Acute and Chronic Cardiovascular Effects of Hyperkalemia: New Insights Into Prevention and Clinical Management
Peter A. McCullough, MD, MPH, FACC, FACP, FAHA, FCCP, FNKF,1 Thomas M. Beaver, MD, MPH,2 Elliott Bennett-Guerrero, MD,3 Michael Emmett, MD, FASN,4 Gregg C. Fonarow, MD, FACC,5 Abhinav Goyal, MD, MHS, FACC,6 Charles A. Herzog, MD, FACC, FAHA,7 Mikhail Kosiborod, MD, FACC, FAHA,8 Biff F. Palmer, MD, FASN9
1Baylor University Medical Center, Baylor Heart and Vascular Institute, Baylor Jack and Jane Hamilton Heart and Vascular Hospital, Dallas, TX, and The Heart Hospital, Plano, TX; 2University of Florida College of Medicine, Gainesville, FL; 3Duke Clinical Research Institute, Durham, NC; 4Baylor University Medical Center, Dallas, TX; 5Ahmanson-UCLA Cardiomyopathy Center, Los Angeles, CA; 6Emory University School of Medicine, Atlanta, GA; 7Hennepin County Medical Center, University of Minnesota, Minneapolis, MN; 8Mid-America Heart Institute, St. Lukes Hospital, University of Missouri-Kansas City School of Medicine, Kansas City, MO; 9University of Texas Southwestern Medical Center, Dallas, TX
The plasma pool of potassium is a partial reflection of the overall body, transient cellular shifts, and potassium elimination regulated by the kidneys. Potassium concentrations elevating above the upper limit of normal (> 5.0 mEq/L) have become more common in cardiovascular practice due to the growing population of patients with chronic kidney disease and the broad applications of drugs that modulate potassium excretion by either reducing production of angiotensin II (angiotensin-converting enzyme inhibitors, direct renin inhibitors, beta-adrenergic receptor antagonists), blocking angiotensin II receptors (angiotensin receptor blockers), or antagonizing the action of aldosterone on mineralocorticoid receptors (mineralocorticoid receptor antagonists). In addition, acute kidney injury, critical illness, crush injuries, and massive red blood cell transfusions can result in hyperkalemia. Progressively more severe elevations in potassium are responsible for abnormalities in cardiac depolarization and repolarization and contractility. Untreated severe hyperkalemia results in sudden cardiac death. Traditional management steps have included reducing dietary potassium and discontinuing potassium supplements; withdrawal of exacerbating drugs; acute treatment with intravenous calcium gluconate, insulin, and glucose; nebulized albuterol; correction of acidosis with sodium bicarbonate for short-term shifts out of the plasma pool; and, finally, gastrointestinal ion exchange with oral sodium polystyrene sulfonate in sorbitol, which is mainly used in the hospital and is poorly tolerated due to gastrointestinal adverse effects. This review explores hyperkalemia as a complication in cardiovascular patients and highlights new acute, chronic, and preventative oral therapies (patiromer calcium, cross-linked polyelectrolyte, ZS-9) that could potentially create a greater margin of safety for vulnerable patients with combined heart and kidney disease.
[Rev Cardiovasc Med. 2014;15(1):11-23 doi:10.3909/ricm0727]
© 2014 MedReviews®, LLC
Acute and Chronic Cardiovascular Effects of Hyperkalemia: New Insights Into Prevention and Clinical Management
Peter A. McCullough, MD, MPH, FACC, FACP, FAHA, FCCP, FNKF,1 Thomas M. Beaver, MD, MPH,2 Elliott Bennett-Guerrero, MD,3 Michael Emmett, MD, FASN,4 Gregg C. Fonarow, MD, FACC,5 Abhinav Goyal, MD, MHS, FACC,6 Charles A. Herzog, MD, FACC, FAHA,7 Mikhail Kosiborod, MD, FACC, FAHA,8 Biff F. Palmer, MD, FASN9
1Baylor University Medical Center, Baylor Heart and Vascular Institute, Baylor Jack and Jane Hamilton Heart and Vascular Hospital, Dallas, TX, and The Heart Hospital, Plano, TX; 2University of Florida College of Medicine, Gainesville, FL; 3Duke Clinical Research Institute, Durham, NC; 4Baylor University Medical Center, Dallas, TX; 5Ahmanson-UCLA Cardiomyopathy Center, Los Angeles, CA; 6Emory University School of Medicine, Atlanta, GA; 7Hennepin County Medical Center, University of Minnesota, Minneapolis, MN; 8Mid-America Heart Institute, St. Lukes Hospital, University of Missouri-Kansas City School of Medicine, Kansas City, MO; 9University of Texas Southwestern Medical Center, Dallas, TX
The plasma pool of potassium is a partial reflection of the overall body, transient cellular shifts, and potassium elimination regulated by the kidneys. Potassium concentrations elevating above the upper limit of normal (> 5.0 mEq/L) have become more common in cardiovascular practice due to the growing population of patients with chronic kidney disease and the broad applications of drugs that modulate potassium excretion by either reducing production of angiotensin II (angiotensin-converting enzyme inhibitors, direct renin inhibitors, beta-adrenergic receptor antagonists), blocking angiotensin II receptors (angiotensin receptor blockers), or antagonizing the action of aldosterone on mineralocorticoid receptors (mineralocorticoid receptor antagonists). In addition, acute kidney injury, critical illness, crush injuries, and massive red blood cell transfusions can result in hyperkalemia. Progressively more severe elevations in potassium are responsible for abnormalities in cardiac depolarization and repolarization and contractility. Untreated severe hyperkalemia results in sudden cardiac death. Traditional management steps have included reducing dietary potassium and discontinuing potassium supplements; withdrawal of exacerbating drugs; acute treatment with intravenous calcium gluconate, insulin, and glucose; nebulized albuterol; correction of acidosis with sodium bicarbonate for short-term shifts out of the plasma pool; and, finally, gastrointestinal ion exchange with oral sodium polystyrene sulfonate in sorbitol, which is mainly used in the hospital and is poorly tolerated due to gastrointestinal adverse effects. This review explores hyperkalemia as a complication in cardiovascular patients and highlights new acute, chronic, and preventative oral therapies (patiromer calcium, cross-linked polyelectrolyte, ZS-9) that could potentially create a greater margin of safety for vulnerable patients with combined heart and kidney disease.
[Rev Cardiovasc Med. 2014;15(1):11-23 doi:10.3909/ricm0727]
© 2014 MedReviews®, LLC
Acute and Chronic Cardiovascular Effects of Hyperkalemia: New Insights Into Prevention and Clinical Management
Peter A. McCullough, MD, MPH, FACC, FACP, FAHA, FCCP, FNKF,1 Thomas M. Beaver, MD, MPH,2 Elliott Bennett-Guerrero, MD,3 Michael Emmett, MD, FASN,4 Gregg C. Fonarow, MD, FACC,5 Abhinav Goyal, MD, MHS, FACC,6 Charles A. Herzog, MD, FACC, FAHA,7 Mikhail Kosiborod, MD, FACC, FAHA,8 Biff F. Palmer, MD, FASN9
1Baylor University Medical Center, Baylor Heart and Vascular Institute, Baylor Jack and Jane Hamilton Heart and Vascular Hospital, Dallas, TX, and The Heart Hospital, Plano, TX; 2University of Florida College of Medicine, Gainesville, FL; 3Duke Clinical Research Institute, Durham, NC; 4Baylor University Medical Center, Dallas, TX; 5Ahmanson-UCLA Cardiomyopathy Center, Los Angeles, CA; 6Emory University School of Medicine, Atlanta, GA; 7Hennepin County Medical Center, University of Minnesota, Minneapolis, MN; 8Mid-America Heart Institute, St. Lukes Hospital, University of Missouri-Kansas City School of Medicine, Kansas City, MO; 9University of Texas Southwestern Medical Center, Dallas, TX
The plasma pool of potassium is a partial reflection of the overall body, transient cellular shifts, and potassium elimination regulated by the kidneys. Potassium concentrations elevating above the upper limit of normal (> 5.0 mEq/L) have become more common in cardiovascular practice due to the growing population of patients with chronic kidney disease and the broad applications of drugs that modulate potassium excretion by either reducing production of angiotensin II (angiotensin-converting enzyme inhibitors, direct renin inhibitors, beta-adrenergic receptor antagonists), blocking angiotensin II receptors (angiotensin receptor blockers), or antagonizing the action of aldosterone on mineralocorticoid receptors (mineralocorticoid receptor antagonists). In addition, acute kidney injury, critical illness, crush injuries, and massive red blood cell transfusions can result in hyperkalemia. Progressively more severe elevations in potassium are responsible for abnormalities in cardiac depolarization and repolarization and contractility. Untreated severe hyperkalemia results in sudden cardiac death. Traditional management steps have included reducing dietary potassium and discontinuing potassium supplements; withdrawal of exacerbating drugs; acute treatment with intravenous calcium gluconate, insulin, and glucose; nebulized albuterol; correction of acidosis with sodium bicarbonate for short-term shifts out of the plasma pool; and, finally, gastrointestinal ion exchange with oral sodium polystyrene sulfonate in sorbitol, which is mainly used in the hospital and is poorly tolerated due to gastrointestinal adverse effects. This review explores hyperkalemia as a complication in cardiovascular patients and highlights new acute, chronic, and preventative oral therapies (patiromer calcium, cross-linked polyelectrolyte, ZS-9) that could potentially create a greater margin of safety for vulnerable patients with combined heart and kidney disease.
[Rev Cardiovasc Med. 2014;15(1):11-23 doi:10.3909/ricm0727]
© 2014 MedReviews®, LLC
KEY WORDS
Hyperkalemia • Potassium concentration • Chronic kidney disease • Cardiovascular effects of kidney disease
KEY WORDS
Hyperkalemia • Potassium concentration • Chronic kidney disease • Cardiovascular effects of kidney disease
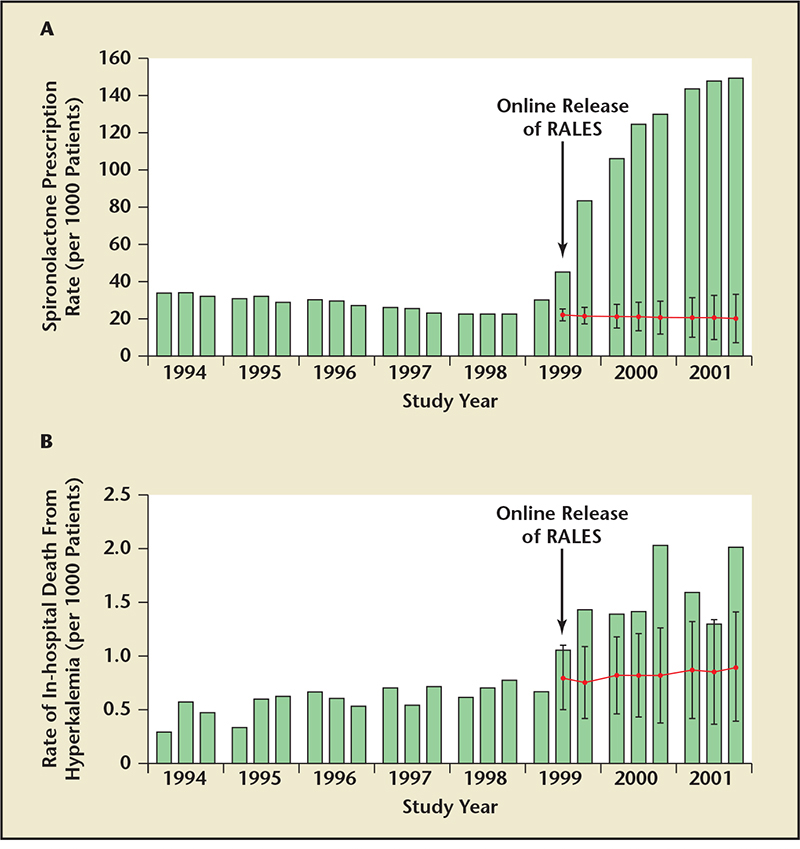
Figure 1. Frequency of spironolactone prescriptions in Canada increased from , 40/1000 to .140/1000 -during the years after publication of the Randomized Aldactone Evaluation Study (RALES) study in 1999
(A). This was associated with a rise in the rates of hospitalization for hyperkalemia from 2.4 to 11.0/1000 patients
(B). Reproduced with permission from Juurlink DN et al.12
Data suggest that there may be a narrow therapeutic window to lower serum potassium in those with emerging hyperkalemia in the setting of AMI.
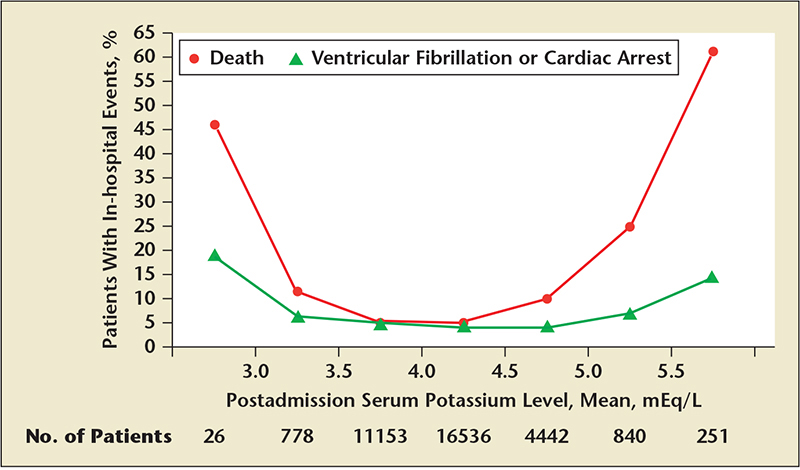
Figure 2. Independent association with ventricular fibrillation and death at both the lower and the upper ranges.Each x-axis interval is equal to or greater than the lower limit of the interval and less than the upper limit. The first interval includes all serum potassium levels less than 3.0 mEq/L; the last interval includes all levels equal to or greater than 5.5 mEq/L. Reproduced with permission from Goyal A et al.25

Figure 2. Independent association with ventricular fibrillation and death at both the lower and the upper ranges.Each x-axis interval is equal to or greater than the lower limit of the interval and less than the upper limit. The first interval includes all serum potassium levels less than 3.0 mEq/L; the last interval includes all levels equal to or greater than 5.5 mEq/L. Reproduced with permission from Goyal A et al.25

Figure 3. Key principles in the development of hyperkalemia. Reproduced with permission from Palmer BF.50

Figure 4. Diagram of channels regulating the movement of ions, including potassium, during the action potential and myocardial contraction. Reproduced with permission from Paulev PE, Zubieta-Callej G. Cardiac action potentials and arrhythmias. In: New Human Physiology, 2nd ed. Copenhagen: University of Copenhagen; 2004.
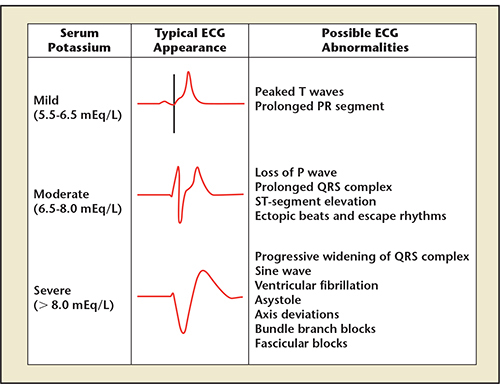
Figure 5. Extracellular concentrations of potassium result in the classic electrocardiographic (ECG) changes. Reproduced from ECG images in hyperkalemia. Hyperkalemia Blog Web site. http://hyperkalemiablog.blogspot.com/2013_12_01_archive.html. Accessed February 20, 2014.

Figure 5. Extracellular concentrations of potassium result in the classic electrocardiographic (ECG) changes. Reproduced from ECG images in hyperkalemia. Hyperkalemia Blog Web site. http://hyperkalemiablog.blogspot.com/2013_12_01_archive.html. Accessed February 20, 2014.
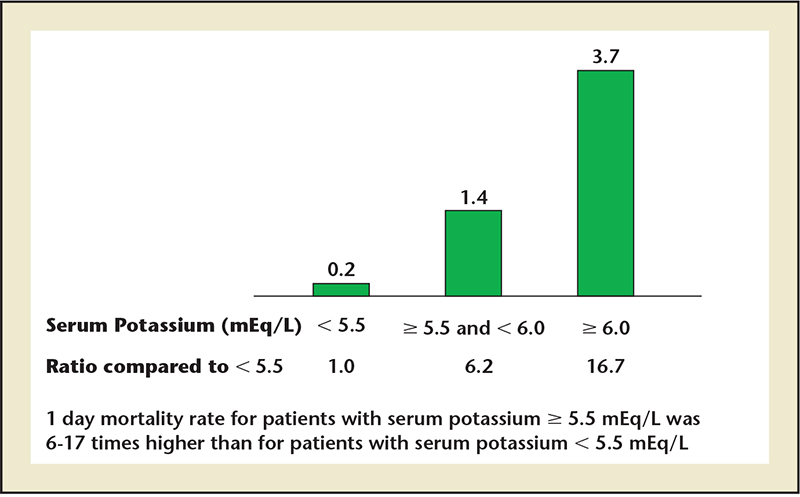
Figure 6. Percentage mortality rate within 1 day of hyperkalemic event. Data from Einhorn LM et al.37
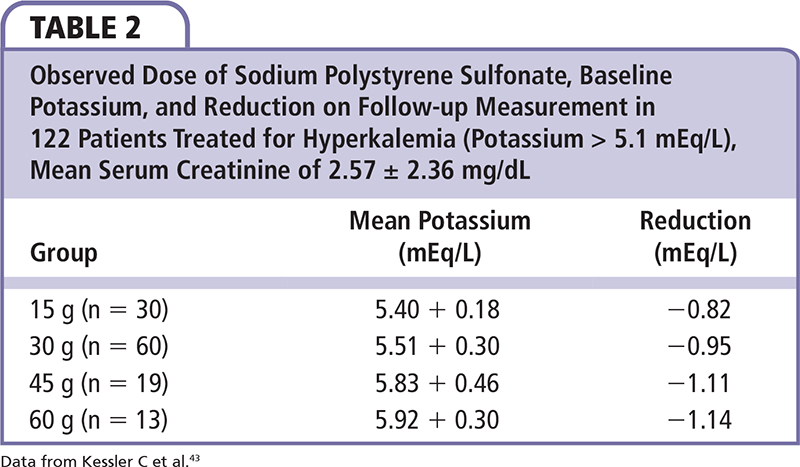

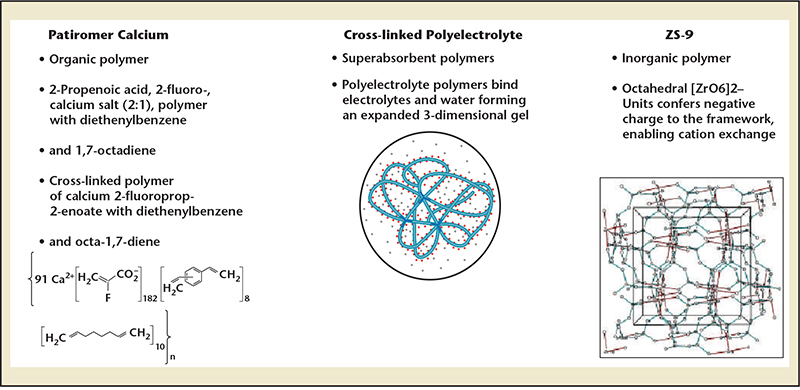
Figure 7. Novel treatments in development for the treatment of both acute and chronic hyperkalemia. Data from Ash SR.49
Vigilance with laboratory monitoring is critical to diagnose hyperkalemia because ECG changes are unreliable, particularly in those with CKD.
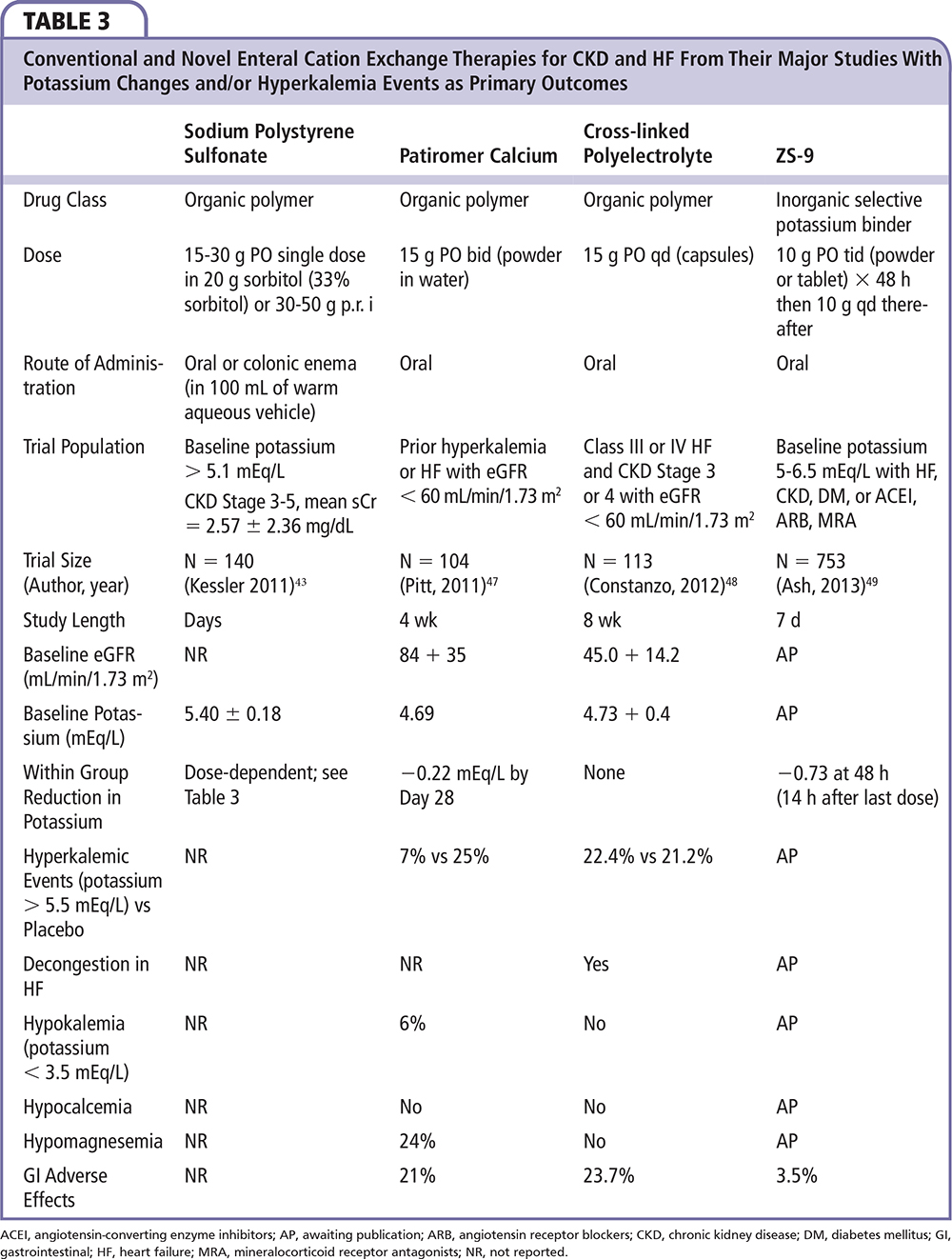

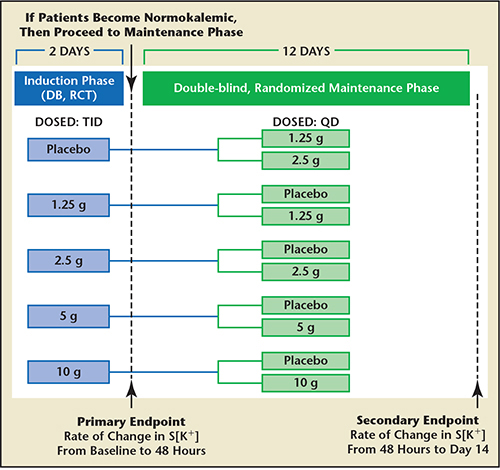
Figure 8. Study design for the double-blind, placebo-controlled ZS-003 trial. A total of 753 patients with hyperkalemia (potassium levels 5.0-.5 mEq/L), including patients with chronic kidney disease, heart failure, diabetes, and those on ACEI, ARB, or MRA, were randomized to receive one of four doses of ZS-9 (1.25 g, 2.5 g, 5 g, or 10 g) or placebo, administered three times daily for the initial 48 hours (acute phase). S[K+], serum potassium. Data from Ash SR.49
Main Points
• Potassium concentrations elevating above the upper limit of normal (> 5.0 mEq/L) have become more common in cardiovascular practice due to the growing population of patients with chronic kidney disease and the broad applications of drugs that modulate potassium excretion by either reducing production of angiotensin II (angiotensin-converting enzyme inhibitors, direct renin inhibitors, beta-adrenergic receptor antagonists), blocking angiotensin II receptors (angiotensin receptor blockers), or antagonizing the action of aldosterone on mineralocorticoid receptors (mineralocorticoid receptor antagonists).
• Progressively more severe elevations in potassium are responsible for abnormalities in cardiac depolarization and repolarization and contractility. Untreated severe hyperkalemia results in sudden cardiac death.
• Traditional management steps have included reducing dietary potassium and discontinuing potassium supplements; withdrawal of exacerbating drugs; acute treatment with intravenous calcium gluconate, insulin, and glucose; nebulized albuterol; correction of acidosis with sodium bicarbonate for short-term shifts out of the plasma pool; and, finally, gastrointestinal ion exchange with oral sodium polystyrene sulfonate in sorbitol, which is mainly used in the hospital and is poorly tolerated due to gastrointestinal adverse effects.
• New acute, chronic, and preventative oral therapies (patiromer calcium, cross-linked polyelectrolyte, ZS-9) could potentially create a greater margin of safety for vulnerable patients with combined heart and kidney disease.
Main Points
• Potassium concentrations elevating above the upper limit of normal (> 5.0 mEq/L) have become more common in cardiovascular practice due to the growing population of patients with chronic kidney disease and the broad applications of drugs that modulate potassium excretion by either reducing production of angiotensin II (angiotensin-converting enzyme inhibitors, direct renin inhibitors, beta-adrenergic receptor antagonists), blocking angiotensin II receptors (angiotensin receptor blockers), or antagonizing the action of aldosterone on mineralocorticoid receptors (mineralocorticoid receptor antagonists).
• Progressively more severe elevations in potassium are responsible for abnormalities in cardiac depolarization and repolarization and contractility. Untreated severe hyperkalemia results in sudden cardiac death.
• Traditional management steps have included reducing dietary potassium and discontinuing potassium supplements; withdrawal of exacerbating drugs; acute treatment with intravenous calcium gluconate, insulin, and glucose; nebulized albuterol; correction of acidosis with sodium bicarbonate for short-term shifts out of the plasma pool; and, finally, gastrointestinal ion exchange with oral sodium polystyrene sulfonate in sorbitol, which is mainly used in the hospital and is poorly tolerated due to gastrointestinal adverse effects.
• New acute, chronic, and preventative oral therapies (patiromer calcium, cross-linked polyelectrolyte, ZS-9) could potentially create a greater margin of safety for vulnerable patients with combined heart and kidney disease.
There are approximately 500,000 to 900,000 individuals in the United States with stage 4 chronic kidney disease (CKD), with an estimated glomerular filtration rate (eGFR) of 15 to 29 mL/min/ 1.73 m2, and who move in a relatively short period of time into stage 5 CKD (eGFR < 15 mL/min/ 1.73 m2).1 It is well recognized that CKD is prevalent among patients with heart disease and that CKD increases the risks and consequences of all major categories of cardiovascular illness including coronary artery disease, cardiomyopathy, valvular disease, and arrhythmias.2 Retention of potassium due to a decrease in renal elimination is somewhat predictable at an eGFR < 10/min/1.73 m2.3 At this decreased level of renal filtration, average dietary intake (120 mEq/day) commonly exceeds renal clearance, and therefore, the mainstay of management is further reductions in dietary intake including all forms of food and dietary potassium supplements (salt substitutes) down to a target of 40 mEq/day.4 In clinical practice, approximately ∽ 60% of patients with stage 5 CKD are under the care of a nephrologist and are amenable to educational efforts, including dietary counseling, in preparation for chronic renal replacement therapy.5 However, those who present late to a nephrologist are at higher risk for poorly controlled metabolic abnormalities including hyperkalemia.6 In contrast, there are approximately 15.5 million Americans with stage 3 CKD (eGFR 30-59 mL/min/1.73 m2), most of whom are actively treated with agents to slow the progression of CKD, including angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB).1 Additionally, patients with myocardial disease or heart failure (HF) are often treated with ACEI or ARB, mineralocorticoid receptor antagonists (MRA), beta-adrenergic antagonists, and in many cases, combinations of these agents. These agents, which are associated with chronic hyperkalemia, also elevate the risk of acute hyperkalemia if there is an inciting event or rapid reduction in eGFR. Observational data have suggested the time period from initiation of one or more of these agents and the onset of hyperkalemia is approximately 1 week, but is clearly dependent on the baseline serum creatinine and potassium concentrations.7 It is somewhat unclear in terms of understanding the burden of hyperkalemia in this broader population of patients who could be considered to have a type 4 cardiorenal syndrome that is CKD and is contributing to or worsening chronic HF.8
Since 1999, the mandate for use of MRAs (eg, spironolactone, eplerenone) in HF has become intensified with a bevy of large clinical trials demonstrating benefit in severe HF with reduced ejection fraction followed by left ventricular dysfunction postmyocardial infarction and less severe HF with reduced ejection fraction.9-11 Juurlink and colleagues reported that the frequency of spironolactone prescriptions in Canada increased from < 40/1000 to > 140/1000 during the years after publication of the Randomized Aldactone Evaluation Study (RALES) study in 1999 (Figure 1); however, this was associated with a rise in the rate of hospitalization for hyperkalemia from 2.4 to 11.0/1000 patients (Figure 1).12 Other observational studies suggest that although baseline creatinine and potassium are present in medical records following hospitalization and initiation on MRA therapy, a large proportion of patients did not have follow-up measurements of potassium concentration within an appropriate timeframe (recommended at 3 and 7 days in current guidelines), and, hence, are at risk for presentation of hyperkalemia in the days to weeks after initiation of therapy.13 Multiple analyses have found that, despite the higher risks of hyperkalemia at lower eGFR, patients with more severe CKD in these trials benefit from MRA therapy.14,15 However, fear about hyperkalemia has limited the use of MRA therapy despite strong evidence for benefit in terms of reduction in hospitalization and death.16-18 Furthermore, a growing body of literature has demonstrated that dosing of ACEIs, ARBs, and MRAs are frequently not optimized in patients with HF in the clinical setting, often due to concerns about renal function and hyperkalemia.19-22 This is partially due to guidelines that recommend MRA initiation as a class I indication only if serum creatinine ≤ 2.5 mg/dL or ≤ 2.0 mg/dL (or eGFR > 30 mL/min/1.73 m2) in men and women, respectively, and potassium < 5.0 mEq/L.23 Conversely, the guidelines advise against the use of MRA when these parameters are not met (class III recommendation) given the potential harms of hyperkalemia as demonstrated in cohorts of patients where MRAs have been initiated.24
There is an emerging epidemiology concerning presentation potassium concentrations in the setting of acute myocardial infarction (AMI). Health Facts database indicates that among 38,689 patients with confirmed AMI, 2094 (5.4%) and 1369 (3.5%) presented with admission potassium levels of 5.0 to < 5.5 mEq/L and ≥ 5.5 mEq/L, respectively. The mean eGFR in these groups were 45.2 and 34.7 mL/min/1.73 m2, respectively, and 121 (5.8%) and 158 (11.5%) were receiving renal replacement therapy prior to admission.25 Potassium was found to have a Gaussian distribution on admission, and mean postadmission potassium levels conferred an independent association with ventricular fibrillation and death at both the lower and the upper ranges (Figure 2). A mean postadmission serum potassium ≥ 5.5 mEq/L during the hospitalization was associated with death in > 60% of patients, corresponding to a 12-fold risk compared with a serum potassium level between 3.5 to 4.5 mEq/L. Upon analysis of admission potassium (vs mean postadmission potassium), this U-shaped relationship was confirmed and further strengthens the potential causality interpretation. Whereas some of this association could be ascribed to the well-known impact of CKD on myocardial disease, even after adjustment for eGFR and the development of acute kidney injury, elevated potassium was associated with worsened out-comes. These data suggest that there may be a narrow therapeutic window to lower serum potassium in those with emerging hyperkalemia in the setting of AMI. Furthermore, even potassium concentrations between 4.5 and 5.0 mEq/L (which are within the “acceptable” 4.0-5.0 mEq/L target range endorsed by some guidelines) were associated with a twofold increased risk of in-hospital mortality compared with patients with potassium concentrations between 3.5 and 4.5 mEq/L.26,27 This twofold increased risk was not attenuated even after multivariable adjustment for patient- and hospital-level factors. Thus, patients with AMI may have a narrower window of optimal potassium concentration, and levels above or below appear to be associated with increased risk for arrhythmias and death.
Pathophysiology
Potassium is the most abundant cation in the human body, with 98% intracellular (140 mEq/L) and 2% extracellular (3.8-5.0 mEq/L). The pathophysiology of hyperkalemic states is complicated and involves dietary and supplemental intake, neurohumoral systems, acid-base balance, and, most importantly, function of the principal cell in the collecting duct of the kidney. For the sake of this review, which is oriented toward cardiologists, we will not cover the relatively rare syndromes of hyporeninemic hypoaldosteronism, pseudohypoal-dosteronism I and II (Gordon's syndrome), and adrenal insufficiency. Instead, we focus on the predialysis CKD patient who has either chronic or acute cardiovascular disease, most commonly myocardial dysfunction. There are several key principles in the development of hyperkalemia in these patients (Figure 3). Any known cause of a reduction in the secretion of renin can begin a cascade of biochemical events worsened by ACEIs, ARBs, and MRAs, ultimately leading to less angiotensin II stimulation of the zona glomerulosa cells within the adrenal glands, and reduced production and circulation of aldosterone. The principal cell in the collecting duct is the major regulator of urinary potassium excretion and the epithelial sodium channel (ENaC) located on its luminal surface recovers sodium from the urine and under normal conditions leads to the lumen-negative potential essential for potassium and proton secretion. Aldosterone is the most important stimulus to the principal cell via MRA receptors and signal transduction to ENaC resulting in sodium reabsorption and to renal outer medullary potassium channels (ROMK) signaling potassium excretion into urine, the net result being sodium reabsorption and potassium excretion. Thus, in patients with CKD, which is a proxy for fewer principal cells, reducing aldosterone activity on MRA by any means can result in a failure to excrete potassium and hyperkalemia. A particularly high-risk subgroup is patients with type 1 diabetes and distal type 4 renal tubular acidosis. In these patients, potassium is shifted out of cells in the systemic circulation and, at the same time, the principal cells in the collecting duct are sensing low sodium delivery and, in the setting of ACEI, ARB, MRA, are at very high risk, over a period as little as a few days, for the development of severe hyperkalemia after initiation of one of these agents.28 As shown in Figure 3, multiple drugs have been implicated in the development of both acute and chronic hyperkalemia. Furthermore, because loop diuretics create more availability of urinary sodium at the level of the principal cell, aldosterone has a relatively magnified impact in terms of sodium resorption and potassium loss. Hence, loop diuretics tend to mask a tendency toward hyperkalemia and when reduced in dose or discontinued, elevations in potassium may manifest.
All myocytes in the body are dependent on extracellular potassium for repolarization. Conduction system cells have a greater reliance on extracellular potassium along with calcium in both depolarization and repolarization. When serum potassium is elevated to critical levels, normal gradients cannot be maintained across cell membranes, and classic signs and symptoms are observed. For example, hyperkalemia can lead to weakness, with the most dramatic example being hyperkalemic periodic paralysis.29 All cardiac cells have channels regulating the movement of ions, including potassium, during the action potential and myocardial contraction (Figure 4). Nine major potassium channels are listed in Table 1. Of note, the inwardly rectifying (IK1, IK.G IK.Ach, IK.Ade) and delayed rectifier (IKr) currents are most impacted by extracellular concentrations of potassium, and, hence, in response to hyperkalemic conditions, their action can result in the classic electrocardiographic (ECG) changes shown in Figure 5.
The typical ECG findings in hyperkalemia progress from tall, “peaked” T waves with shortened duration and a shortened QT interval, to lengthening PR interval and loss of P waves, and then to widening of the QRS complex culminating in a “sine wave” morphology. With the sine wave morphology, it is important to recognize that either electromechanical failure or ventricular fibrillation is imminent. Unfortunately, depending on the time course of hyperkalemia and the ability of potassium channels to adjust to higher concentrations of extracellular and intracellular potassium, ECG changes are not a reliable indicator of serum potassium levels.30 With adaptation, patients with end-stage renal disease (ESRD) can tolerate higher concentrations of potassium without ECG changes, and this may indicate a relative protection against lethal arrhythmias as a result of hyperkalemia. Montague and coworkers studied 90 patients with potassium > 6.0 mEq/L and simultaneous ECG tracings, and found that only 16 (18%) had classic ECG findings of peaked T waves assessed in lead V4 and in the lead with the greatest R-wave amplitude. To complicate matters, in this study, 43% of subjects had baseline conduction abnormalities, which are common in CKD, and there was poor concordance among clinical readers about peaked T-waves.30 In patients on hemo- or peritoneal dialysis, ECG findings typically occur at much higher serum potassium levels than in those with more preserved renal function. Thus, in a dialysis patient, peaked T waves can be seen, but additional serial shortening in the T-wave duration, loss of P waves, or progressive QRS widening can be harbingers of severe unexpected hyperkalemia.31 In pacemaker patients, hyperkalemic ECG changes can present clinical challenges in the interpretation of paced cardiac rhythms.32 In addition, hyperkalemia can be detected in some cases by changes in lead impedance measured by automatic implantable defibrillators (ICD) giving clinicians a warning of periodic elevations in potassium.33 As expected, hyperkalemia has been the cause of sufficient changes on the intra-cardiac electrogram to trigger ICD defibrillation discharge, primarily by oversensing peaked T waves.34
Clinical Outcomes
The outcomes of patients who are discovered to have periodic or persistent hyperkalemia are consistently poor across the care continuum. In a study from Korea, 1803/282,832 (0.6%) of hospitalizations were associated with hyperkalemia.35 A total of 923 nonhospice cases with complete data were analyzed with a mean age of 61 years, 41% with diabetes, 70% with CKD, and 17% on dialysis. In those patients hospitalized for or with acute hyperkalemia (potassium ≥ 6.5 mEq/L), 40% had hyperkalemia upon presentation and 60% developed it sometime during the hospitalization, with 70% displaying typical ECG changes. The most common presenting symptom was cardiac arrest with asystole or sinus arrest, followed by other arrhythmias, and skeletal muscle weakness. A total of 52% had underlying CKD and 22% had superimposed acute kidney injury (AKI). As a part of resuscitation, 24% had hyperkalemia-causing drugs discontinued and 27% underwent some form of renal replacement therapy. Severe hyperkalemia improved in 715 patients (77.5%), and a total of 283 patients (30.7%) died. Infection, volume depletion, and bleeding were significantly associated with a higher case fatality rate. Furthermore, the development of AKI in patients with normal baseline renal function was a predictor of increased mortality (odds ratio [OR] 5.23; 95% confidence interval [CI], 3.75-7.30; P < .001). In contrast, the mortality rate was lower in patients with AKI superimposed on CKD (OR 0.53; 95% CI, 0.40-0.70; P < .001), suggesting CKD-related adaptation to chronically higher levels of potassium may have been protective. However, Jain and colleagues found that both CKD stage and severity of hyperkalemia were both independently associated with mortality among a large cohort (n = 15,803) treated with cardiovascular drugs.36
Einhorn and colleagues analyzed 66,259 hyperkalemic (potassium ≥ 5.5 mEq/L) events (3.2% of a representative sample of hospitalization events) from the Veterans Administration and found that 47% were detected on outpatient encounters.37 As expected, ACEIs, ARBs, and MRAs were associated with hyperkalemia. The 1-day risk of a hyperkalemic event are shown in Figure 6 and were noted to be higher in those without preexisting CKD as compared with those with CKD, which was consistent with the findings of An and colleagues.35
Similar to the findings of Goyal and coworkers in AMI,25 Torlén and colleagues found among a large peritoneal dialysis cohort (N = 10,468) that there was a U-shaped relationship between serum potassium level and cardiovascular, infection-related, and all-cause mortality38 Of note, a time-averaged potassium ≥ 5.5 mEq/L was associated with 50% excess of both cardiovascular and all cause mortality in this cohort.
McMahon and colleagues studied 39,705 adult patients over 10 years with a mean age of 63 years, 16% of whom with AMI, who were hospitalized in the intensive care unit.39 Higher admission potassium values were associated with AKI and ESRD, but otherwise occurred in a broad spectrum of patients. The highest potassium concentration on the day of critical care initiation was an independent predictor of 30-day death in a graded manner compared with the referent group of K = 4.0 to 4.5 mEq/L: K = 4.5-5.0 mEq/L, OR 1.25 (95% CI, 1.16-1.35; P < .0001); K = 5.0-5.5 mEq/L, OR 1.42 (95% CI, 1.29-1.56; P < .0001); K = 5.5-6.0 mEq/L, OR 1.67 (95% CI, 1.47-1.89; P < .0001); K = 6.0-6.5 mEq/L, OR 1.63 (95% CI, 1.36-1.95; P < .0001); and K > 6.5 mEq/L, OR 1.72 (95% CI, 1.49-1.99; P < .0001). Interestingly, in patients whose potassium concentration declined ≥ 1 mEq/L after 48 hours in the intensive care unit, the association between hyperkalemia and mortality was no longer statistically significant, suggesting either treatment of hyperkalemia or natural resolution was favorable in terms of mortality.
Management
Potassium monitoring is the cornerstone of management and is associated with lower rates of hyperkalemia among patients who are at risk.40 However, when a patient develops or presents with acute hyperkalemia, emergency department care and hospitalization are frequently warranted. The initial focus is on hemodynamic stabilization and rapid correction of potassium by causing shifts from the extracellular to intracellular compartment, followed by finding a more durable solution over the next several hours to days. In a patient with acidosis, intravenous acute sodium bicarbonate can mediate a shift of potassium from the extracellular to intracellular compartment. Likewise, chronic acidosis can be treated with oral sodium bicarbonate therapy. For both acute and chronic therapy, sodium polystyrene sulfonate and calcium polystyrene sulphonate (available in Europe) are adjunctive therapies for severe hyperkalemia. Sodium polystyrene sulfonate, an organic enteral potassium-sodium exchange resin, nonselectively binds potassium and other cations (especially divalent cations like calcium and magnesium). Sodium polystyrene sulfonate was approved by the US Food and Drug Administration (FDA) in 1958 with very little clinical data, 4 years before the Kefauver-Harris amendment that required new therapies to have proven efficacy.41 Sodium polystyrene sulfonate is given in sorbitol, which results in loose stools or diarrhea. Diarrhea itself works to lower potassium; however, it is an uncomfortable side effect for hospitalized patients. In 2006, the FDA advised against the use of a 70% sorbitol solution given concerns regarding bowel injury primarily with retention enemas, and in 2009, the agency further recommended that sorbitol not be added to sodium polystyrene sulfonate, thus making the premixed solution the standard hospital formulary item.42 Today, approximately 5 million doses are given per year, most commonly in a formulation of 15 g sodium polystyrene sulfonate that is either given in 20 g of sorbitol (33% sorbitol) with the FDA warning as mentioned earlier, or mixed with water or syrup, and usually administered at 15 to 30 g per dose. In a single-center observational study, Kessler and coworkers found a range of sodium polystyrene sulfonate doses was associated with a graded decrease in the potassium concentration (Table 2).43 As noted by Watson and colleagues, sodium polystyrene sulfonate is associated with frequent adverse effects and carries the risk of acute bowel necrosis both as an oral solution and as a retention enema, particularly in critically ill and postsurgical patients.44 In addition, hypernatremia has been reported as a response to excessive (∼240 g) short-term use.45 Thus, sodium polystyrene sulfonate is infrequently prescribed as a chronic oral therapy by internists and cardiologists because of diarrhea and concerns over hypokalemia and sodium accumulation.
Fortunately, there are novel treatments in development for the treatment of both acute and chronic hyperkalemia (Figure 7). Patiromer calcium (RLY5016; Relypsa, Inc, Redwood City, CA) is a novel potassium exchange resin formulated as a dry, odorless powder for suspension in small amounts of water. It occurs substantially in a spherical bead form, and has a lower viscosity and higher yield than polymeric drugs made in bulk and ground into a powder. Patiromer is insoluble in typical solvents and passes through the gastrointestinal tract without degradation. It is being developed as a chronic therapy to limit hyperkalemia seen with higher doses of spironolactone (≥ 50 mg/d).46 The Polymeric Potassium Binder, in a Double-blind, Placebo-controlled Study in Patients with Chronic Heart Failure (PEARL-HF) study included 195 patients with HF and a history of hyperkalemia resulting in discontinuation of ACEI, ARB, or beta-adrenergic receptor antagonist, or CKD confirmed by an eGFR of < 60 mL/min/1.73 m2, who were randomized to double-blind treatment with 30 g/d RLY5016 or placebo for 4 weeks. Spironolactone, initiated at 25 mg/d, was increased to 50 mg/d on Day 15 if potassium was ≤ 5.1 mEq/L.47 Compared with placebo, RLY5016 had significantly lower serum potassium levels (—0.45 mEq/L; P < .001); a lower rate of hyperkalemia (potassium > 5.5 mEq/L; 7.3% RLY5016 vs 24.5% placebo; P = .015); and a higher proportion of patients receiving spironolactone 50 mg/d (91% RLY5016 vs 74% placebo; P = .019). The most common adverse events were gastrointestinal disorders (eg, flatulence, diarrhea, constipation, and vomiting), which were reported with higher frequency in the RLY5016 group (21% vs 6%, respectively). Serum magnesium < 1.8 mg/dL during the treatment period was seen in 13 (24%) RLY5016-treated patients and in 1 (2.1%) placebo-treated patient.
In another trial focusing on patients with HF, fluid overload, and risks for hyperkalemia, a novel cross-linked polyelectrolyte polymer (CLP, CLP-1001; Sorbent Therapeutics, Inc., Sunnyvale, CA), was given orally to absorb both water and electrolytes (sodium and potassium) in the gastrointestinal tract with eventual elimination in the feces.48 A total of 113 subjects with HF and eGFR ∼ 45 mL/min/1.73 m2 were randomized to CLP capsules versus placebo. The primary outcome was the change in potassium over time. At 8 weeks, there was no difference in the change in serum potassium between the groups. The two groups were similar in terms of incidence of hyperkalemia (potassium > 5.5 mEq/L), 13 (22.4%) versus 11 (21.2%), and hyperkalemia prompting discontinuation of study drug, 6 (10.2%) versus 5 (9.3%). At the end of Week 4, the percentages of patients eligible to increase their daily spironolactone dose to 50 mg because their serum potassium level was ≤ 5.1 mEq/L were similar in the CLP and placebo groups (64.4% vs 73.1%; P = .327). Weight loss and functional capacity as secondary end-points were significantly improved in the CLP group. The rates of gastrointestinal adverse events were 14 (23.7%) and 7 (13.5%) in the CLP and placebo groups, respectively. Although CLP-1001 was not effective for potassium control, it demonstrated efficacy in terms of sodium and fluid removal enter-ally as a proof of concept for future applications of polymers in HF. A modification of CLP, CLP-1004, is now beginning trials as a treatment for hyperkalemia.
Neither patiromer nor CLP has been studied as a treatment for acute hyperkalemia (Table 3). A novel agent, ZS-9 (ZS Pharma Inc., Coppell, TX) is being developed as a treatment for both acute and long-term chronic hyperkalemia. ZS-9 is an inorganic cation exchanger engineered to have a highly selective, high-capacity crystalline lattice structure to preferentially entrap monovalent cations (specifically excess potassium ions) over divalent cations (eg, calcium and magnesium). ZS-9 is available as a tasteless, odorless tablet or powder; requires no special handling, refrigeration, or special preparation; and does not have to be given in solution or with cathartics. In the largest double-blind, placebo-controlled clinical trial in patients with hyperkalemia to date (ZS-003), a total of 753 patients with hyperkalemia (potassium levels 5.0-6.5 mEq/L)—which included patients with CKD, heart failure, diabetes, and those on ACEIs, ARBs, or MRAs—were randomized to receive one of four doses of ZS-9 (1.25 g, 2.5 g, 5 g, or 10 g) or placebo, administered three times daily for the initial 48 hours (acute phase) (Figure 8).49 The primary endpoint was the rate of change in serum potassium from baseline throughout the 48-hour acute phase. Results from the acute phase and subsequent extended period have been published as of the time of this writing. Thus, ZS-9 and potentially patiromer appear to be the first oral therapies that are safe and efficacious for the treatment of hyperkalemia as an alternative to sodium polystyrene sulfonate, and is well positioned to be studied as an adjunct to ACEIs, ARBs, and MRAs in patients with CKD and HF. Control of hyperkalemia in this setting may in the future lead to expanded use and improved medication adherence and reduce the hazards of hyperkalemic events.
Conclusions
Both acute and chronic hyperkalemia complicate the management of CKD, HF, and AKI. Vigilance with laboratory monitoring is critical to diagnose hyperkalemia because ECG changes are unreliable, particularly in those with CKD. Cardiac arrest is the most frequent presentation for acute hyperkalemia. Novel therapies in development are warranted both as acute remedies and as adjunctive thera pies allowing greater use of ACEIs, ARBs, and MRAs in these vulner able populations. ![]()
Publication of this article was made possible through an educational grant from ZS Pharma.
References
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038-2047.
- McCullough PA. Interface between renal disease and cardiovascular illness. In: Zipes DP, Libby P, Bonow RO, Braunwald E, eds. Braunwald’s Heart Disease, 8th ed. New York: WB Saunders; 2006.
- Palmer BF. A physiologic-based approach to the evaluation of a patient with hyperkalemia. Am J Kidney Dis. 2010;56:387-393.
- Yip T, Wan W, Hui PC, et al. Severe hyperkalemia in a peritoneal dialysis patient after consumption of salt substitute. Perit Dial Int. 2012;32:206-208.
- Kurella Tamura M, Li S, Chen SC, et al. Educational programs improve the preparation for dialysis and survival of patients with chronic kidney disease [published online ahead of print September 25, 2013]. Kidney Int. doi:10.1038/ki.2013.369.
- Agrawal V, Ghosh AK, Barnes MA, McCullough PA. Perception of indications for nephrology referral among internal medicine residents: a national online survey. Clin J Am Soc Nephrol. 2009;4: 323-328.
- Park IW, Sheen SS, Yoon D, et al. Onset time of hyperkalaemia after angiotensin receptor blocker initiation: when should we start serum potassium monitoring? J Clin Pharm Ther. 2014;39:61-68.
- Ronco C, McCullough PA, Anker SD, et al; Acute Dialysis Quality Initiative (ADQI) consensus group. Cardiorenal syndromes: an executive summary from the consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol. 2010;165: 54-67.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASISHF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11-21.
- Pitt B, Remme W, Zannad F, et al; Eplerenone PostAcute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309-1321.
- Pfeffer MA. Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT). Circulation. 2013;128: 2704-2722. [Abstract]
- Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543-551.
- Allen LA, Shetterly S, Peterson PN, et al. Guideline Concordance of testing for hyperkalemia and kidney dysfunction during initiation of mineralocorticoid receptor antagonist therapy in patients with heart failure. Circ Heart Fail. 2014;7:43-50.
- Rossignol P, Cleland JG, Bhandari S, et al. Determinants and consequences of renal function variations with aldosterone blocker therapy in heart failure patients after myocardial infarction: insights from the Eplerenone PostAcute Myocardial Infarction Heart Failure Efficacy and Survival Study Circulation. 2012;125:271-279.
- Vardeny O, Wu DH, Desai A, et al; RALES Investigators. Influence of baseline and worsening renal function on efficacy of spironolactone in patients with severe heart failure: insights from RALES (Randomized Aldactone Evaluation Study). J Am Coll Cardiol. 2012;60:2082-2089.
- Albert NM, Yancy CW, Liang L, et al. Use of aldosterone antagonists in heart failure. JAMA. 2009;302:1658-1665.
- Fonarow GC, Yancy CW, Albert NM, et al. Heart failure care in the outpatient cardiology practice setting: findings from IMPROVE HF. Circ Heart Fail. 2008;1:98-106.
- Rao KK, Enriquez JR, de Lemos JA, et al. Use of aldosterone antagonists at discharge after myocardial infarction: results from the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry-Get with the Guidelines (GWTG). Am Heart J. 2013;166:709-715.
- Komajda M, Follath F, Swedberg K, et al; Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology. The EuroHeart Failure Survey programme—a survey on the quality of care among patients with heart failure in Europe. Part 2: treatment. Eur Heart J. 2003;24:464-474.
- Echemann M, Zannad F, Briançon S, et al. Determinants of angiotensin-converting enzyme inhibitor prescription in severe heart failure with left ventricular systolic dysfunction: the EPICAL study. Am Heart J. 2000;139:624-631.
- Lenzen MJ, Boersma E, Reimer WJ, et al. Underutilization of evidence-based drug treatment in patients with heart failure is only partially explained by dissimilarity to patients enrolled in landmark trials: a report from the Euro Heart Survey on Heart Failure. Eur Heart J. 2005;26:2706-2713.
- Bungard TJ, McAlister FA, Johnson JA, Tsuyuki RT. Underutilisation of ACE inhibitors in patients with congestive heart failure. Drugs. 2001;61:2021-2033.
- Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/ AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-e239.
- Hernandez AF, Mi X, Hammill BG, et al. Associations between aldosterone antagonist therapy and risks of mortality and readmission among patients with heart failure and reduced ejection fraction. JAMA. 2012;308:2097-2107.
- Goyal A, Spertus JA, Gosch K, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307:157-164.
- Antman EM, Anbe DT, Armstrong PW, et al. ACC/ AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction). J Am Coll Cardiol. 2004;44:E1-E211.
- Cohn JN, Kowey PR, Whelton PK, Prisant LM. New guidelines for potassium replacement in clinical practice: a contemporary review by the National Council on Potassium in Clinical Practice. Arch Intern Med. 2000;160:2429-2436.
- Karet FE. Mechanisms in hyperkalemic renal tubular acidosis. J Am Soc Nephrol. 2009;20:251-254.
- Renaud JM, Hayward LJ. Lessons learned from muscle fatigue: implications for treatment of patients with hyperkalemic periodic paralysis. Recent Pat Biotechnol. 2012;6:184-191.
- Montague BT, Ouellette JR, Buller GK. Retrospective review of the frequency of ECG changes in hyperkalemia. Clin J Am Soc Nephrol. 2008;3:324-330.
- Nemati E, Taheri S. Electrocardiographic manifestations of hyperkalemia in hemodialysis patients. Saudi J Kidney Dis Transpl. 2010;21:471-477.
- Bahl A, Swamy A, Jeevan H, et al. ECG changes of hyperkalemia during paced rhythm. Indian Heart J. 2009;61:93-94.
- Kuriachan V, Tedrow U, Antman E, Epstein LM. Acute hyperkalemia detected by alert from implantable cardioverter-defibrillator. Pacing Clin Electrophysiol. 2012;35:e276-e279.
- Hosaka Y, Chinushi M, Iijima K, et al. Correlation between surface and intracardiac electrocardiogram in a patient with inappropriate defibrillation shocks due to hyperkalemia. Intern Med. 2009;48:1153-1156.
- An JN, Lee JP, Jeon HJ, et al. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care. 2012;16:R225.
- Jain N, Kotla S, Little BB, et al. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012;109:1510-1513.
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156-1162.
- Torlén K, KalantarZadeh K, Molnar MZ, et al. Serum potassium and cause-specific mortality in a large peritoneal dialysis cohort. Clin J Am Soc Nephrol. 2012;7:1272-1284.
- McMahon GM, Mendu ML, Gibbons FK, Christopher KB. Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med. 2012;38:1834-1842.
- Raebel MA, Ross C, Xu S, et al. Diabetes and drug-associated hyperkalemia: effect of potassium monitoring. J Gen Intern Med. 2010;25:326-333.
- Flinn RB, Merrill JP, Welzant WR. Treatment of the oliguric patient with a new sodium-exchange resin and sorbitol: a preliminary report. N Engl J Med. 1961;264:111-115.
- Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21:733-735.
- Kessler C, Ng J, Valdez K, et al. The use of sodium polystyrene sulfonate in the inpatient management of hyperkalemia. J Hosp Med. 2011;6:136-140.
- Watson M, Abbott KC, Yuan CM. Damned if you do, damned if you don’t: potassium binding resins in hyperkalemia. Clin J Am Soc Nephrol. 2010;5: 1723-1726.
- Nepal M, Bucaloiu ID, Norfolk ER. Hypernatremia in a patient treated with sodium polystyrene sulfonate. Int J Nephrol Renovasc Dis. 2010;3:141-143.
- Buysse JM, Huang IZ, Pitt B. PEARLHF: prevention of hyperkalemia in patients with heart failure using a novel polymeric potassium binder, RLY5016. Future Cardiol. 2012;8:17-28.
- Pitt B, Anker SD, Bushinsky DA, et al; PEARLHF Investigators. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARLHF) trial. Eur Heart J. 2011;32:820-828.
- Costanzo MR, Heywood JT, Massie BM, et al. A double-blind, randomized, parallel, placebo-controlled study examining the effect of cross-linked polyelectrolyte in heart failure patients with chronic kidney disease. Eur J Heart Fail. 2012;14: 922-930.
- Ash SR. ZS9, a novel, first-in-class hyperkalemia therapy: results from a multicenter, double-blind, placebo-controlled, multiple-dose study to evaluate the effects of ZS9 in patients with chronic kidney disease and hyperkalemia. Presented at: ASN Kidney Week 2013; Atlanta, GA; November 510, 2013.
- Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004;351:585-592.