Cancer Chemotherapy Update
Nivolumab and Olaparib
Dominic A. Solimando, Jr, MA, FAPhA, FASHP, BCOP, and J. Aubrey Waddell,
PharmD, FAPhA, BCOP
Cancer Chemotherapy Update
Nivolumab and Olaparib
Dominic A. Solimando, Jr, MA, FAPhA, FASHP, BCOP, and J. Aubrey Waddell,
PharmD, FAPhA, BCOP
Cancer Chemotherapy Update
Nivolumab and Olaparib
Dominic A. Solimando, Jr, MA, FAPhA, FASHP, BCOP, and J. Aubrey Waddell,
PharmD, FAPhA, BCOP
The complexity of cancer chemotherapy requires pharmacists be familiar with the complicated regimens and highly toxic agents used. This column reviews various issues related to preparation, dispensing, and administration of antineoplastic therapy, and the agents, both commercially available and investigational, used to treat malignant diseases. Questions or suggestions for topics should be addressed to Dominic A. Solimando, Jr, President, Oncology Pharmacy Services, Inc., 4201 Wilson Blvd #110-545, Arlington, VA 22203, e-mail: OncRxSvc@comcast.net; or J. Aubrey Waddell, Professor, University of Tennessee College of Pharmacy; Oncology Pharmacist, Pharmacy Department, Blount Memorial Hospital, 907 E. Lamar Alexander Parkway, Maryville, TN 37804, e-mail: waddfour@charter.net.
Name: Nivolumad
Synonyms: Opdivo, Anti-PD-1; Anti-PD-1 antibody; BMS-936558; ONO-4538
The complexity of cancer chemotherapy requires pharmacists be familiar with the complicated regimens and highly toxic agents used. This column reviews various issues related to preparation, dispensing, and administration of antineoplastic therapy, and the agents, both commercially available and investigational, used to treat malignant diseases. Questions or suggestions for topics should be addressed to Dominic A. Solimando, Jr, President, Oncology Pharmacy Services, Inc., 4201 Wilson Blvd #110-545, Arlington, VA 22203, e-mail: OncRxSvc@comcast.net; or J. Aubrey Waddell, Professor, University of Tennessee College of Pharmacy; Oncology Pharmacist, Pharmacy Department, Blount Memorial Hospital, 907 E. Lamar Alexander Parkway, Maryville, TN 37804, e-mail: waddfour@charter.net.
Name: Nivolumad
Synonyms: Opdivo, Anti-PD-1; Anti-PD-1 antibody; BMS-936558; ONO-4538
The complexity of cancer chemotherapy requires pharmacists be familiar with the complicated regimens and highly toxic agents used. This column reviews various issues related to preparation, dispensing, and administration of antineoplastic therapy, and the agents, both commercially available and investigational, used to treat malignant diseases. Questions or suggestions for topics should be addressed to Dominic A. Solimando, Jr, President, Oncology Pharmacy Services, Inc., 4201 Wilson Blvd #110-545, Arlington, VA 22203, e-mail: OncRxSvc@comcast.net; or J. Aubrey Waddell, Professor, University of Tennessee College of Pharmacy; Oncology Pharmacist, Pharmacy Department, Blount Memorial Hospital, 907 E. Lamar Alexander Parkway, Maryville, TN 37804, e-mail: waddfour@charter.net.
Name: Nivolumad
Synonyms: Opdivo, Anti-PD-1; Anti-PD-1 antibody; BMS-936558; ONO-4538
Hosp Pharm 2013;50(5):356–366
2015 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj5005-356




MECHANISM OF ACTION
Programmed death 1 protein (PD-1) is a T-cell inhibitory receptor similar to the cytotoxic T-lymphocyte-associated antigen (CTLA-4). PD-1 is involved in modulating the initial stages of T-cell activation. Nivolumab is a monoclonal antibody that blocks the PD-1 receptor on T-cells. Inhibiting the interaction between PD-1 and the PD-1 ligand and CD80 enhances T-cell response, potentiating immune response.1-5
PHARMACOKINETICS
The time to peak concentration (Tmax) is 1 to 4 hours after the beginning of the infusion. The maximum concentration (Cmax) is dose-related.6 Following doses of 0.1 to 20 mg/kg, the volume of distribution (Vd) is 8 L, with total clearance of 9.5 mL/h.7 The elimination half-life (T½) is 26.7 days; clearance is increased with increased body weight.7
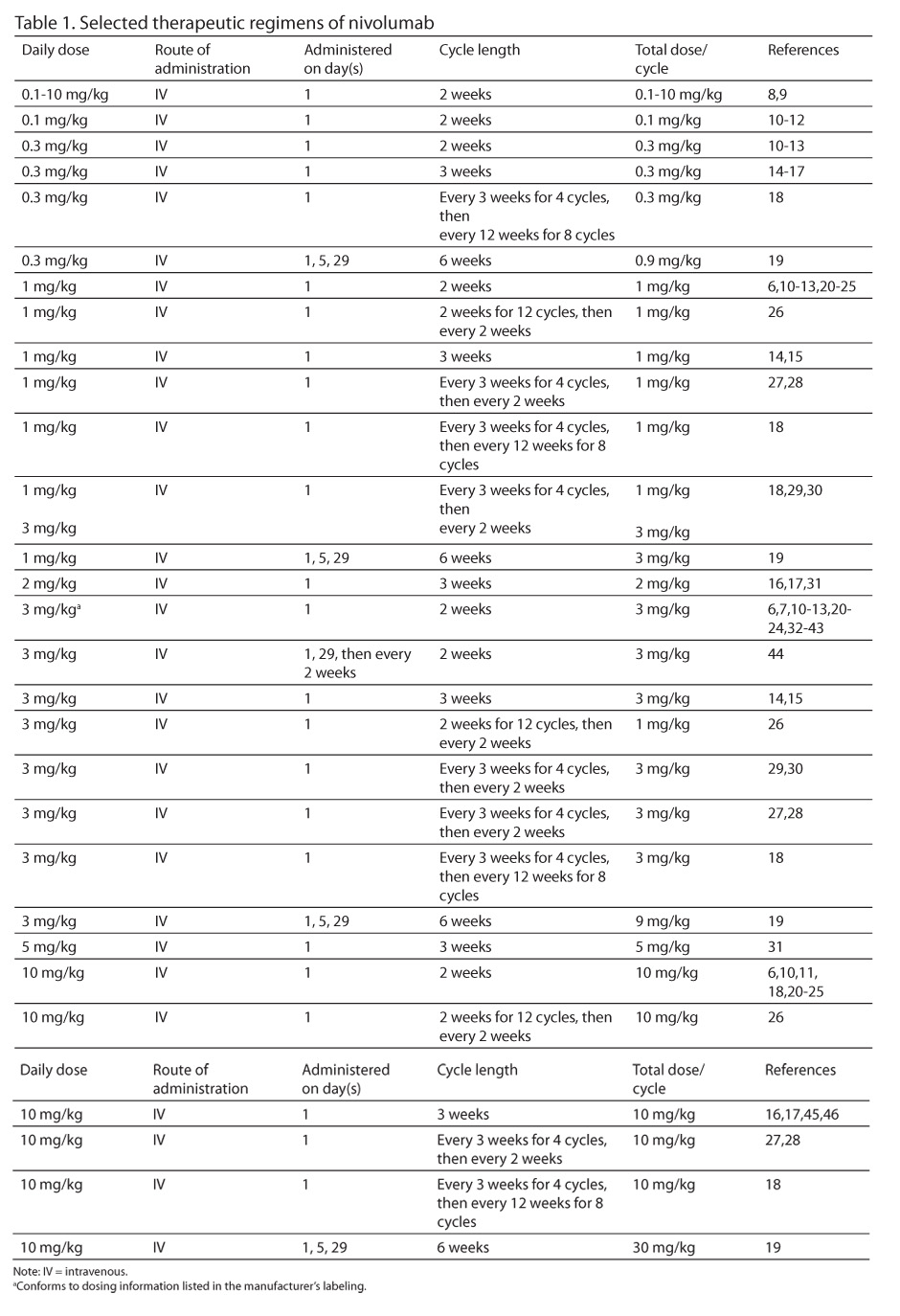
Selected therapeutic regimens of nivolumab appear in Table 1.
PREPARATION
A. Follow institutional policies for preparation of hazardous medications when preparing nivolumab.
B. Use nivolumab injection, 10 mg/mL.
C. Dilute with 100 to 250 mL 0.9% sodium -chloride (NS) or 5% dextrose in water (D5W).
STABILITY
Solutions for infusion are stable to 4 hours at room temperature, or 24 hours under refrigeration (2ºC to 8ºC [36ºF-46ºF]).
ADMINISTRATION
Niovolumab is administered as a 1-hour intravenous (IV) infusion.
TOXICITIES (3 mg/kg every 2 weeks)
A. Constitutional: Asthenia 10%,32 fatigue 2% to 20%.10,32
B. Dermatologic: Pruritus 16% to 17%,6,32 (grade 3 or 4) 0.5%32; rash 15% to 20%,6,32 (grade 3 or 4) 0.5% to 5%32,37; rash, macular 2%6; rash, pruritic 2%6; urticatia 6%6; vitiligo 4% to 11%.6,32
C. Endocrine/Metabolic: Hyperglycemia (grade 3 or 4) 5%,37 hyperthyroidism 2%,6 hypothyroid-ism 2%,6 increased thyroid-stimulating hormone (TSH) 4%,6 unspecified endocrine disorders (grade 3 or 4) 2%.10
D. Gastrointestinal: Constipation 11%32; diarrhea 16% to 19%,6,32 (grade 3 or 4) 1% to 5%10,32,36; increased lipase 2%10; nausea 17%32; vomiting 6%,32 (grade 3 or 4) 0.5%32; weight loss (grade 3 or 4) 5%.36
E. Hepatic: Hepatitis (grade 3 or 4) 1%10; increased alanine aminotransferase (ALT) 4%,6 (grade 3 or 4) 5% to 10%36,37; increased aspartate aminotransferase (AST) 4%,6 (grade 3 or 4) 2% to 5%.6,36,37
F. Hematologic: Lymphopenia 3%.10
G. Hypersensitivity: Hypersensitivity/infusion reactions 6%.6
H. Pulmonary: Pneumonitis 2%.6
I. Renal: Nephritis (grade 2) 5%.36
DOSE ADJUSTMENT
A. Hepatic
- Mild hepatic impairment, no adjustment required.7
- Moderate hepatic impairment (ALT or AST greater than 3 times the upper limit of normal [ULN] and less than or equal to 5 times the ULN)7:
a. Delay therapy up to 12 weeks.
b. If no resolution at 12 weeks, discontinue drug.
- Severe hepatic impairment (ALT or AST greater than 5 times the ULN or bilirubin greater than 3 times the ULN), discontinue the drug.7
B. Renal
- Mild renal impairment, no adjustment required.7
- Moderate renal impairment (serum creatinine greater than 1.5 times the ULN and less than or equal to 6 times the ULN)7:
a. Delay therapy up to 12 weeks.
b. If no resolution at 12 weeks, discontinue drug.
- Life threatening renal impairment (serum creatinine greater than 6 times the ULN), discontinue the drug.7
Name: Olaparib
Synonyms: Lynparza;AZD2281; CBT-1
MECHANISM OF ACTION
Olaparib is an inhibitor of poly(ADP-ribose) polymerase (PARP) enzymes (PARP-1, PARP-2, PARP-3). PARPs are enzymes needed for repair of single-strand DNA breaks. Inhibition of this pathway leads to accumulation of single-strand breaks, resulting in double-strand breaks in the DNA molecule.47-49
PHARMACOKINETICS
Following oral administration, absorption is rapid, with the peak concentration (Cmax) between 1 and 3 hours.50-52 The drug is approximately 82% protein bound.52 The Cmax was reported to range from 4.7 mcg/mL to 9.1 mcg/mL; the time to maximum concentration (Tmax) was 1 to 2 hours and 2 to 3 hours.53-55 One study found a Cmax of 2,399.8 ± 1.371.5 mcg/L.56 For oral doses ranging from 100 mg daily to 600 mg twice a day, the mean Vd is 40.3 L.50 Following a 400 mg oral dose, the Vd is reported to be 167 ± 196 L.52
The AUC following 200 mg and 400 mg doses was 25.8 mcg•h/L and 58.1 mcg•h/L, respectively.53 Following daily administration of 10 mg to 80 mg per day for 14 days, the AUC increased by approximately 26%. After twice-daily dosing with 60 mg to 600 mg per day for 14 days, the mean AUC increase was 49%.50 Administration with a high-fat meal slows the Tmax, but does not significantly increase the AUC.52,56 Plasma clearance is 4.6 L/h; the elimination T½ is 5 to 11 hours.50,51
Olaparnib is metabolized by oxidation followed by glucuronide or sulfate conjugation in the liver by CYP3A4.52 Renal excretion accounts for about 44% of the drug, primarily as unchanged drug; about 15% is excreted as metabolites.52 Forty-two percent of the drug is excreted in the feces, primarily as -metabolites.52
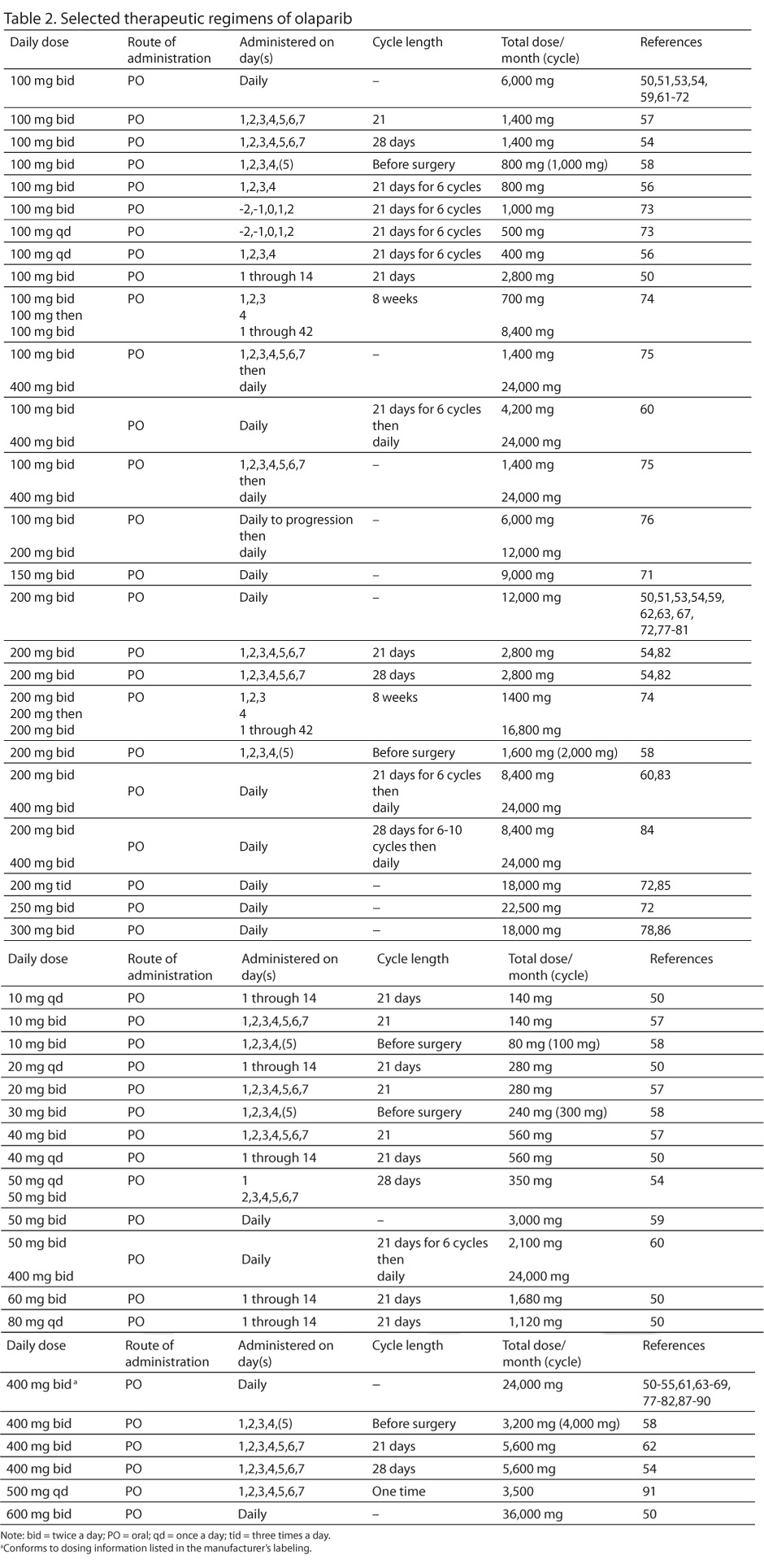
Selected therapeutic regimens of olaparib appear in Table 2.
PREPARATION
A. Follow institutional policies for preparation of hazardous medications when dispensing olaparib.
B. Olaparib is available as 50 mg capsules.
C. Olaparib should be stored at 25°C (77°F).
STABILITY
A. Olaparib is stored at controlled room temperature (20°C to 25°C [68°F to 77°F]).
B. Brief (less than 24 hours) exposure to temperatures up to 30°C (86°F) is acceptable.
ADMINISTRATION
A. Olaparib is administered orally, usually twice a day.
B. Patients should be advised to avoid grapefruit and Seville oranges while taking olaparib.
TOXICITIES (400 mg twice daily)
A. Central Nervous System: Dizziness 13%,89 (grade 1) 10%,88 (grade 2) 2%,88 (grade 1 or 2) 6% to 16%,61,87 (grade 3 or 4) 12%50; headache 21% to 25%,53,89 (grade 1) 9% to 12%,77,88 (grade 2) 7%,88 (grade 1 or 2) 6% to 7%.61,64
B. Constitutional: Asthenia 14% to 50%,54,89 (grade 1) 7%,88 (grade 2) 4%,88 (grade 1 or 2) 6% to 34%,61,82 (grade 3 or 4) <1%89; fatigue 7% to 75%,51,53,81,89 (grade 1) 24% to 30%,77,88 (grade 2) 15% to 18%,77,88 (grade 1 or 2) 30% to 62%,50,61,64-66,82,87,90 (grade 3) 11%,77 (grade 3 or 4) 3% to 15%61,64,66,67,78,82,87-89; hot flushes (grade 1) 3%,88 (grade 2) 1%.88
C. Dermatologic: Pruritus 33%51; rash 17%,51 (grade 1 or 2) 9%.61,82
D. Endocrine/Metabolic: Hyperglycemia 17%,51 hypothyroidism (grade 2) 2%.77
E. Gastrointestinal: Abdominal distension 13%,89 (grade 1) 10%,88 (grade 2) 1%,88 (grade 1 or 2) 19%87; anorexia 21% to 33%,51,54,89 (grade 1) 13%,88 (grade 2) 6%,88 (grade 1 or 2) 11% to 37%,64,87 (grade 3 or 4) 1%87; constipation 17% to 21%,51,89 (grade 1) 9%,88 (grade 2) 4%,88 (grade 1 or 2) 7% to 16%64,82; diarrhea 25% to 27%,51,89 (grade 1) 2% to 23%,77,88 (grade 2) 4%,88 (grade 1 or 2) 12% to 38%,50,61,64,82,87 (grade 3 or 4) 2% to 3%87-89; dysgeusia 25% to 33%,51,53,89 (grade 1) 13%,88 (grade 2) 2%,88 (grade 1 or 2) 12% to 17%50,87; dyspepsia 18%,89 (grade 1) 14%,88 (grade 2) 2%,88 (grade 1 or 2) 6% to 12%50,61,64; flatulence (grade 1 or 2) 7%64; gastritis (grade 1 or 2) 6%61; gastroesophageal reflux (grade 1 or 2) 7% to 9%61,64; mucosal inflammation 25%53; nausea 67% to 75%,51,53,54,89 (grade 1) 48% to 52%,77,88 (grade 2) 14% to 26%,77,88 (grade 1 or 2) 26% to 72%,61,64-66,82,87,90 (grade 3) 7%,65 (grade 3 or 4) 2% to 15%50,61,64,66,78,82,87-89; stomatitis 92%,54 (grade 3 or 4) 17%54; vomiting 17% to 34%,51,54,89 (grade 1) 20%,88 (grade 2) 10%,88 (grade 1 or 2) 9% to 47%,61,64,66,82,87,90 (grade 3 or 4) 1% to 12%50,61,64,82,87-89; xerostomia 25%.51
F. Hematologic: Anemia 21% to 50%,51,89 (grade 1) 2% to 13%,77,88 (grade 2) 4% to 10%,77,88 (grade 1 or 2) 4% to 19%,61,64-66,82 (grade 3 or 4) 3% to 25%61,64,66,67,78,82,88-90; decreased hematocrit 33%51; decreased hemoglobin 33%51; decreased red blood cell count 33%51; decreased white blood cells (grade 1) 2%,77 (grade 2) 7%77; leukopenia 50%,51 (grade 3) 5%65; lymphopenia 33%,51 (grade 3 or 4) 12%50; neutropenia 5% to 17%,54,89 (grade 1) 2%,77 (grade 2) 9%,77 (grade 3 or 4) <1%% to 17%54,61,67,89; thrombocytopenia 33%,51 (grade 1) 7%,77 (grade 3 or 4) 20%.67
G. Hepatic: Increased ALT 17%,51 increased AST 17%,51 increased gamma-glutamyltransferase (GGT) 17%.51
H. Hypersensitivity: (grade 3 or 4) 13%.67
I. Infections: Urinary tract (grade 1 or 2) 34%.82
J. Musculoskeletal: Arthralgia 17%,89 (grade 1) 7%,88 (grade 2) 4%,88 (grade 3 or 4) <1%.89
K. Neurologic: Palmar-plantar erythrodysesthesia 33%,54 (grade 3 or 4) 8%.54
L. Pain: Abdominal 25%,89 (grade 1) 8%,88 (grade 2) 8%,88 (grade 1 or 2) 7% to 25%,61,64,82,87 (grade 3 or 4) 2%87-89; back pain 16%,89 (grade 1) 7%,88 (grade 2) 3%,88 (grade 3 or 4) 2%88,89; upper abdominal pain 18%,89 (grade 1) 9%,88 (grade 2) 4%.88
M. Respiratory: Cough 18%,89 (grade 1) 10%,88 (grade 2) 3%88; dyspnea (grade 1 or 2) 3%,87 (grade 3 or 4) 3%87; nasopharyngitis 15%,89 (grade 1) 9%,88 (grade 2) 4%.88
N. Renal: Increased blood urea nitrogen (BUN) 67%.51
DOSE ADJUSTMENT
A. Hepatic: No information available.52
B. Renal:
- Creatinine clearance greater than or equal to 50 mL/min and less than or equal to 80 mL/minute, no adjustment needed.52
- Creatinine clearance less than 50 mL/min, no information available.52
REFERENCES
- Okazaki T, Honjo T. PD-1 and PD-1 ligands: From discovery to clinical application. Int Immunol. 2007;19:813-824.
- Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365-1369.
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member lease to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027-1034.
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8:793-800. [Erratum, Nat Med. 2002;8:1039].
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207-212.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454.
- Opdivo [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Co.; 2014.
- Topalian SL, Sznol M, Brahmer JR, et al. Nivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients with advanced solid tumors: Survival and long-term safety in a phase 1 trial [abstract 3002]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/113545-132. Accessed February
4, 2015. - Grosso J, Horak CE, Inzunza D, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538) [abstract 3016]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/113904-132. Accessed February 4, 2015.
- Sznol M, Kluger HM, Hodi FS, et al. Survival and long-term follow-up of safety and response in patients (pts) with advanced melanoma (MEL) in a phase I trial of nivolumab. (anti-PD-1; BMS-936558; ONO-4538) [abstract CRA9006]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/113607-132. Accessed February 4, 2015.
- Hodi FS, Sznol M, Kluger HM, et al. Long-term survival of ipilimumab-naïve patients (pts) with advanced melanoma (MEL) treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) in a phase I trial [abstract 9002. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/125578-144. Accessed February 4, 2015.
- Sangro B, Crocenzi TS, Welling TH, et al. Phase I escalation of nivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients (pts) with advanced hepatocellular carcinoma (HCC) with or without chronic viral hepatitis [abstract TPS3111]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/113499-132. Accessed February 4, 2015.
- Porkka K, Mauro MJ, Lipton JH. Et al. An open-label, phase Ib, dose-escalation study (CA180-373) of dasatinib plus nivolumab, an investigational anti-programmed cell death 1 (PD-1) antibody, in patients (pts) with previously treated chronic myeloid leukemia (CML) [abstract TPS7110]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/127155-144. Accessed February 4, 2015.
- Wolchok JD, Kluger HM, Callahan MK, et al. safety and activity of nivolumab (anti-PD1, BMS-936558, OJO-4538) in combination with ipilimumab in patients (pts) with advanced melanoma (MEL) [abstract 9012]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/107862-132. Accessed February 4, 2015.
- Sznol M, Kluger HM Callahan MK, et al. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO_4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL) [abstract LBA9003]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/126008-144. Accessed February 4, 2015.
- Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma (mRCC): Results of a randomized, dose-ranging phase II trial [abstract 5009]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/125893-144. Accessed February 4, 2015.
- Choueiri TK, Fishman MN, Escudier BJ, et al. Immunotherapy activity of nivolumab in previously treated and untreated metastatic renal cell carcinoma (mRCC): Biomarker-based results from a randomized clinical trial [abstract 5012]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/125914-144. Accessed February 4, 2015.
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122-133.
- Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and efficacy of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455-2465.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020-1030.
- Hamanishi J, Mandai M, Ikeda T, et al. Efficacy and safety of anti-PD-1 antibody (Nivolumab; BMS-936558; ONO-4538) in patients with platinum-resistant ovarian cancer [abstract 5511]. Proc Am Soc Clin Oncol. 2014.). http://meetinglibrary.asco.org/content/130814-144. Accessed -February
4, 2015. - Lesokhin AM, Gutierrez M, Halwani AS, et al. A phase I dose-escalation study evaluating the effects of nivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients with select relapsed or refractory hematologic malignancies [abstract TPS3113]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/113548-132. Accessed February 4, 2015.
- Gibney GT, Weber JS, Kudchadkar RR, et al. Safety and efficacy of adjuvant anti-PD-1 therapy (nivolumab) in combination with vaccine in resected high-risk metastatic melanoma [abstract 9056]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/112620-132. Accessed February 4, 2015.
- Weber JS, Kudchadkar RR, Gibney GT, et al. Phase I/II trial of PD-1 antibody nivolumab with peptide vaccine in patients naïve to or that failed ipilimumab [abstract 9011]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/112287-132. Accessed February 4, 2015.
- Drake CG, McDermott DF, Sznol M, et al. Survival, safety, and response duration results of nivolumab (anti-PD-1; BMS-936558; ONO-4538) in a phase I trial in patients with previously treated metastatic renal cell carcinoma (mRCC): Long term patient follow-up [abstract 4514]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/113579-132. Accessed February 4, 2015.
- Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or –naïve melanoma. J Clin Oncol. 2013;31(34):
4311-4318. - Hammers HJ, Plimack ER, Infante JR, et al. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC) [abstract 4504]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/129458-144. Accessed February 4, 2015.
- Antonia SJ, Gettinger SN, Chow LQM, et al. Nivolumab (anti-PD-1; BMS-936558; Ono-4538) and ipilimumab in first-line NSCLC: Interim phase I results [abstract 8023]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/125736-144. Accessed February 4, 2015.
- Sampson JH, Vlahovic G, Desjardins A, et al. Randomized phase IIb study of nivolumab (anti-{D-1; BMS-036558; ONO-4538) alone or in combination with ipilumumab versus bevacizumab in patients (pts) with recurrent glioblastoma (GBM) [abstract YPS2101]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/125698-144. Accessed February 4, 2015.
- Callahan MK, Bendell JC, Chan E, et al. Phase I/II open-label study of nivolumab (anti-PD-1; BMS-936558; ONO-4538) as monotherapy or combined with ipilimumab in advanced or metastatic solid tumors [abstract TPS3114]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/125706-144. Accessed February 4, 2015.
- Amin A, Plimack ER, Infante JR, et al. Nivolumab (anti-PD-1; BMS-936558; ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC) [abstract 5010]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/125881-144. Accessed February 4, 2015.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320-330.
- Chow LQM, Gordon MS, Logan TF, et al. Phase I dose escalation study of recombinant interleukin-21 (rIL-21; BMS-982470) in combination with nivolumab (anti-PD-1; BMS-936558;ONO-4538) in patients (pts) with advanced or metastatic solid tumors [abstract TPS3112]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/113524-132. Accessed February 4, 2015.
- Sosman JA, Martin-Algarra S, Wolchok JD, et al. An exploratory study of the biologic effects of nivolumab (anti-PD-1; BMS-936558; ONO-4538) treatment in patients (pts) with advanced (unresectable or metastatic) melanoma (MEL) [abstract TPS3114]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/113623-132. Accessed -February 4, 2015.
- Motzer RJ, Bono P, Hides GR, et al. A phase III comparative study of nivolumab (anti-PD-1; BMS-936558; Ono-4538) versus everolimus in patients (pts) with advanced or metastatic renal cell carcinoma (mRCC) previously treated with antiangiogenic therapy [abstract TPS4592]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/113341-132 URL. Accessed February 4, 2015.
- Rizvi NA, Chow LQM, Borghaei H, et al. Safety and response with nivolumab (anti-PD-1; BMS-936558; ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC [abstract 8022]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/125751-144. Accessed February
4, 2015. - Gettinger SN, Shepherd FA, Antonia SJ, et al. First-line nivolumab (anti-PD-1; BMS-936558; ONO-4538) monotherapy in advanced NSCLC: Safety, efficacy, and correlation of outcomes with PD-L1 status [abstract 8024]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/125745-144. Accessed February 4, 2015.
- Segal NH, Hodi S, Sanborn RE, et al. A phase I dose escalation and cohort expansion study of lirilumab (anti-KIR; BMS-986015) in combination with nivolumab (anti-PD-1; BMS-936558; ONO-4538) in advanced solid tumors [abstract TPS3115]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/125708-144. Accessed February 4, 2015.
- Gettinger SN, Brahmer JR, Rizvi NA, et al. A phase III comparative study of nivolumab (anti-PD-1; BMS-936558; ONO-4538) versus docetaxel in patients (pts) with previously treated advances/metastatic nonsquamous non-small-cell lung cancer NSCLC) [abstract TPS8121]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/112426-132. Accessed February 4, 2015.
- Borghaei H, Lynch TJ, Rizvi NA, et al. A phase III comparative study of nivolumab (anti-PD-1; BMS936558; Ono-4538) versus docetaxel in patients with previously treated advanced or metastatic squamous cell non-small cell lung cancer (NSCLC) [abstract TPS8122]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/113595-132. Accessed
February 4, 2015. - Carbone DP, Socinski MA, Chen AC, et al. A phase III, randomized, open-label trial of nivolumab (andi-PD-1; BMS-936558; ONO-4538) versus investigator’s choice chemotherapy (ICC) as first-line therapy for stage IV or recurrent PD-L1+non-small cell lung cancer (NSCLC) [abstract TPS8128]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/125773-144. Accessed February 4, 2015.
- Chmielowski B, Hamid O, Minor DR, et al. A phase III open-label study of nivolumab (anti-PD-1; BMS-936558; ONO-4538) versus investigator’s choice in advanced melanoma patients progressing post anti-CTLA-4 therapy [abstract TPS9105]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/112577-132. Accessed February 4, 2015.
- Hodi FS, Baudelet C, Chen AC, et al. An open-label, randomized, phase II study of nivolumab (anti-PD-1; BMS-936558; ONO-4538) given sequentially with ipilimumab in patients (pts) with advanced or metastatic melanoma (MEL) [abstract TPS9107]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/117295-132. Accessed February 4, 2015.
- Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 -blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. New Engl J Med. 2015;372(4):311-319.
- Rizvi NA, Antonia SJ, Chow LQM, et al. A phase I study of nivolumab (anti-PD-1; BMS-936558; ONO-4538) plus platinum-based doublet chemotherapy (PT-doublet) chemotherapy-naïve non-small cell lung cancer (NSCLC) patients (pts) [abstract 8072]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/113504-132. Accessed February
4, 2015. - Antonia SJ, Brahmer JR, Gettinger SN, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with platinum-based doublet chemotherapy (PT-DC) in advanced non-small cell lung cancer (NSCLC) [abstract 8113]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/125962-144. Accessed February 4, 2015.
- Amé JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26(8):882-893.
- Dantzer F. de La Rubia G, Ménissier-De Murcia J, et al. Base excision repair is impaired in mammalian cells lacking poly(ADP-ribose) polymerase-1. Biochemistry. 2000;39(25):7559-7569.
- McCabe N, Turner NC, Lord JC, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66(16):8109-8115.
- Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123-134.
- Yamamoto N, Nokihara H, Yamada Y, et al. A phase I, dose-finding and pharmacokinetic study of olaparnib (AZD2281) in Japanese patients with advanced solid tumors. Cancer Sci. 2012;103(3):504-509.
- Lynparza [prescribing information]. Wilmington, DE: Astra-Zeneca Pharmaceuticals LP; 2014.
- Dean E, Middleton MR, Pwint T, et al. Phase I study to assess the safety and tolerability of olaparib in combination with bevacizumab in patients with advanced solid tumors.
Br J Cancer. 2012;106(3):468-474. - Del Conte G, Sessa C, von Moos R, et al. Phase I study of olaparib in combination with liposomal doxorubicin in patients with advanced solid tumors. Br J Cancer. 2014;111(4):651-659.
- Rajan A, Carter CA, Kelly RJ, et al. A phase I combination study of olaparnib with cisplatin and gemcitabine in adults with solid tumors. Clin Cancer Res. 2012;18(8):2344-2351.
- Vergote I, Rutten A, Rolfo CD, et al. Effect of food on the pharmacokinetics (PK) of olaparib after oral dosing of the capsule formulation [abstract 2599]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/133511-144. Accessed February 4, 2015.
- Kahn OA, Gore M, Lorigan P, et al. A phase I study of the safety and tolerability of olaparib (AZD2281, KU0059436) and dacarbazine in patients with advanced solid tumors. Br J Cancer. 2011;104(5):750-755.
- Bundred N, Gardovska J, Jaskiewicz, et al. Evaluation of the pharmacodynamics and pharmacokinetics of the PARP inhibitor olaparib: A phase I multicenter trial in patients scheduled for elective breast cancer surgery. Invest New Drugs. 2013;31(4):949-958.
- Samol J, Ranson M, Scott E, et al. Safety and tolerability of the poly(ADP-ribose) polymerase (PARP) inhibitor, olaparnib (AZD2281) in combination with topotecan for the treatment of patients with advanced solid tumors: A phase I study. Invest New Drugs. 2012;30(4):1493-1500.
- Balmaña J, Tung NM, Isakoff SJ, et al. Phase I, open-label study of olaparib plus cisplatin in patients with advanced solid tumors [abstract 1009]. Proc Am Soc Clin Oncol. 2012. http://meetinglibrary.asco.org/content/95087-114. Accessed February
4, 2015. - Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376(9737):
245-251. - Lee JM, Hays JL, Annunziata CM, et al. Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2 mutation-associated breast or ovarian cancer with biomarker analyses. J Natl Cancer Inst. 2014;106(6):dju089.
- Liu JF, Tolaney SM, Birrer M, et al. A phase 1 trial of the PARP inhibitor olaparib (AZD2281) in combination with the anti-angiogenic cedranib (AZD2171) in recurrent epithelial ovarian or triple-negative breast cancer. Eur J Cancer. 2013;49(14):2972-2978.
- Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparnib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet. 2010;376(9737):235-244.
- Audeh MW, Penson RT, Friedlander M, et al. Phase II trial of the oral PARP inhibitor olaparib (AZD2281) in BRCA-deficient advanced ovarian cancer [abstract 5500]. Proc Am Soc Clin Oncol. 2009. http://meetinglibrary.asco.org/content/30781-65. Accessed February 4, 2015.
- Tutt A, Robson M, Garber JE, et al. Phase II trial of the oral PARP inhibitor olaparib in BRCA-deficient advanced breast cancer [abstract CRA501]. Proc Am Soc Clin Oncol. 2009. http://meetinglibrary.asco.org/content/30774-65. Accessed February 4, 2015.
- Lee J, Annunziata CM, Minasian LM, et al. Phase I study of the PARP inhibitor olaparib (O) in combination with carboplatin (C) in BRCA1/2 mutation carriers with breast (Br) or ovarian (Ov) cancer (Ca) [abstract 2520]. Proc Am Soc Clin Oncol. 2011. http://meetinglibrary.asco.org/content/ 81876-102. Accessed February 4, 2015.
- Liu J, Fleming GF, Tolaney SM, et al. A phase I trial of the PARP inhibitor olaparib (AZD2281) in combination with the antiangiogenic cedranib (AZD2171) in recurrent ovarian or triple-negative breast cancer [abstract 5028]. Proc Am Soc Clin Oncol. 2011. http://meetinglibrary.asco.org/content/80769-102. Accessed February 4, 2015.
- Van der Noll, Ang JE, Jager A, et al. Phase I study of olaparib in combination with carboplatin and/or paclitaxel in patients with advanced solid tumors [abstract 2579]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/111375-132. Accessed February 4, 2015.
- Matulonis U, Wulf GM, Birrer MJ, et al. Phase I study of oral BKM120 and oral olaparib for high-grade serous ovarian cancer (HGSC) or triple-negative breast cancer (TNBC) [abstract 2510]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/130302-144. Accessed February 4, 2015.
- Rivkin SE, Iriarte D, Sloan H, et al. Phase Ib/II with expansion of patients at the mTD study of olaparib plus weekly (metronomic) carboplatin and paclitaxel in relapsed ovarian cancer patients [abstract 5527]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/134486-144. Accessed February 4, 2015.
- Campelo RG, Felip E, Massuti B, et al. Phase IB study to evaluate efficacy and tolerability of olaparib (AZD2281) plus gefitinib in patients (P) with epidermal growth factor (EGFR) mutation positive advanced non-small cell lung cancer (NSCLC) (NCT=1513174/GECP-GOAL) [abstract 8079]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/133231-144. Accessed February 4, 2015.
- Giaccone G, Rajan A, Kelly RJ, et al. A phase I combination study of olaparib (AZD2281); KU-0059436) and cisplatin (C) plus gemcitabine (G) in adults with solid tumors [abstract 3027]. Proc Am Soc Clin Oncol. 2010. http://meetinglibrary.asco.org/content/51495-74. Accessed February 4, 2015.
- Chalmers AJ, Jackson A, Swaisland H, et al. Results of stage I of the oparatic trial: A phase I study of olaparib in combination with temozolomide in patients with relapsed glioblastoma [abstract 2025]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/126101-144. Accessed February 4, 2015.
- Lee J, Annunziata CM, Hays JL, et al. Phase I/Ib study of the PARP inhibitor olaparib (O) with carboplatin (C) in BRCA1/2 mutation carriers with breast or ovarian cancer (Bb/OvCa) (NCT00647062) [abstract 2514]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/112646-132. Accessed February 4, 2015.
- Bang YJ, IM SA, Lee KW, et al. Olaparib plus paclitaxel in patients with recurrent or metastatic gastric cancer: A randomized double-blind phase I trial [abstract 4013]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/111323-132. Accessed February 4, 2015.
- Liu JF, Barry WT, Birrer M, et al. Combination cediranib and olaparnib versus olaparnib alone for women with recurrent platinum-sensitive ovarian cancer: A randomised phase 2 study. Lancet Oncol. 2014;15(11):1207-1214.
- Molife LR, Mateo J, McGoldrick T, et al. Safety and efficacy results from two randomized expansions of a phase I study of a tablet formulation of the PARP inhibitor, olaparib, in ovarian and breast cancer patients with BRCA1/2 mutations [abstract 3048]. Proc Am Soc Clin Oncol. 2012. http://meetinglibrary.asco.org/content/95143-114. Accessed February 4, 2015.
- Gupta A, Moreno V, Dean EJ, et al. Phase I study to determine the bioavailability and tolerability of a tablet formulation of the PARP inhibitor olaparib in patients with advanced solid tumors: Dose-escalation phase [abstract 3051]. Proc Am Soc Clin Oncol. 2012. http://meetinglibrary.asco.org/content/96456-114. Accessed February 4, 2015.
- Lee JM, Liu J, Choyke PL, et al. Biomarker correlates from the randomized phase 2 trial of the PARP inhibitor olaparib (O) with or without the antiangiogenic TKI cedrinib (C) in recurrent platinum-sensitive ovarian cancer (NCT01116648) [abstract 5535]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/132327-144. Accessed February 4, 2015.
- Liu J, Barry WT, Birrer MJ, et al. A randomized phase 2 trial comparing efficacy of the combination of the PARP inhibitor olaparib and the antiangiogenic cediranib against olaparib alone in recurrent platinum-sensitive ovarian cancer [abstract LBA5500]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/130933-144. Accessed February 4, 2015.
- Kaye SB, Lubinski J, Matulonis U, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly(ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30(4):372-379.
- Oza AM, Cibula D, Oaknin A, et al. Olaparib plus paclitaxel plus carboplatin (P/C) followed by olaparib maintenance treatment in patients (pts) with platinum-sensitive recurrent serous ovarian cancer (PSR SOC): A randomized, open-label phase II study [abstract 5001]. Proc Am Soc Clin Oncol. 2012. http://meetinglibrary.asco.org/content/94600-114. Accessed February 4, 2015.
- Dent RA, Lindeman GJ, Clemons M, et al. Phase I trial of the oral PARP inhibitor olaparib in combination with paclitaxel for first- or second-line treatment of patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2013;15(5):R88
- Massuti B, Campelo RG, Abreu DR, et al. Open, phase II randomized trial of gefitinib alone versus olaparib (AZD2281) plus gefitinib in advanced non-small cell lung cancer (NSCLC) patients (P) with epidermal growth factor receptor (EGFR) mutations: Spanish Lung Cancer Group trial (NCT=1513174/GECP-GOL) [abstract TPS8127]. Proc Am Soc Clin Oncol. 2014. http://meetinglibrary.asco.org/content/134492-144. Accessed February 4, 2015.
- Moore KN, DiSilvestro P, Lowe ES, et al. SOLO1 andOSLO2: Randomized phase III trials of olaparib in patients (pts) with ovarian cancer and a BRCA1/2 mutation (BRCAm) [abstract TPS5616]. Proc Am Soc Clin Oncol. 2014.). http://meetinglibrary.asco.org/content/131636-144. Accessed February 4, 2015.
- Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: A phase 2, multicenter, open-label, non-randomized study. Lancet Oncol. 2011;12(9):852-861.
- Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382-1392.
- Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomized phase 2 trial. Lancet Oncol. 2014;15(8):852-861.
- Kaufmann B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germ-line BRCA1/2 mutation: An open-label phase II study [abstract 11024]. Proc Am Soc Clin Oncol. 2013. http://meetinglibrary.asco.org/content/112220-132. Accessed February 4, 2015.
- Kelly RJ, Robey RW, Chen CC, et al. A pharmacodynamics study of the P-glycoprotein antagonist CBT-1 in combination with paclitaxel in solid tumors. Oncologist. 2012;17(4):
512-e523.