ISMP Medication Error Report Analysis
Ambiguous Course Dosing Leads to Errors
Farxiga and Fetzima Mix-Ups
Transdermal Patches and Heat Sources
Patient-Controlled Analgesia Pump Security Issue
Bloxiverz and Vazculep Mix-Ups
Michael R. Cohen, RPh, MS, ScD,* and Judy L. Smetzer, RN, BSN†
ISMP Medication Error Report Analysis
Ambiguous Course Dosing Leads to Errors
Farxiga and Fetzima Mix-Ups
Transdermal Patches and Heat Sources
Patient-Controlled Analgesia Pump Security Issue
Bloxiverz and Vazculep Mix-Ups
Michael R. Cohen, RPh, MS, ScD,* and Judy L. Smetzer, RN, BSN†
ISMP Medication Error Report Analysis
Ambiguous Course Dosing Leads to Errors
Farxiga and Fetzima Mix-Ups
Transdermal Patches and Heat Sources
Patient-Controlled Analgesia Pump Security Issue
Bloxiverz and Vazculep Mix-Ups
Michael R. Cohen, RPh, MS, ScD,* and Judy L. Smetzer, RN, BSN†
These medication errors have occurred in health care facilities at least once. They will happen again—perhaps where you work. Through education and alertness of personnel and procedural safeguards, they can be avoided. You should consider publishing accounts of errors in your newsletters and/or presenting them at your inservice training programs.
Your assistance is required to continue this feature. The reports described here were received through the Institute for Safe Medication Practices (ISMP) Medication Errors Reporting Program. Any reports published by ISMP will be anonymous. Comments are also invited; the writers’ names will be published if desired. ISMP may be contacted at the address shown below.
Errors, close calls, or hazardous conditions may be reported directly to ISMP through the ISMP Web site (www.ismp.org), by calling 800-FAIL-SAFE, or via e-mail at ismpinfo@ismp.org. ISMP guarantees the confidentiality and security of the information received and respects reporters’ wishes as to the level of detail included in publications.
These medication errors have occurred in health care facilities at least once. They will happen again—perhaps where you work. Through education and alertness of personnel and procedural safeguards, they can be avoided. You should consider publishing accounts of errors in your newsletters and/or presenting them at your inservice training programs.
Your assistance is required to continue this feature. The reports described here were received through the Institute for Safe Medication Practices (ISMP) Medication Errors Reporting Program. Any reports published by ISMP will be anonymous. Comments are also invited; the writers’ names will be published if desired. ISMP may be contacted at the address shown below.
Errors, close calls, or hazardous conditions may be reported directly to ISMP through the ISMP Web site (www.ismp.org), by calling 800-FAIL-SAFE, or via e-mail at ismpinfo@ismp.org. ISMP guarantees the confidentiality and security of the information received and respects reporters’ wishes as to the level of detail included in publications.
These medication errors have occurred in health care facilities at least once. They will happen again—perhaps where you work. Through education and alertness of personnel and procedural safeguards, they can be avoided. You should consider publishing accounts of errors in your newsletters and/or presenting them at your inservice training programs.
Your assistance is required to continue this feature. The reports described here were received through the Institute for Safe Medication Practices (ISMP) Medication Errors Reporting Program. Any reports published by ISMP will be anonymous. Comments are also invited; the writers’ names will be published if desired. ISMP may be contacted at the address shown below.
Errors, close calls, or hazardous conditions may be reported directly to ISMP through the ISMP Web site (www.ismp.org), by calling 800-FAIL-SAFE, or via e-mail at ismpinfo@ismp.org. ISMP guarantees the confidentiality and security of the information received and respects reporters’ wishes as to the level of detail included in publications.
Hosp Pharm 2015;50(5):347–350
2015 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj5005-347
AMBIGUOUS COURSE DOSING LEADS TO ERRORS
An incorrect intravenous immune globulin (IVIG) dose was entered into a pharmacy computer and administered to a patient. A pharmacist took a telephone order for IVIG 150 g for 5 days for a 75 kg patient with myasthenia gravis. The pharmacist checked several reliable references to make sure the dose was appropriate. For that indication, Facts & Comparisons mentions a “total dose of 1-2 g/kg, given over 2-5 days.” Three pharmacists misinterpreted this as 2 g/kg/day for 2 to 5 days. The patient received one dose of 150 g on the first day. Fortunately, on the second day, a pharmacist questioned the dose. The original order was from a hospitalist who received the recommendation from a neurologist. The pharmacist called the neurologist who confirmed that the dose was supposed to be a total of 150 g divided over 5 days.
Telephone orders can sometimes be misinterpreted even when the order is read back to the prescriber. References also allow for misinterpretation based on inconsistent wording. For IVIG, some doses read “dose/ kg/day for a set amount of days,” “dose/kg given in divided doses over a set amount of days,” or “dose/kg over a set amount of days.” Like the chemotherapy errors of the 1990s in which cisplatin or cyclophosphamide were ambiguously ordered as the entire course dose given over a period of days (eg, cyclophosphamide 4 g/m2 x 4 days, rather than 1 g/m2 daily on day 1, day 2, day 3, and day 4), errors can easily happen with IVIG and other drugs or biologics that are given over several days.
Doses should be prescribed as “dose/ kg/day for a set amount of days” to avoid misinterpretation. In this case, rather than a total dose being expressed, it is safer to communicate “intravenous immune globulin 30 g IV daily” and to specify each date of administration. Similar errors can occur when a dose is expressed as “mg/kg/day every 8 hours in divided doses.” A safer alternative is mg/kg/dose given every 8 hours. Telephone orders should be avoided. Drug information publishers should take notice and clarify their monographs if needed.
FARXIGA AND FETZIMA MIX-UPS
The US Food and Drug Administration (FDA) is aware of several reported mix-ups due to name confusion between 2 medications—Farxiga (dapagliflozin) and Fetzima (levomilnacipran). Farxiga was approved in January 2014 to lower blood glucose levels in adults with type 2 diabetes when used along with diet and exercise. It is available in 5 and 10 mg tablets. Fetzima was approved in July 2013. It is a selective norepinephrine and serotonin reuptake inhibitor for major depressive disorder. The drug is available in 20, 40, 80, and 120 mg extended-release capsules.
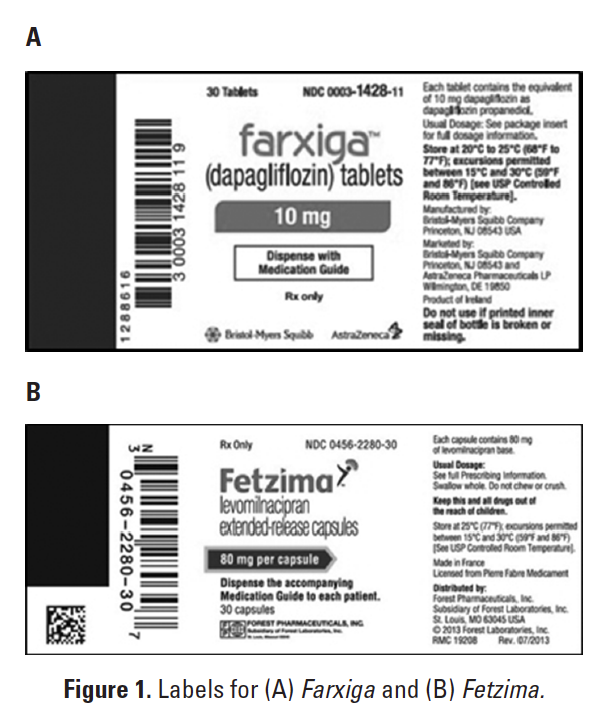
Upon review of the 5 medication error reports received by FDA, it is believed that the errors can largely be attributed to the drugs being approved and marketed within 6 months of one another. Both drug names begin with the letter F and end with the letter A and are of the same length and number of -syllables. Prescribers and pharmacists may choose the wrong item from computer screens. Furthermore, the container labels might appear similar because both display the proprietary name of the product in red font (Figures 1A and B).
Farxiga and Fetzima have been added to the ISMP List of Confused Drug Names. The updated is available at: http://www.ismp.org/Tools/confuseddrugnames.pdf. If these drugs are used in your hospital, consider adding computer alerts to verify the indication for these medications. Prescribers should include the indication with orders or prescriptions. Community pharmacists should counsel all patients before dispensing these drugs to confirm the indication. As practitioners become more familiar with the 2 products, we hope that name confusion errors will diminish.
TRANSDERMAL PATCHES AND HEAT SOURCES
While hospitalized, a woman with multiple myeloma was placed on transdermal fentanyl (Duragesic) 25 mcg per hour for back pain management. The patient had previously suffered 2 vertebral compression fractures. During her first 2 weeks at home, she was doing well. But soon thereafter, a family member noticed that the patient seemed disoriented, was losing her balance, and had nausea and vomiting.
A thorough investigation was conducted, and it was discovered that the fentanyl patch was being applied to the patient’s back. The patient routinely sat in her favorite recliner, which vibrates and has a heating component that was activated. The heat from this chair over the area that the patch was applied likely led to the patient’s symptoms of fentanyl -toxicity.
As mentioned in the product labeling, it’s important to remind patients and caregivers to avoid exposing transdermal fentanyl and other transdermal medication patches to heat from heating pads, electric blankets, heat or tanning lamps, sunbathing, hot baths, saunas, hot tubs, heat wraps, and heated water beds, as this could increase the rate of drug delivery from the patch into the body. Also, tight coverings over the patch and strenuous exercise, which can heat the body, should be avoided. The person who reported this wanted us to remind others that heated loungers and even vehicles with heated seats can affect absorption when the patches are exposed to these heat sources for more than a short time. Patients should be reminded to apply patches to nonirritated and nonirradiated skin on the chest, back, flank, or upper arm and to avoid applying the patches to body areas that might come in contact with heat sources. They should understand that the medication works systemically regardless of where the patch is placed on the body, so it does not need to be placed directly on or near the area that hurts.
PATIENT-CONTROLLED ANALGESIA PUMP SECURITY ISSUE
Did you know it’s possible to purchase keys on eBay or Amazon to unlock patient-controlled analgesia (PCA) pumps? They may be available on other Web sites, too. When we searched, we found keys for CADD-Solis pumps, Hospira LifeCare PCA 3, Baxter PCA II syringe pump, and several other current and older model infusion pumps. Some pumps may have other security features such as software codes to activate locking mechanisms and not just a key. Be sure to engage or activate these other security features whenever possible. If the volume in an opioid infusion bag on a PCA pump begins to dwindle unexpectedly, your PCA pump locking mechanism may have been compromised.
BLOXIVERZ AND VAZCULEP MIX-UPS
ISMP is alerting hospitals about the potential for a dangerous mix-up between 2 relatively new presentations of older medications. Bloxiverz, available from Eclat Pharmaceuticals, became the first FDA-approved neostigmine product in 2013. It is a cholinesterase inhibitor indicated for the reversal of nondepolarizing neuromuscular blocking agents after surgery. The company has 5 mg/10 mL and 10 mg/10 mL vials. The issue is with the latter product.
A mix-up between these products could be problematic. Patients on phenylephrine for hypotension could also be on paralytics; neostigmine could reverse these paralytics if given accidentally. To complicate the matter, nurses may invite a problem by requesting a bag of “neo” (referring to phenylephrine by the brand name Neo-Synephrine), which could be confused with neostigmine if staff are unfamiliar with the Neo-Synephrine brand of phenylephrine. Also, neostigmine is given IV push as a weight-based dose. If someone has a phenylephrine vial in hand and doses the drug by volume as they have with -neostigmine, the patient could receive too much phenylephrine, risking a cardiac event.
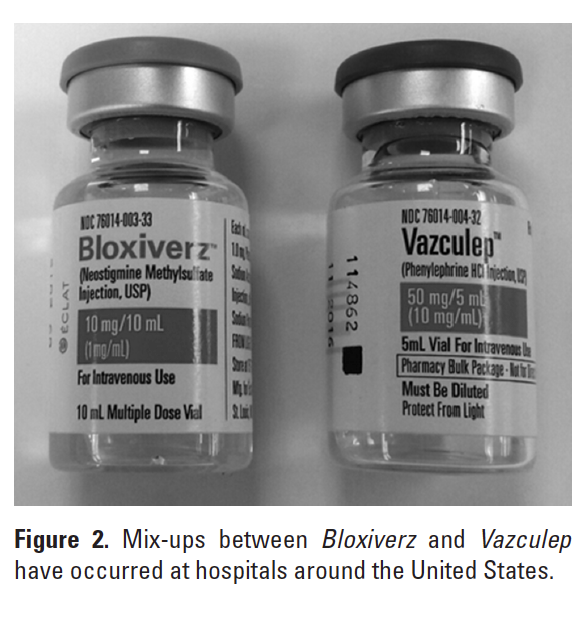
Several hospitals have reported that cartons of the 10 mg product were found mixed in with the company’s phenylephrine 50 mg/5 mL Vazculep, which was approved last year and recently became available as the only FDA-approved phenylephrine injection available in 3 vial sizes (1 mL, 5 mL, 10 mL). The cartons look somewhat similar in size, color, and design (Figure 2).
There have also been several close calls in which the wrong product was used during sterile compounding but the error was identified during an independent check by a second person. In the process of preparing phenylephrine infusions for a patient in the intensive care unit (ICU), staff accidentally removed several vials of neostigmine that were stored with the phenylephrine supply. The infusion bags were prepared but a technician and pharmacist noticed the vials of neostigmine during the checking process. All of the infusions had to be remade. Another hospital experienced a similar situation. The neostigmine was also found mixed together in the phenylephrine bin. An IV technician pulled neostigmine assuming it was phenylephrine, but caught the mix-up prior to compounding.
It is possible that the letters N and P, being close alphabetically, may put these drugs in close -proximity to one another on a shelf, thereby increasing the risk of the wrong product being selected. To prevent mix-ups, keep supplies of these drugs separated and alert staff to the potential risk of confusion. Consider using a different brand of phenylephrine injection so the vials don’t look similar. For example, a 50 mg vial of phenylephrine is also available from Sandoz and a 10 mg vial is available from Westward. ![]()
*President, Institute for Safe Medication Practices, 200 Lakeside Drive, Suite 200, Horsham, PA 19044; phone: 215-947-7797; fax: 215-914-1492; e-mail: mcohen@ismp.org; Web site: www.ismp.org. †Vice President, Institute for Safe Medication Practices, Horsham, Pennsylvania.