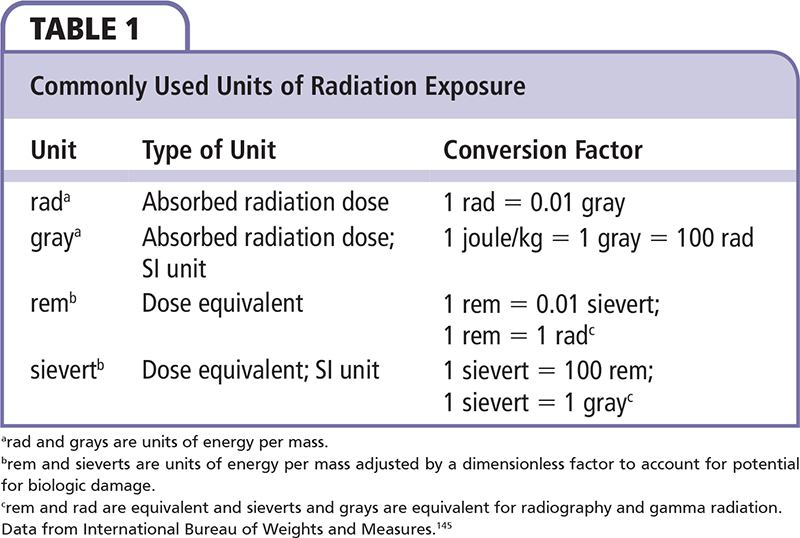
Radiation-induced heart disease (RIHD) is a well-known complication of radiotherapy (RT) for cancer and environmental exposure to radioactive material first described in large-scale human studies in the 1960s.1 The first description of cardiac lesions came from patients who had received mediastinal RT for Hodgkin disease (HD); since then, RIHD has been described in patients who received RT for breast cancer. In patients treated for breast cancer or HD, this may exacerbate and contribute to cardiotoxicity from systemic anthracycline chemotherapy. Additionally, survivors of the atomic bombs in Japan and radiation accidents (such as that in Chernobyl, Ukraine) provide additional evidence of the cardiovascular effects of radiation. In this review, grays (Gy) are used as the units of measurement of absorbed radiation dose; units of radiation dosage are described in Table 1.
Involvement of the pericardium, myocardium, coronary arteries, valves, and conduction system has been observed, depending on the type and amount of radiation received (Table 2). In one autopsy series of 16 young patients (age 15-33 years) who had received > 35 Gy to the heart during mediastinal RT, published in 1981, the majority had pericardial and valvular thickening, and many patients also had myocardial fibrosis and coronary artery stenoses.2 Another autopsy series of patients with likely RIHD found that the majority had pericardial disease, valvular injury, and myocardial fibrosis, whereas a smaller proportion had coronary artery stenoses.3
Radiation therapy is frequently used to treat patients with breast cancer.4 Examination of the US Surveillance, Epidemiology, and End Results (SEER) registry revealed that among patients with early breast cancer, 37% received RT. Meta-analyses of randomized trials of RT for breast cancer have found statistically significant increases in cardiac and vascular mortality.5,6 In the SEER registry there was significant laterality of RIHD; that is, women who received radiation to the left breast had significantly higher cardiovascular mortality than those receiving radiation to the right breast.4 This effect was more prominent as time since radiation increased. In patients who died ≥ 10 years after irradiation, a statistically significant difference in mortality after left versus right breast irradiation was mainly attributed to acute myocardial infarction (MI) and other ischemic heart disease. These findings likely reflect the anterior coronary arteries, including the left anterior descending artery (LAD), being present in the field of radiation of a left breast tumor.
Mediastinal RT is a common treatment for HD.7 In studies following patients treated long term with RT for HD, cardiovascular causes were the third most common cause of death after HD and other cancers, and accounted for 12% to 16% of mortality.7,8 One study that screened patients for cardiac disease by exercise stress test and echocardiography found cardiac disease in 11% of patients.9 Another study that evaluated patients with echocardiography, radionuclide angiocardiography, and cardiac catheterization found cardiac disease in nearly all patients.10 The greater prevalence of heart disease in this study may be attributed to the more extensive work-up, and the majority of patients had normal echocardiogram results. Constrictive pericarditis (including overt or occult) was found in 50% of patients, coronary artery disease (CAD) in 12%, and left ventricular dysfunction in 4%. In patients with HD who received irradiation to a portion of the heart or carotid arteries, there was an increased risk of valve surgery, coronary artery bypass grafting (CABG), or percutaneous coronary intervention (PCI) when compared with the US general population.11
In addition to RIHD secondary to therapeutic radiation, environmental radiation exposure from nuclear power plant accidents and the atomic bombs dropped in Japan in 1945 has previously caused cardiovascular disease. Longitudinal studies that have followed survivors of Hiroshima and Nagasaki have found statistically significant excess risk for heart disease when compared with the population without radiation exposure.12,13 Ischemic heart disease was associated with radiation exposure; however, there were stronger associations between radiation and heart failure or hypertensive heart disease. An excess relative risk for heart disease of 14% was found for each Gy of exposure. Patients were at highest risk for MI when exposed at age < 40 years.14 Workers who were involved in the recovery operation after the Chernobyl accident were also found to have an increased risk in cardiovascular mortality.15 Risk of disease was found to be increased in those who received doses of ≥ 150 mGy over a period of < 6 weeks. Cardiovascular mortality was primarily driven by hypertensive heart disease, and there was no association between radiation dose and ischemic heart disease or acute MI. In addition to those exposed to atomic bomb fallout and nuclear accidents, workers for British Nuclear Fuels were found to have increased risk for ischemic heart disease, although this is not observed in uranium miners.13
The literature regarding radiation exposure from diagnostic studies (ie, conventional films and computed tomography [CT]) focuses primarily on the risk of carcinogenesis rather than discussion of cardiotoxicity.16,17 However, the dose of a typical CT scan is approximately 10 mGy (0.01 Gy), far less than typical RT doses exceeding 10 Gy. Additionally, in large-scale studies of low-dose radiation exposure, doses < 0.5 Gy did not result in increased risk of heart disease.12 This indicates that cardiac toxicity as a result of diagnostic radiation is likely not clinically significant.
Relation of Radiation Field, Dose, and Fractionation to RIHD
The total dose of radiation received by the heart, and fractionation (the division of the total dose into smaller fractions separated by time), have both been studied with respect to risk of subsequent RIHD. Early animal studies provided insight into the dose-response relationship of cardiac irradiation. In rats that received unfractionated radiographic radiation localized to the heart, a dose of 35 to 40 Gy resulted in severe cardiac failure within 15 weeks,18 and animals that received 10 to 15 Gy developed only minor heart failure after 1 year. It is likely that cardiac disease develops with single radiation doses of < 10 Gy. Larger fractions of radiation led to severe heart failure earlier.19 Myocardial cytolysis was observed at 50 to 70 Gy of fractionated radiation, higher than the single dose of 20 Gy causing cytolysis in the former study, indicating that fractionation does play a role in the development of RIHD. In one early autopsy study of RIHD, the mean radiation dose received was 42 Gy (range, 13-75 Gy) in single fractions.3 Only those who received > 30 Gy developed moderate to severe myocardial fibrosis.
The radiation dose that the heart receives varies depending on the type and location of the tumor being treated or environmental exposure. As described above, RT for left-sided breast cancer has resulted in higher risks of cardiac toxicity when compared with RT for right-sided tumors.4 The estimated whole heart dose for left-sided tumors is more than double that received for right-sided tumors.20 The LAD received the greatest radiation dose with left-sided irradiation. Anterior radiation fields result in higher cardiac doses than fields that are tangential to the breast.21 For HD, the historic RT used was mantle field irradiation (lymph nodes in the neck, mediastinum, and axillae) of 35 to 45 Gy.22 This type of radiation results in 27.5 Gy of radiation to the whole heart, and > 35 Gy to some parts of the heart—above the threshold dose for aortic or mitral valve thickening.23,24 Similarly, the majority of deaths due to cardiac causes in follow-up of HD patients occur in those who received > 30 Gy.7 In patients who developed pericardial effusion following RT, the average pericardial dose was 53.25 Gy.25 RT has previously been used to treat peptic ulcer disease and, in these patients, an average total cardiac dose of 2.8 Gy was the lowest dose that resulted in a statistically significant increase in CAD.26
Many changes have been made to contemporary RT regimens for breast cancer in order to reduce the radiation dose received by the heart. Breast cancer radiation may now be performed using CT planning in order ensure the heart is not included in the field (Figure 1); exclusion of the internal mammary chain of lymph nodes from the field also results in reduced cardiac doses.4,27 It is expected that these changes will result in a lower incidence of RIHD; however, the long-term follow-up data for those patients who received RT in the 1990s and later are not yet complete.4 The laterality of CAD observed with left versus right-sided irradiation was most prominent for women who received RT in the 1970s, and decreased steadily after 1979.28 This indicates that RT techniques have improved in recent years with respect to cardiac irradiation. In early randomized controlled trials of RT for breast cancer, the risk of ischemic heart disease was significantly greater in the RT arms than in the surgery-only control arms.21,29 The more recent Danish Breast Cancer Cooperative Group (DBCG) 82b and 82c trials randomized patients to surgery with or without RT, and found no increased risk of ischemic heart disease.30,31 In the DBCG trials, a lower volume of irradiated heart tissue, radiation protection blocks over the heart, and RT treatment planning using ultrasound measurement of chest wall thickness all likely contributed to the absence of cardiotoxicity.
Radiation therapy for HD has also been refined. Stanford University Medical Center (Stanford, CA) began using radiation protection blocks during RT to protect the left ventricle, limiting the total cardiac dose to 15 Gy, and reduced the fraction size in 1972.7,32 The nonacute MI cardiac death relative risk in the era prior to 1972 was 5.3; this decreased to 1.4 in the period after 1972. A more recent change to RT for HD is involved-field radiation therapy, which only includes the lymph nodes that were enlarged prior to initiation of treatment and their surrounding regions, as opposed to extended-field (mantle) radiation.20 Similarly, involved-node radiation therapy involves the involved nodes alone. These techniques significantly decrease the total cardiac dose (Figure 2).23
Fractionation of radiation doses is another potential factor in the development of RIHD. Studies have demonstrated that greater fractionation of the total dose decreases acute pericarditis and myocardial necrosis.33-35 The decrease in non-acute MI cardiovascular mortality in patients who received RT for HD observed at Stanford University Medical Center after 1972 coincided with a reduction in fraction size.31 Fractionation schedules of twice weekly rather than five times weekly have been found to result in increased risk of late complications in normal tissues such as pulmonary pneumonitis and fibrosis and pathologic fractures, whereas cardiac effects were not reported.36 The current standard RT for breast cancer is a total dose of 50 Gy to breast tissue given in 25 fractions.37 However, a recent randomized trial comparing this regimen with hypo-fractionated RT (larger dose fractions given in fewer treatments, in this case a total dose of 42.5 Gy in 16 fractions) found that there were no differences in cardiovascular mortality in 10 years of follow-up.38 Analysis of dose plans finds that patients receiving hypofractionated RT will receive lower radiation dose to the heart than those receiving normofractionated RT.37 These studies indicate that there does not appear to be evidence in favor of either fractionation regimen for breast cancer treatment, whereas in the case of mediastinal irradiation for HD, larger fractions are more detrimental.
Pathology of RIHD and Mechanism of Injury
The distinct gross and microscopic pathology of RIHD has been characterized in human autopsy studies.2,3 Many of these patients were young, making it unlikely that age-related degenerative changes or CAD was responsible for the lesions observed. The majority (70%-100%) of RIHD autopsy pericardium specimens had some form of pericardial disease. Pericardial effusion was the most common finding, followed by constrictive pericarditis, fibrinous pericardial adhesions, and obliterative pericardial fibrosis. Pericardial thickening with fibrous tissue was also found in most patients. The mural endocardium and valves were also thickened in the majority of patients. Several valve specimens in one study were surgically removed mitral and aortic valves, and most of these were moderately to severely fibrotic and stenotic. Across the spectrum of autopsied patients, diffuse fibrosis was noted in each of the four valves, with calcification present in some. Interstitial fibrosis of the myocardium was present in > 50% of patients. Damage to or necrosis of myocardial cells was not observed in either study. Finally, 15% to 37% of autopsies revealed severe coronary artery stenosis (> 75% narrowing) attributable to radiation. Further microscopic examination showed atherosclerosis and intimal fibrosis; however, fibrous tissue dominated in the majority of sections. In addition, in most patients, the media was infiltrated by fibrous tissue.
A complex set of causes contributes to the development of RIHD. There is an early finding of increased vascular permeability and fluid extravasation, as well as inflammatory cell infiltration.18,19,39 This finding correlates with the development of pericardial fibrin exudates and effusions. After 1 year, fibrous tissue within the myocardium is more prevalent. Coagulation necrosis is seen near arteries occluded by fibrointimal proliferation, indicating focal infarctions rather than direct radiation damage to myocardium.
Myocardial fibrosis after radiation exposure is mediated by multiple cytokines and inflammatory cells. Following irradiation there is an increase in both type I and III collagen in the ventricles.40,41 Tumor necrosis factor-α and interleukin-1 secreted by macrophages following irradiation contribute to fibrosis in lung tissue.41,42 Transforming growth factor-β (TGF-β) promotes fibroblast activity and proliferation, and has been found to correlate with radiation-induced fibrosis in several tissue types.42 TGF-β gene expression is upregulated after irradiation in cultured endothelial cells, fibroblasts, and myocytes.43 Expression of other fibrosis-promoting factors, such as fibroblast growth factor-2, is also found to be elevated. The level of gene expression, however, is independent of radiation dose, indicating that this initial change in gene expression may not be the primary insult leading to fibrosis. The authors hypothesize that hypoxia after radiation-induced vascular damage may play a greater role. Indeed, late interstitial fibrosis is often perivascular, and capillary injury precedes fibrosis, providing support that vascular damage causes radiation-induced fibrosis.18,19,39,42-44 There is also evidence that intestinal radiation injury is caused primarily by endothelial apoptosis.45 Deposition of von Willebrand factor (vWF) deposition in myocardial capillaries is followed by fibrosis in the ventricles and atria.46,47 This deposition is further evidence of endothelial damage and may result in capillary obstruction. Additionally, histopathologic findings in RIHD are most evident in the subendocardial layer, as in myocardial ischemia due to CAD. However, the intercalated discs and mitochondria of myofibers are damaged following irradiation, which appears to be unrelated to ischemia.48-51 Additionally, there is evidence of chronic TGF-β activity after irradiation continuing to stimulate fibrosis.52 Cardiac mitochondrial respiration is also inhibited by ionizing radiation, which results in elevated reactive oxygen species (ROS) production.53 These findings are associated with radiation-induced myocardial dysfunction.41,48-54 Finally, radiation-induced oxidative stress activates the renin-angiotensin-aldosterone system, and angiotensin II is a potent stimulator of fibrosis.55,56 Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are protective against radiation-induced fibrosis in multiple tissue types, including heart.55-57 The kallikreinkinin system promotes inflammatory cell recruitment within the heart following radiation, although this may have both protective and injurious effects.58,59
Cardiac radiation exposure results in endothelial injury and atherosclerosis. Within several days after irradiation of the carotid and coronary arteries, lysosomal enzymes are activated within the intima and media, and cholesterol plaques form in the intimal layer.60-63 This intimal infiltration of lipids is a result of increased endothelial permeability following radiation.64,65 The plaques that develop following irradiation are often inflammatory plaques that are vulnerable to thrombosis.66,67 These lesions contain infiltrates with macrophages and neutrophils, and occasional plaque hemorrhages were present.66 Microvascular atherosclerosis is also observed.68 In addition to plaques, fibrosis is present throughout the intima, media, and adventitia.2,3 Generation of ROS is important to the pathogenesis of radiation-induced endothelial damage.69 Release of vWF from endothelial cells is increased, thrombomodulin production is decreased, and adhesiveness of endothelial cells is found to increase, contributing to thrombosis.46,70-72 Additionally, endothelium-dependent vasodilation is impaired by radiation, which may worsen small vessel thrombosis.73 In patients with whole-body radiation exposure, such as survivors of the atomic bombs or nuclear accidents, injury to the kidney can cause hypertension, further worsening atherosclerosis.13
Because valvular tissue lacks vasculature, ischemia secondary to atherosclerosis is unlikely to cause radiation-induced valvular disease.74 Radiation does have a direct effect on interstitial valve cells. When aortic valve interstitial cells are grown in culture and exposed to 10 Gy of radiation, these cells exhibit a conversion to cells resembling osteoblasts.75 Among the changes in phenotype observed are an increased expression of alkaline phosphatase, bone morphogenetic protein 2, and osteopontin. This is likely an important step in the development of calcific aortic stenosis and other radiation-induced valvular disease.
Pericardial Disease
Pericardial disease is a well-known outcome after RT, and includes a spectrum of disease ranging from acute pericardial effusions to constrictive pericarditis. Among the first case reports of RIHD were of pericardial effusions and adhesions following thoracic radiation, as well as extensive fibrosis of the pericardium resulting in cardiac tamponade.76 In one of the early series of RIHD patients who had received RT, pericardial disease was commonly observed.77 The cases consisted primarily of acute pericarditis. Most of these patients subsequently recovered; however, some developed constrictive physiology later on. Their clinical presentation was identical to that of viral pericarditis, including chest pain and a friction rub.77,78 The onset of acute pericarditis was not necessarily immediately following RT, ranging from immediately after RT to 2 years later.77 Many patients developed pericardial effusion, and several of these patients developed tamponade. Treatment was similar to viral pericarditis, consisting of nonsteroidal anti-inflammatory drugs and pericardiocentesis for large effusions.
Some patients develop chronic pericarditis with effusion or constrictive pericarditis.77-79 In a series of patients with constrictive pericarditis, up to one-third of cases were caused by RT, although this statistic likely varies by institution.80-82 Constrictive pericarditis can occur in patients of any age, including children.83 Patients with constrictive pericarditis following RT often have jugular venous distention or a pericardial knock on examination, and hepatomegaly was common in children.77-79,83 Many have symptomatic pulmonary congestion or edema. Electrocardiographic findings include low QRS voltage and T-wave abnormalities. The average time from irradiation to clinical presentation ranged from 14 to 88 months in several studies.3,77,83,84 There have also been cases of constrictive pericarditis developing over 20 years after RT for HD.85 Diagnosis can be aided with echocardiography or CT scan, revealing pericardial thickening, although cardiac catheterization showing elevated end-diastolic pressure is the most sensitive modality.86 Pericardiocentesis may be indicated for large effusions, especially for stabilization prior to surgery.77 When fibrosis of the pericardium has progressed to the degree that diastolic filling is significantly impaired, radical or partial pericardiectomy is performed to remove the constrictive portions of the pericardium.86,87 The prognosis after pericardiectomy after radiation is significantly worse than that for pericardiectomy performed for other reasons (5-year survival of 64.3% vs 11.0%; P < .001).87 This is likely because, with radiation-induced constriction, the entire heart is involved rather than the pericardium alone.86,87 However, at least one institution has achieved 100% survival in a small cohort after 8 years, and early pericardiectomy is recommended if constrictive pericarditis is diagnosed.86
Cardiomyopathy
As a result of the involvement of myocardium by vascular compromise or direct radiation injury and fibrosis, cardiomyopathy and heart failure is a potential complication of cardiac radiation exposure. Dogs that developed severe congestive heart failure (CHF) following irradiation often also had myocytolysis on histopathologic examination.19 Systolic CHF with depressed ejection fraction occurs, presenting similarly to nonradiation CHF, with dyspnea.88 MRI in patients with radiation-induced CHF may demonstrate myocardial fibrosis. For patients receiving RT alone for treatment of HD, one study found that, although the average ejection fraction of all patients was within the normal range (56%), it was significantly lower than that of healthy control subjects (62%; P < .05).89 Of the patients in this study, 90% were in New York Heart Association class I heart failure, whereas the other 10% were in class II heart failure. In addition to systolic failure, diastolic failure due to restrictive cardiomyopathy may also develop following radiation.90,91 This develops secondary to both endocardial and myocardial fibrosis. Because patients receiving RT for breast cancer or HD also often receive anthracyclines, there is the risk for enhanced cardiotoxicity.1 Mediastinal irradiation potentiates doxorubicin cardiotoxicity, even when the two are not administered during the same time period.92-94
The treatment for CHF secondary to mediastinal irradiation is similar to treatment for CHF not caused by radiation exposure, including β-blockers and ACE inhibitors.95 As with CHF due to other causes, orthotopic heart transplantation (OHT) is the final option in cases that are refractory to medical therapy.96,97 This may be preferable to CABG or pericardiectomy, given that the severe fibrosis found in the mediastinum or diffuse CAD may impair operative success. One study had a 100% survival rate at a mean follow-up time of 48 months, indicating that OHT has the potential to be safe and feasible.97 However, OHT in this population also carries significant risks, with 33% of patients dying in the perioperative period in another study.96 Although OHT is an option for end-stage RIHD, patients should be carefully selected given the significant perioperative risk.
Valvular Disease
A wide range of disease affecting any of the cardiac valves may occur as a result of cardiac irradiation. Valvular disease has a high prevalence 20 years or more after mediastinal radiation, affecting the majority of these patients.98 Most patients are asymptomatic. Aortic regurgitation greater than trace levels was present in 60%, aortic stenosis in 16%, and mitral regurgitation in 52%. Calcification of the aortic or mitral valves was present in 90% of these patients. Regurgitation is more common than stenosis in RIHD; however, clinically significant valvular disease is more likely to be stenosis.3,11,99,100 The aortic and mitral valves are the most commonly affected, and tricuspid or pulmonary disease is less frequently reported.3,11 Risk factors for asymptomatic valvular disease include > 25 Gy of radiation to the heart.101 In addition to mediastinal RT exposure, valvular disease, including aortic stenosis, aortic regurgitation, and mitral regurgitation, has been reported in a man who was exposed to radiation in Chernobyl.102 Patients, when symptomatic, often present with heart failure symptoms depending on the valve involved.11,99,100,103,104 The presentation is similar to that of non-RIHD-associated valvular disease, and occurs an average of 22 years after radiation exposure.
Treatment of severe radiation-induced valvular disease is primarily surgical, consisting of valve replacement or occasionally percutaneous intervention.99 In patients with RIHD undergoing valve surgery (including aortic, mitral, tricuspid, and multiple), the 30-day mortality rate is 12%, and the 5-year survival rate is 66%.105,106 The most important predictor for perioperative (30-day) mortality was the presence of constrictive pericarditis. These patients had a 30-day mortality rate of 40%. One possible reason for this increased mortality is “frozen mediastinum,” referring to severe fibrosis of the mediastinum that may interfere with the operation.104-107 In addition, constrictive pericarditis may be limited to patients who have received higher doses of radiation and have greater overall cardiac injury. In patients with extensive mediastinal fibrosis or who are otherwise poor surgical candidates, transcatheter valve replacement is also an option. One case report described a woman with severe radiation-induced aortic stenosis who was successfully treated with the Edwards SAPIEN XT (Edwards Lifesciences, Irvine, CA) stent-mounted prosthetic valve.108 Another reported a patient with pulmonary stenosis secondary to RT who was treated with balloon angioplasty and stent placement, achieving reduction in the stenosis and pressure gradient.103 Intervention for radiation-induced valvular disease should be individually tailored to the valve involved and the patient's other cardiac disease and noncardiac comorbidities.
Coronary Artery Disease
CAD is rapidly accelerated in patients exposed to radiation, and is the most common clinically significant manifestation of RIHD (Figure 3).11 The majority of patients who develop CAD have at least one Framingham risk factor. However, radiation alone may cause CAD. In multiple cases, young patients without traditional cardiac risk factors who had received mediastinal radiation for HD have had acute MI.77,109 The distribution of CAD depends on the areas irradiated, as vessels exposed to higher doses have more stenoses.110 In patients with left-sided breast cancer treated with RT, the risk of severe stenosis in the mid and distal LAD as well as the distal diagonal is significantly increased.111 In patients who have received mediastinal RT, over 75% of asymptomatic patients have coronary artery stenoses.112 The distribution of CAD in these patients is often extensive; 30% of patients screened for CAD with coronary angiography have two- or three-vessel disease with stenosis of ≥ 70%.112-114 Ostial lesions in the left, right, or both coronary arteries were also common. In addition to de novo CAD following RT, mediastinal RT for HD can increase the risk of in-stent restenosis following PCI previous to irradiation.115 Interestingly, intracoronary radiation therapy in the form of iridium-192 seeds embedded within a ribbon is being used to treat in-stent restenosis.116 The Washington Radiation for In-Stent Restenosis Trial (WRIST) randomized study evaluating this therapy found a reduced major adverse cardiac event rate at 5 years when compared with placebo (46.2% vs 69.2%; P = .008), primarily driven by reduced target lesion revascularization. The proposed mechanism is inhibition of neointimal proliferation associated with medial fibrosis and cell death of the proliferative cells within the media.116,117 These inconsistencies in the effects of radiation on vasculature may be due to the fact that external RT uses repetitive and higher doses as opposed to the single, lower dose used with intracoronary radiation therapy.117
The clinical presentation of radiation-induced CAD is primarily angina, as with typical CAD.113 This may be exertional angina, rest angina, chest pain due to acute MI, or heart failure secondary to acute MI. Hibernating myocardium associated with a significant stenosis has also been observed in a patient treated for HD.118 Electrocardiographic (ECG) and cardiac biomarkers such as troponin and creatine kinase-MB are useful for the diagnosis of acute MI.109 Coronary angiography is still the standard of care for diagnosis and localization of the lesion in patients who present with angina associated with cardiac irradiation.113 There are also several other validated diagnostic approaches to CAD associated with radiation; perfusion imaging using technetium-99m tetrofosmin is one such method.119,120 However, it should be noted that some lesions observed with perfusion imaging are irreversible or do not correspond to coronary artery territories, suggesting that they are due to damage to microvasculature rather than coronary arteries. CT angiography (CTA) with or without coronary artery calcium (CAC) scoring is another technique.121-123 CAC scoring alone with Agatston and volume scores over 200 was fairly specific for CAD, although not necessarily sensitive.121 Using CTA in addition to CAC scoring adds sensitivity to the test.123
Although guidelines for the medical management of acute coronary syndromes (ACS) and stable CAD do not make mention of RIHD specifically, it is reasonable to follow the existing recommendations for conventional CAD and ACS.124,125 For revascularization of coronary arteries, both PCI and CABG may be used.126 Because radiation may cause multiple types of heart disease in one patient, CABG has the potential of being done during the same operation as pericardiectomy or valve surgery to reduce reoperation.127 As discussed above, mediastinal fibrosis may interfere with cardiac surgery.107 There is concern that the internal mammary artery (IMA) may also be affected by irradiation, and that this makes it an inferior conduit for CABG in patients with radiation-induced CAD.128 However, one study of 125 patients who had previously received RT did not find any evidence of radiation-induced injury to IMA grafts, and the authors state that avoiding use of the IMA as a bypass graft is not necessary.129 Both CABG and PCI are reasonable options and the appropriate use criteria for coronary revascularization should be considered when managing significant coronary artery stenoses in patients with a history of irradiation.130,131
Conduction Abnormalities
Damage to myocardium and coronary arteries also can result in conduction abnormalities, such as bundle branch blocks and atrioventricular (AV) block.98,132 In asymptomatic patients, left or right bundle branch block (LBBB, RBBB), and first-degree AV block may be detected incidentally on ECG.98 The prevalence of these defects is comparable with Framingham studies. In a case series of patients with symptomatic conduction defects, clinical presentation was most commonly syncope.132 The average time interval from irradiation until presentation was 12 years, and intermittent complete infranodal AV block was present in all patients, with interval ECGs showing LBBB or RBBB. One reported cause of AV block after RT is exercise-induced ischemia of the AV node, due to ostial stenosis of the right coronary artery.133,134 In addition, fibrosis throughout the heart can disrupt the bundle branches, the AV node, and the conduction system proximal to the AV node.135,136 Pacemaker therapy is indicated for high-degree AV blocks.132,133 In addition to conduction blocks, young patients treated for cancer with anthracyclines and cardiac irradiation were at increased risk for premature ventricular contractions, supraventricular tachycardia, and ventricular tachycardia.137
Prevention and Screening
The most important method to prevent RIHD is preventing or reducing radiation exposure to the heart. This is accomplished with radiation protection blocks covering the heart, decreasing the radiation field, and altering total dose or dose fraction. There is a paucity of literature regarding the prevention of RIHD post-exposure. Several preclinical studies have found that ACE inhibitors and ARBs mitigate injury to noncardiac tissues, and another study found that patients who were incidentally on ACE inhibitors had a decreased risk of radiation pneumonitis.138-140 The mechanism of this protective effect appears to be the prevention of fibrosis within these tissues. Given that fibrosis is also commonly observed in the heart following irradiation, the use of ACE inhibitors or ARBs may be similarly protective for RIHD; however, prospective trials would need to evaluate this.
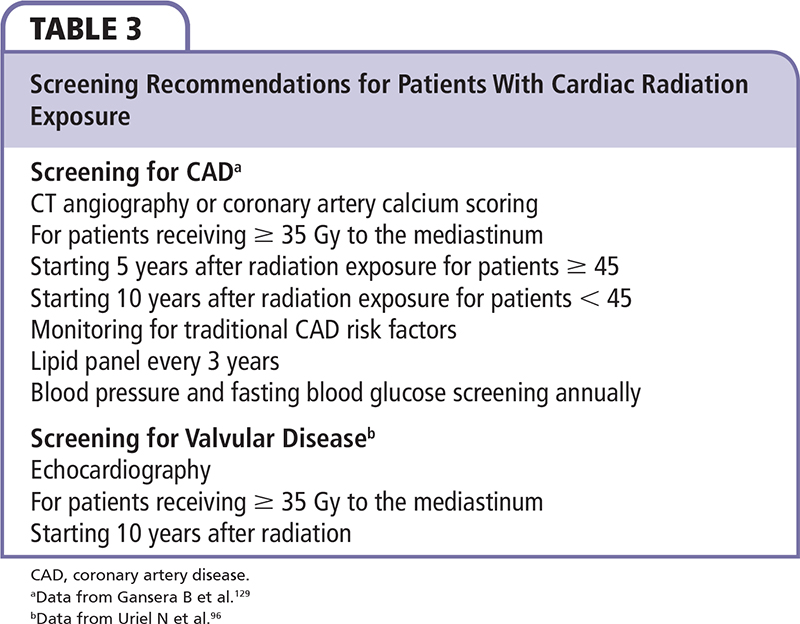
Troponin I and brain natriuretic peptide have been found to increase following RT for breast cancer141; however, further studies should be conducted to determine the capability of these biomarkers to predict future cardiomyopathy. There are recommendations to screen for valvular disease with echocardiography 10 years after RT.98,142 The benefit of screening echocardiograms is to determine which patients are candidates for endocarditis prophylaxis. Proposed recommendations for screening for CAD are to screen asymptomatic patients aged 45 years or older 5 years following mediastinal RT ≥ 35 Gy, or 10 years following RT for younger patients (Table 3).111,132 These authors recommended CTA or CAC scoring as a screening technique. Patients with abnormalities on these screening tests then proceed to coronary angiography. It is also important to carefully monitor and treat traditional CAD risk factors such as hypertension, dyslipidemia, diabetes, and smoking, even in young patients.93 Recommendations for lipid screening include a lipid panel every 3 years, and annual blood pressure and fasting blood glucose testing.143
Conclusions
RIHD is a significant cause of long-term morbidity and mortality in survivors of HD and breast cancer, with a prevalence of 6.7% after treatment for HD.77 High radiation doses portend a poor prognosis and refractoriness to treatment. It is prudent for the majority of patients with significant radiation exposure to be followed by a cardiologist for life, as CAD, valve disease, and heart failure have a high prevalence and are progressive.144 Additionally, screening for valvular disease with echocardiography, and for CAD with CAC scoring and CT angiography, may be warranted. As RT is refined to reduce cardiac doses, the incidence of RIHD may decline. ![]()
References
- Stewart JR, Fajardo LF. Radiation-induced heart disease: an update. Prog Cardiovasc Dis. 1984;27: 173-194.
- Brosius FC 3rd, Waller BF, Roberts WC. Radiation heart disease: analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3,500 rads to the heart. Am J Med. 1981;70:519-530.
- Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol. 1996;27:766-773.
- Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557-565.
- Early Breast Cancer Trialists Collaborative Group. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet. 2000;355:1757-1770.
- Cuzick J, Stewart H, Rutqvist L, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12:447-453.
- Hoppe RT. Hodgkin’s disease: complications of therapy and excess mortality. Ann Oncol. 1997;8(suppl 1):115-118.
- Mauch PM, Kalish LA, Marcus KC, et al. Long-term survival in Hodgkin’s disease relative impact of mortality, second tumors, infection, and cardiovascular disease. Cancer J Sci Am. 1995;1:33-42.
- Piovaccari G, Ferretti RM, Prati F, et al. Cardiac disease after irradiation for Hodgkin’s disease: incidence in 108 patients with long follow-up. Int J Cardiol. 1995; 49:39-43.
- Applefeld MM, Wiernik PH. Cardiac disease after radiation therapy for Hodgkin’s disease: analysis of 48 patients. Am J Cardiol. 1983;51:1679-1681.
- Hull MC, Morris CG, Pepine CJ, Mendenhall NP. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831-2837.
- Preston DL, Shimuzu Y, Pierce DA, et al. Studies of atomic bomb survivors. Report 13: solid cancer and noncancer disease mortality: 1950-1997. Radiation Res. 2003;160:381-407.
- Shimizu Y, Kodama K, Nishi N, et al. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950-2003. BMJ. 2010;340:b5349.
- Yamada M, Wong L, Fujiwara S, et al. Noncancer disease incidence in atomic bomb survivors, 1958-1998. Radiation Res. 2004;161:622-632.
- United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation. UNSCEAR 2008 Report to the General Assembly with Scientific Annexes. Volume II: Scientific Annexes C, D and E. Vienna, Austria: United Nations Publications; 2011.
- Davies HE, Wathen CG, Gleeson FV. Risks of exposure to radiological imaging and how to minimise them. BMJ. 2011;342:d947.
- Brenner DJ, Hall EJ. Computed tomography – an increasing source of radiation exposure. N Engl J Med. 2007;357:2277-2284.
- Lauk S, Kiszel Z, Buschmann J, Trott K. Radiation-induced heart disease in rats. Int J Radiation Oncol Biol Phys. 1985;11:801-808.
- Gillette SM, Gillette EL, Shida T, et al. Late radiation response of canine mediastinal tissues. Radiother Oncol. 1992;23:41-52.
- Taylor CW, Nisbet A, McGale P, Darby SC. Cardiac exposures in breast cancer radiotherapy: 1950s-1990s. Int J Radiation Oncol Biol Phys. 2007:69:1484-1495.
- Rutqvist LE, Lax I, Fornander T, Johansson H. Cardiovascular mortality in a randomized trial of adjuvant radiation therapy versus surgery alone in primary breast cancer. Int J Radiation Oncol Biol Phys. 1992;22:887-896.
- Hodgson DC. Late effects in the era of modern therapy for Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011:323-329.
- Maraldo MV, Brodin NP, Vogelius IR, et al. Risk of developing cardiovascular disease after involved node radiotherapy versus mantle field for Hodgkin lymphoma. Int J Radiation Oncol Biol Phys. 2012;83: 1232-1237.
- Glanzmann C, Huguenin P, Lutolf UM, et al. Cardiac lesions after mediastinal irradiation for Hodgkin’s disease. Radiother Oncol. 1994;30:43-54.
- Byhardt R, Brace K, Ruckdeschel J, et al. Dose and treatment factors in radiation-related pericardial effusion associated with the mantle technique for Hodgkin’s disease. Cancer. 1975;35:795-802.
- Carr ZA, Land CE, Kleinerman RA, et al. Coronary heart disease after radiotherapy for peptic ulcer disease. Int J Radiation Oncol Biol Phys. 2005;61:842-850.
- Buchholz TA. Radiation therapy for early-stage breast cancer after breast-conserving surgery. N Engl J Med. 2009;360:63-70.
- Giordano SH, Kuo YF, Freeman JL, et al. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97:419-424.
- Høst H, Brennhovd IO, Loeb M. Postoperative radiotherapy in breast cancer – long-term results from the Oslo study. Int J Radiation Oncol Biol Phys. 1986;12:727-732.
- Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med. 1997;337:949-955.
- Højris I, Overgaard M, Christensen JJ, Overgaard J. Morbidity and mortality of ischaemic heart disease in high-risk breast-cancer patients after adjuvant postmastectomy systemic treatment with or without radiotherapy: analysis of DBCG 82b and 82c randomised trials. Lancet. 1999;354:1425-1430.
- Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. JAMA. 1993;270:1949-1955.
- Lauk S, Rüth S, Trott KR. The effects of dose-fractionation on radiation-induced heart disease in rats. Radiother Oncol. 1987;8:363-367.
- Schultz-Hector S, Sund M, Thames HD. Fractionation response and repair kinetics of radiation-induced heart failure in the rat. Radiother Oncol. 1992;23:33-40.
- Cosset JM, Henry-Amar M, Girinski T, et al. Late toxicity of radiotherapy in Hodgkin’s disease: the role of fraction size. Acta Oncologica. 1988;27:123-129.
- Overgaard M, Bentzen SM, Christensen JJ, Madsen EH. The value of the NSD formula in equation of acute and late radiation complications in normal tissue following 2 and 5 fractions per week in breast cancer patients treated with postmastectomy irradiation. Radiother Oncol. 1987;9:1-11.
- Appelt AL, Vogelius IR, Bentzen SM. Modern hypofractionation schedules for tangential whole breast irradiation decrease the fraction size-corrected dose to the heart. Clin Oncol. 2013;25:147-152.
- Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513-520.
- McChesney SL, Gillette EL, Powers BE. Radiation-induced cardiomyopathy in the dog. Radiat Res. 1988;113:120-132.
- Chello M, Mastroroberto P, Romano R, et al. Changes in the proportion of types I and III collagen in the left ventricular wall of patients with post-irradiative pericarditis. Cardiovasc Surg. 1996;4:222-226.
- Krüse J, Zurcher C, Strootman EG, et al. Structural changes in the auricles of the rat heart after local ionizing irradiation. Radiother Oncol. 2001;58:303-311.
- Rodemann HP, Bamberg M. Cellular basis of radiation- induced fibrosis. Radiother Oncol. 1995;35: 83-90.
- Boerma M, Bart CI, Wondergem J. Effects of ionizing radiation on gene expression in cultured rat heart cells. Int J Radiat Biol. 2002;78:219-225.
- Fajardo LF, Stewart JR. Capillary injury preceding radiation-induced myocardial fibrosis. Radiology. 1971;101:429-433.
- Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293-297.
- Boerma M, Kruse JJ, van Loenen M, et al. Increased deposition of von Willebrand factor in the rat heart after local ionizing irradiation. Strahlenther Onkol. 2004;180:109-116.
- Verheij M, Dewit LG, Boomgaard MN, et al. Ionizing radiation enhances platelet adhesion to the extracellular matrix of human endothelial cells by an increase in the release of von Willebrand factor. Radiation Res. 1994;137:202-207.
- Cilliers GD, Harper IS, Lochner A. Radiation-induced changes in the ultrastructure and mechanical function of the rat heart. Radiother Oncol. 1989;16:311-326.
- Yang VV, Stearner SP, Tyler SA. Radiation-induced changes in the fine structure of the heart: comparison of fission neutrons and 60Co gamma rays in the mouse. Radiat Res. 1976;67:344-360.
- Kahn MY. Radiation-induced cardiomyopathy. I. An electron microscopic study of cardiac muscle cells. Am J Pathol. 1973;73:131-146.
- Maeda, S. Pathology of experimental radiation pancarditis. II. Correlation between ultrastructural changes of the myocardial mitochondria and succinic dehydrogenase activity in rabbit heart receiving a single dose of X-ray irradiation. Acta Pathol Jpn. 1982;32:199-218.
- Barcellos-Hoff MH. How do tissues respond to damage at the cellular level? The role of cytokines in irradiated tissues. Radiat Res. 1998;150:S109-S120.
- Barjaktarovic Z, Schmaltz D, Shyla A, et al. Radiation-induced signaling results in mitochondrial impairment in mouse heart at 4 weeks after exposure to X-rays. PLoS One. 6(12):e27811.
- Schultz-Hector S, Böhm M, Blöchel A, et al. Radiation- induced heart disease: morphology, changes in catecholamine synthesis and content, β-adrenoceptor density, and hemodynamic function in an experimental model. Radiat Res. 1992;129:281-289.
- Robbins ME, Diz DI. Pathogenic role of the reninangiotensin system in modulating radiation-induced late effects. Int J Radiat Oncol Biol Phys. 2006;64: 6-12.
- Wu R, Zeng Y. Does angiotensin II–aldosterone have a role in radiation-induced heart disease? Med Hypotheses. 2009;72:263-266.
- Yarom R, Harper IS, Wynchank S, et al. Effect of captopril on changes in rats’ hearts induced by long-term irradiation. Radiat Res. 1993;133:187-197.
- Sridharan V, Tripathi P, Sharma SK, et al. Cardiac inflammation after local irradiation is influenced by the kallikrein-kinin system. Cancer Res. 2012;72: 4984-4992.
- Boerma M, Wang J, Wondergem J, et al. Influence of mast cells on structural and functional manifestations of radiation-induced heart disease. Cancer Res. 2005;65:3100-3107.
- Amromin GD, Gildenhorn HL, Solomon RD, Nadkarni BB. The synergism of X-irradiation and cholesterol- fat feeding on the development of coronary artery lesions. J Atheroscler Res. 1964;4:325-334.
- Konings AW, Hardonk MJ, Wieringa RA, Lamberts HB. Initial events in radiation-induced atheromatosis I. Activation of lysosomal enzymes. Strahlentherapie. 1975;150:444-448.
- Konings AW, Smit Sibinga CT, Aarnoudse MW, et al. Initial events in radiation-induced atheromatosis. II. Damage to intimal cells. Strahlentherapie. 1978;154:795-800.
- Konings AW, Smit Sibinga CT, Lamberts HB. Initial events in radiation-induced atheromatosis. IV. Lipid composition of radiation-induced plaques. Strahlentherapie. 1980;156:134-138.
- Konings AW, de Wit SS, Lamberts HB. Initial events in radiation-induced atheromatosis. III. Effect on lipase activity. Strahlentherapie. 1979;155:655-657.
- Evans ML, Graham MM, Mahler PA, Rasey JS. Changes in vascular permeability following thorax irradiation in the rat. Radiat Res. 1986;107:262-271.
- Stewart FA, Heeneman S, Te Poele J, et al. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE -/- mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am J Pathol. 2006;168:649-658.
- Hoving S, Heeneman S, Gijbels MJ, et al. Single-dose and fractionated irradiation promote initiation and progression of atherosclerosis and induce an inflammatory plaque phenotype in ApoE(-/-) mice. Int J Radiat Oncol Biol Phys. 2008;71:848-857.
- Gabriels K, Hoving S, Seemann I, et al. Local heart irradiation of ApoE(-/-) mice induces microvascular and endocardial damage and accelerates coronary atherosclerosis. Radiother Oncol. 2012;105:358-364.
- Tribble DL, Barcellos-Hoff MH, Chu BM, Gong EL. Ionizing radiation accelerates aortic lesion formation in fat-fed mice via SOD-inhibitable processes. Arterioscler Thromb Vasc Biol. 1999;19:1387-1392.
- Zhou Q, Zhao Y, Li P, et al. Thrombomodulin as a marker of radiation-induced endothelial cell injury. Radiat Res. 1992;131:285-289.
- Stewart FA, Hoving S, Russell NS. Vascular damage as an underlying mechanism of cardiac and cerebral toxicity in irradiated cancer patients. Radiat Res. 2010;174:865-869.
- Khaled S, Gupta KB, Kucik DF. Ionizing radiation increases adhesiveness of human aortic endothelial cells via a chemokine-dependent mechanism. Radiat Res. 2012;177:594-601.
- Beckman JA, Thakore A, Kalinowski BH, et al. Radiation therapy impairs endothelium-dependent vasodilation in humans. J Am Coll Cardiol. 2001;37:761-765.
- Stewart FA. Mechanisms and dose–response relationships for radiation-induced cardiovascular disease. Ann ICRP. 2012;41:72-79.
- Nadlonek NA, Weyant MJ, Yu JA, et al. Radiation induces osteogenesis in human aortic valve interstitial cells. J Thorac Cardiovasc Surg. 2012;144:1466-1470.
- Hurst DW. Radiation fibrosis of pericardium, with cardiac tamponade. Can Med Assoc J. 1959;81: 377-380.
- Cohn KE, Stewart JR, Fajardo LF, Hancock EW. Heart disease following radiation. Medicine (Baltimore). 1967;46:281-298.
- Ruckdeschel JC, Chang P, Martin RG, et al. Radiationrelated pericardial effusions in patients with Hodgkin’s disease. Medicine (Baltimore). 1975;54:245-259.
- Kumar PP. Pericardial injury from mediastinal irradiation. J Natl Med Assoc. 1980;72:591-594.
- Cameron J, Oesterle SN, Baldwin JC, Hancock EW. The etiologic spectrum of constrictive pericarditis. Am Heart J. 1987;113:354-360.
- Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100:1380-1386.
- Talreja DR, Edwards WD, Danielson GK, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 2003;108:1852-1857.
- Greenwood RD, Rosenthal A, Cassady R, et al. Constrictive pericarditis in childhood due to mediastinal irradiation. Circulation. 1974;50:1033-1039.
- Applefeld MM, Cole JF, Pollock SH, et al. The late appearance of chronic pericardial disease in patients treated by radiotherapy for Hodgkin’s disease. Ann Intern Med. 1981;94:338-341.
- Kane GC, Edie RN, Mannion JD. Delayed appearance of effusive-constrictive pericarditis after radiation for Hodgkin lymphoma. Ann Intern Med. 1996;124: 534-535.
- Barbetakis N, Xenikakis T, Paliouras D, et al. Pericardiectomy for radiation-induced constrictive pericarditis. Hellenic J Cardiol. 2010;51:214-218.
- George TJ, Arnaoutakis GJ, Beaty CA, et al. Contemporary etiologies, risk factors, and outcomes after pericardiectomy. Ann Thorac Surg. 2012;94:445-451.
- O H-Icí D, Garot J. Radiation-induced heart disease. Circ Heart Fail. 2011;4:e1-e2.
- Tsai HR, Gjesdal O, Wethal T, et al. Left ventricular function assessed by two-dimensional speckle tracking echocardiography in long-term survivors of Hodgkin’s lymphoma treated by mediastinal radiotherapy with or without anthracycline therapy. Am J Cardiol. 2011;107:472-477.
- Kushwaha SS, Fallon JT, Fuster V. Restrictive cardiomyopathy. N Engl J Med. 1997;336:267-276.
- Heidenreich PA, Hancock SL, Vagelos RH, et al. Diastolic dysfunction after mediastinal irradiation. Am Heart J. 2005;150:977-982.
- Billingham ME, Bristow MR, Glatstein E, et al. Adriamycin cardiotoxicity: endomyocardial biopsy evidence of enhancement by irradiation. Am J Surg Pathol. 1977;1:17-23.
- Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878-1886.
- Shapiro CL, Hardenbergh PH, Gelman R, et al. Cardiac effects of adjuvant doxorubicin and radiation therapy in breast cancer patients. J Clin Oncol. 1998;16:3493-3501.
- Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
- Uriel N, Vainrib A, Jorde UP, et al. Mediastinal radiation and adverse outcomes after heart transplantation. J Heart Lung Transplant. 2010;29:378-381.
- Handa N, McGregor CG, Daly RC, et al. Heart transplantation for radiation-associated end-stage heart failure. Transpl Int. 2000;13:162-165.
- Heidenreich PA, Hancock SL, Lee BK, et al. Asymptomatic cardiac disease following mediastinal irradiation. J Am Coll Cardiol. 2003;42:743-749.
- Adabag AS, Dykoski R, Ward H, Anand IS. Critical stenosis of aortic and mitral valves after mediastinal irradiation. Catheter Cardiovasc Interv. 2004;63:247-250.
- Carlson RG, Mayfield WR, Normann S, Alexander JA. Radiation-induced valvular disease. Chest. 1991; 99:538-545.
- Cella L, Liuzzi R, Conson M, et al. Dosimetric predictors of asymptomatic heart valvular dysfunction following mediastinal irradiation for Hodgkin’s lymphoma. Radiother Oncol. 2011;101:316-321.
- Bose AS, Shetty V, Sadiq A, et al. Radiation induced cardiac valve disease in a man from Chernobyl. J Am Soc Echocardiogr. 2009;22:973.e1-3.
- Bruhl SR, Sheikh M, Adlakha S, et al. Endovascular therapy for radiation-induced pulmonary artery stenosis: a case report and review of the literature. Heart Lung. 2012;41:87-89.
- Janelle GM, Mnookin SC, Thomas JJ, et al. Surgical approach for a patient with aortic stenosis and a frozen mediastinum. J Cardiothorac Vasc Anesth. 2003;17:770-772.
- Handa N, McGregor CG, Danielson GK, et al. Valvular heart operation in patients with previous mediastinal radiation therapy. Ann Thorac Surg. 2001;71: 1880-1884.
- Crestanello JA, McGregor CG, Danielson GK, et al. Mitral and tricuspid valve repair in patients with previous mediastinal radiation therapy. Ann Thorac Surg. 2004;78:826-831.
- Veeragandham RS, Goldin MD. Surgical management of radiation-induced heart disease. Ann Thorac Surg.
- Latib A, Montorfano M, Figini F, et al. Percutaneous valve replacement in a young adult for radiation- induced aortic stenosis. J Cardiovasc Med. 2012;13:397-398.
- Letsas KP, Korantzopoulos P, Evangelou D, et al. Acute myocardial infarction with normal coronary arteries in a patient with Hodgkin’s disease: a late complication of irradiation and chemotherapy. Tex Heart Inst J. 2006;33:512-514.
- Annest LS, Anderson RP, Li W, Hafermann MD. Coronary artery disease following mediastinal radiation therapy. J Thorac Cardiovasc Surg. 1983;85: 257-263.
- Nilsson G, Holmberg L, Garmo H, et al. Distribution of coronary artery stenosis after radiation for breast cancer. J Clin Oncol. 2012;30:380-386.
- Heidenreich PA, Schnittger I, Strauss HW, et al. Screening for coronary artery disease after mediastinal irradiation for Hodgkin’s disease. J Clin Oncol. 2007;25:43-49.
- Santoro F, Ferraretti A, Centola A, et al. Early clinical presentation of diffuse, severe, multi-district atherosclerosis after radiation therapy for Hodgkin lymphoma. Int J Cardiol. 2013;165:373-374.
- Orzan F, Brusca A, Conte MR, et al. Severe coronary artery disease after radiation therapy of the chest and mediastinum: clinical presentation and treatment. Br Heart J. 1993;69:496-500.
- Schomig K, Ndrepepa G, Mehilli J, et al. Thoracic radiotherapy in patients with lymphoma and restenosis after coronary stent placement. Catheter Cardiovasc Interv. 2007;70:359-365.
- Waksman R, Ajani AE, White RL, et al. Five-year follow-up after intracoronary gamma radiation therapy for in-stent restenosis. Circulation. 2004;109: 340-344.
- Wiedermann JG, Marboe C, Amols H, et al. Intracoronary irradiation markedly reduces restenosis after balloon angioplasty in a porcine model. J Am Coll Cardiol. 1994;23:1491-1498.
- Ellis GR, Penny WJ. Hibernating myocardium caused by isolated, radiation induced left main stem coronary artery stenosis. Heart. 1997;78:419-420.
- Seddon B, Cook A, Gothard L, et al. Detection of defects in myocardial perfusion imaging in patients with early breast cancer treated with radiotherapy. Radiother Oncol. 2002;64:53-63.
- Marks LB, Yu X, Prosnitz RG, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63:214-223.
- Andersen R, Wethal T, Günther A, et al. Relation of coronary artery calcium score to premature coronary artery disease in survivors >15 years of Hodgkin’s lymphoma. Am J Cardiol. 2010;105:149-152.
- Kupeli S, Hazirolan T, Varan A, et al. Evaluation of coronary artery disease by computed tomography angiography in patients treated for childhood Hodgkin’s lymphoma. J Clin Oncol. 2010;28:1025-1030.
- Rademaker J, Schoder H, Ariaratnam NS, et al. Coronary artery disease after radiation therapy for Hodgkin’s lymphoma: coronary CT angiography findings and calcium scores in nine asymptomatic patients. Am J Roentgenol. 2008;191:32-37.
- Wright RS, Anderson JL, Adams CD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;57:e215-367.
- Gibbons RJ, Abrams J, Chatterjee K, et al; American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina). ACC/AHA 2002 guideline update for the management of patients with chronic stable angina— summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina). J Am Coll Cardiol. 2003;41:159-168.
- Salemi VM, Dabarian AL, Nastari L, et al. Treatment of left main coronary artery lesion after late thoracic radiotherapy. Arq Bras Cardiol. 2011;97:e53-e55.
- Aqel RA, Lloyd SG, Gupta H, Zoghbi GJ. Three-vessel coronary artery disease, aortic stenosis, and constrictive pericarditis 27 years after chest radiation therapy: a case report. Heart Surg Forum. 2006;9:E728-E730.
- Khan MH, Ettinger SM. Post mediastinal radiation coronary artery disease and its effects on arterial conduits. Cathet Cardiovasc Interv. 2001;52:242-248.
- Gansera B, Schmidtler F, Angelis I, et al. Quality of internal thoracic artery grafts after mediastinal irradiation. Ann Thorac Surg. 2007;84:1479-1484.
- Handler CE, Livesey S, Lawton PA. Coronary ostial stenosis after radiotherapy: angioplasty or coronary artery surgery? Br Heart J. 1989;61:208-211.
- Patel MR, Dehmer GJ, Hirshfeld JW, et al. ACCF/ SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 Appropriate use criteria for coronary revascularization focused update: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2012;59:857-881.
- Slama MS, Le Guludec D, Sebag C, et al. Complete atrioventricular block following mediastinal irradiation: a report of six cases. Pacing Clin Electrophysiol. 1991;14:1112-1118.
- de Waard DE, Verhorst PM, Visser CA. Exerciseinduced syncope as late consequence of radiotherapy. Int J Cardiol. 1996;57:289-291.
- Orzan F, Brusca A, Gaita F, et al. Associated cardiac lesions in patients with radiation-induced complete heart block. Int J Cardiol. 1993;39:151-156.
- Cohen SI, Bharati S, Glass J, Lev M. Radiotherapy as a cause of complete atrioventricular block in Hodgkin’s disease. An electrophysiological-pathological correlation. Arch Intern Med. 1981;141:676-679.
- Santoro F, Ieva R, Lupo P, et al. Late calcification of the mitral-aortic junction causing transient complete atrio-ventricular block after mediastinal radiation of Hodgkin lymphoma: multimodal visualization. Int J Cardiol. 2012;155:e49-e50.
- Larsen RL, Jakacki RI, Vetter VL, et al. Electrocardiographic changes and arrhythmias after cancer therapy in children and young adults. Am J Cardiol. 1992;70:73-77.
- Moulder JE, Fish BL, Cohen EP. Radiation nephropathy is treatable with an angiotensin converting enzyme inhibitor or an angiotensin II type-1 (AT1) receptor antagonist. Radiother Oncol. 1998;46:307-315.
- Kohl RR, Kolozsvary A, Brown SL, et al. Differential radiation effect in tumor and normal tissue after treatment with ramipril, an angiotensin-converting enzyme inhibitor. Radiat Res. 2007;168:440-445.
- Kharofa J, Cohen EP, Tomic R, et al. Decreased risk of radiation pneumonitis with incidental concurrent use of angiotensin-converting enzyme inhibitors and thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84:238-243.
- Nellessen U, Zingel M, Hecker H, et al. Effects of radiation therapy on myocardial cell integrity and pump function: which role for cardiac biomarkers? Chemotherapy. 2010;56:147-152.
- van Leeuwen-Segarceanu EM, Bos WJ, Dorresteijn LD, et al. Screening Hodgkin lymphoma survivors for radiotherapy induced cardiovascular disease. Cancer Treat Rev. 2011;37:391-403.
- Wethal T, Lund MB, Edvardsen T, et al. Valvular dysfunction and left ventricular changes in Hodgkin’s lymphoma survivors. A longitudinal study. Br J Cancer. 2009;101:575-581.
- Topolnjak R, Borst GR, Nijkamp J, Sonke JJ. Imageguided radiotherapy for left-sided breast cancer patients: geometrical uncertainty of the heart. Int J Radiat Oncol Biol Phys. 2012;82:e647-e655.
- International Bureau of Weights and Measures. United States National Institute of Standards and Technology. The International System of Units (SI). NIST Special Publication 330. Taylor BN, Thompson A, eds. Gaithersburg, MD: National Institute of Standards and Technology; 2008.