Analysis of Gunshot Residue by ICP-MS
Agilent 4500 ICP-MS By Elzbieta (Ela) Bakowska, Peter B. Harrsch, and Thomas J. Gluodenis, Jr.
Abstract
ICP-MS was successfully utilized for elemental analysis of gunshot residues (GSR). The concentrations of antimony, barium, and lead were determined from the GSR collection swab extract solutions. The capabilities of semiquantitative analysis were also demonstrated.
Agilent 7696A Sample Prep WorkBench

Agilent 7696A Sample Prep WorkBench
Agilent 7696A Sample Prep WorkBench

Agilent 7693A Automatic Liquid Sampler

Agilent 7696A Sample Prep WorkBench
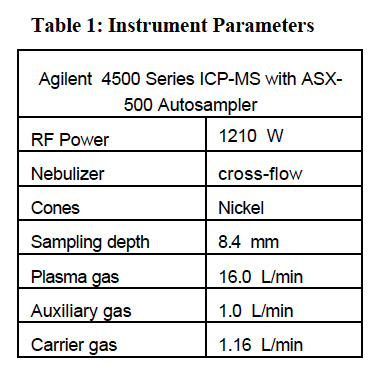

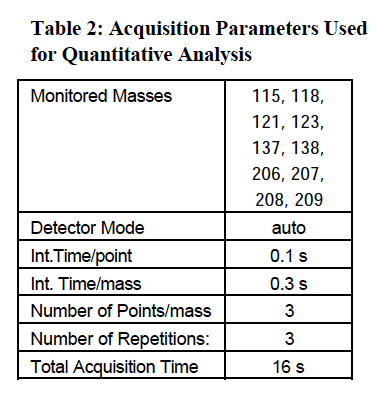

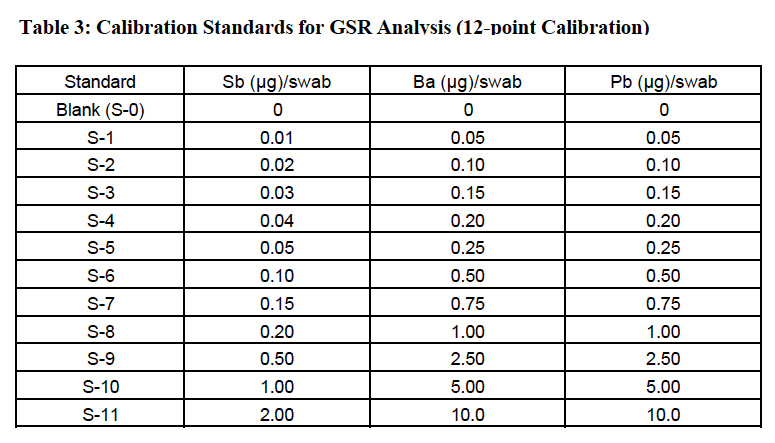

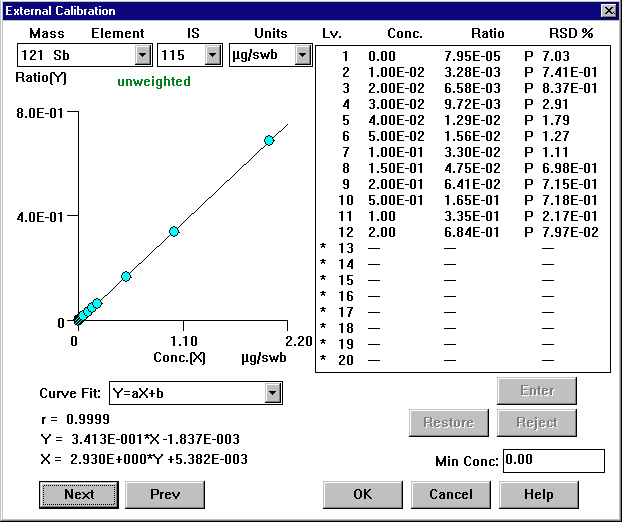
Figure 1. Calibration Curve: Antimony

Figure 1. Calibration Curve: Antimony

Figure 1. Calibration Curve: Antimony
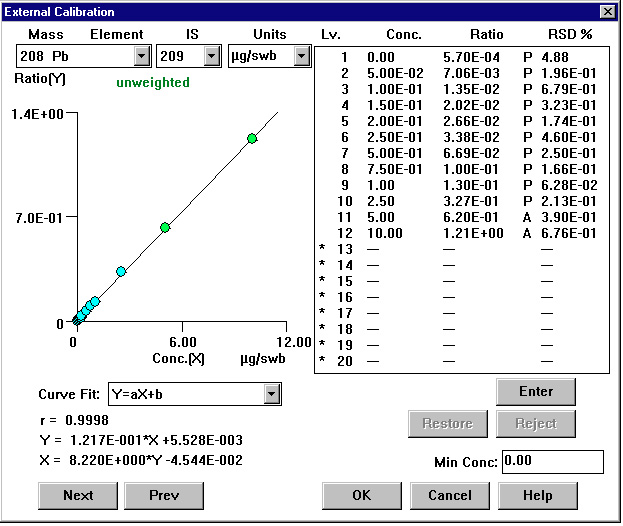
Figure 2. Calibration Curve: Barium

Figure 2. Calibration Curve: Barium

Figure 2. Calibration Curve: Barium
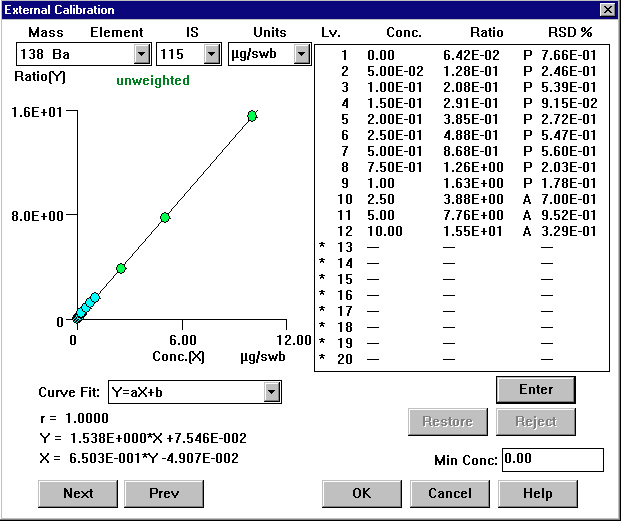
Figure 3. Calibration Curve: Lead

Figure 3. Calibration Curve: Lead

Figure 3. Calibration Curve: Lead
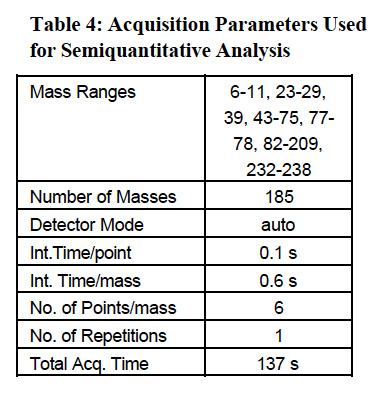

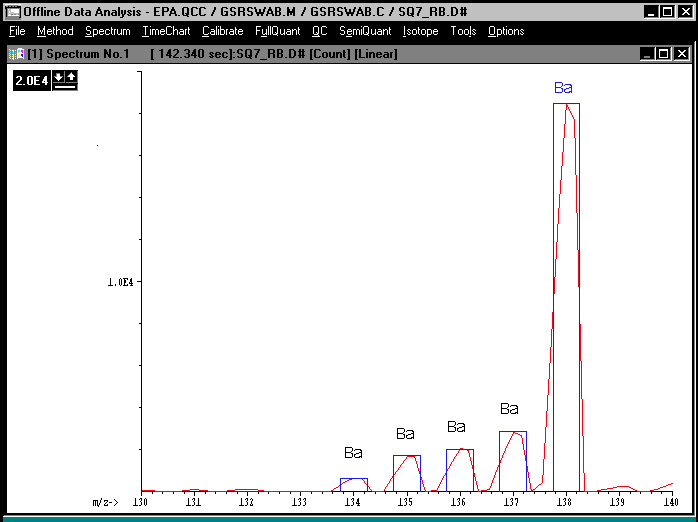
Figure 4. Isotopes of Barium: Semiquantitative Analysis

Figure 4. Isotopes of Barium: Semiquantitative Analysis

Figure 4. Isotopes of Barium: Semiquantitative Analysis

Introduction
The elemental analysis of GSR is being used as one of the tools in interpretation of the criminal event. Some answers to the question of “accepted uniqueness”1 of GSR particles can be given by the determination of lead, antimony and barium with additional information provided by the determination of copper, zinc, and iron. Determination of antimony, barium, and lead from the hands of a suspect was originally performed by the Dermal Nitrate (paraffin cast) technique with diphenylamine used as the testing reagent. This technique detects nitrites in GSR. However, this technique also detects nitrites originating from other sources, such as urine, matches, fertilizer, and some pharmaceuticals. Thus, the diphenylamine test produces numerous false positives and has been abandoned as a means of detecting GSR2. The elemental analysis techniques which replaced the Dermal Nitrate method were neutron activation analysis (NAA) and graphite furnace atomic absorption spectrometry (GFAAS). Both of these techniques suffered from several limitations, especially as a tool for routine, rapid analysis of GSR samples. In the past several years, inductively coupled plasma mass spectrometry (ICP-MS) has gained wide acceptance for trace and ultratrace analysis of liquid and solid samples in variety of application fields, including judicial and regulatory arenas.
Methods of elemental analysis are gaining popularity as forensic tools. The main disadvantage of many of these techniques for the analysis of glass is the required sample preparation: digestion/ dissolution of the samples in HF. This sample preparation method is not only time consuming and requires extra safety precautions, but is also a destructive method, which in many cases may not be acceptable. LA-ICP-MS eliminates the need for extensive sample preparation, provides excellent detection limits, offers unmatched elemental coverage, and exhibits a wide dynamic range. An additional benefit of LA-ICP-MS is that the amount of sample used for a single determination is negligible and leaves the remaining sample available for further tests, if required.
Experimental
GSR samples were collected from the hands of a person who had fired a 9 mm semiautomatic gun (Glock) with 9 mm ammunition (Federal Hydra Shok). The shooting was conducted outdoors, gun was handled twohanded, and the samples were collected approximately 40 minutes after the shooting. GSR samples and calibration solutions were placed on Qtip cotton swabs (a pair of swabs for each sample and standard), placed in 15-mL polypropylene screw-top tubes and dried overnight. Sample preparation consisted of adding of 10.0 mL a 10% (v/v) nitric acid (Fisher Scientific, Optima grade) into each tube, recapping and vortexing for about 1 minute. The nitric acid solution was spiked with 50 μg/L each of indium (In) and bismuth (Bi) as internal standards3. The tubes with caps removed were placed in an oven set at 80°C for 2 hours. Solutions were mixed again, and centrifuged for 5 minutes for extract separation. The extract solution was transferred by pipetting into another polypropylene tube and analyzed.
The solutions were analyzed in unattended mode, employing the ASX- 500 (CETAC) autosampler and the Agilent ChemStation software feature allowing for sequential analysis of the samples. The additional QA/QC software can be applied, to monitor the quality requirements of the analysis.
The operating conditions for Agilent 4500 series ICP-MS instrument are listed in Table 1. Table 2 shows the acquisition parameters employed in quantitative analysis of the swabs.
Quantitation of element concentration was made using 115In as internal standard for Sb and Ba. 209Bi was used as the internal standard for lead (represented by the sum of its three major isotopes: 206Pb + 207Pb + 208Pb). Lead has four naturally occurring isotopes at masses 204, 206, 207 and 208. Three major isotopes (206, 207, and 208), being products of different radio-decay processes, may vary in abundance, depending upon the source of lead. To minimize errors caused by the difference in isotopic distribution, the sum of the signals measured at three major isotopes is used and represented as the lead value. This approach was adapted from the USEPA methods utilizing ICPMS for determination of lead in environmental samples4. Twelve-point calibration curves were created for all analytes. The calibration standard concentrations are listed in Table 3. The internal standard mix contained indium, and bismuth added at 0.05 μg level to all swabs.
The calibration curves for 121Sb, 138Ba and 208Pb are shown in Figure 1, Figure2, and Figure 3, respectively.
The semiquantitative analysis of the samples was performed to demonstrate the unique capability of ICP-MS in providing fast and reliable information for over 70 elements, which can be present in the sample. This additional information can be used for further “fingerprinting” of GSR, similarly to the methods used in other forensic applications5. The acquisition parameters used in semiquantitative analysis are presented in Table 4.
Results
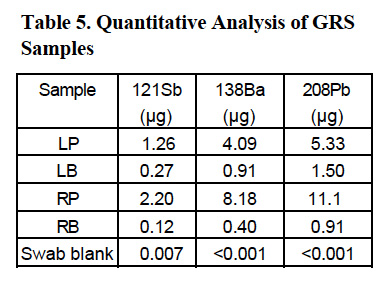
Four samples were analyzed for the determination of Sb, Ba and Pb. They were swaps from the left hand palm, left hand’s bottom, right hand palm, and right hand’s bottom. They are labeled LP, LB, RP, and RB, respectively.
The results of the quantitative analysis of those four samples are presented in Table 5.
The mass range, which includes the analytes of interest, is practically interference-free and monitoring either of the masses (121 or 123) for antimony will give the same results, especially since their relative abundances are almost equal. The isotope 121 was selected for reporting antimony results. For barium the two most abundant isotopes are 137 (11.2%) and 138 (71.7%). The latter offers more than six-fold higher signal, however it can suffer from unpredictable elemental interferences from lanthanum and cerium. Most likely those interferences will be negligible in GRS samples, so the choice of isotope 138 is recommended. For routine analysis, only one isotope of antimony and one isotope of barium need to be quantified. As mentioned before, the lead value is calculated from a sum of signals collected at masses 206, 207 and 208 and represented as a value for isotope 208.
Figure 4 exemplifies the use of the semiquantitative analysis to verify that the signal measured at mass 138 was associated with barium. The software allows for measurement of the signals generated at the large mass range and subsequently fits a template corresponding to the natural abundance of the barium isotopes over the resulting signals. It is clear that there is a perfect fit between the experimental and theoretical values, thus the signal measured at mass 138 is a consequence of barium present in the solution.
Conclusions
ICP-MS was proven to be rapid and reliable analytical method for the determination of antimony, barium and lead in gun shot residue samples. The total analysis time (without sample preparation) was little over 2 minutes, with the actual data acquisition time equal to 16 seconds.
There are additional possible applications of ICP-MS for the evaluation of the origin of the GSR by the determination of other elements like copper, nickel and silver, and measurement of the isotopic ratios of lead.
References
1 A. Zeichner and N. Levin, J. Forensic Sci. 1997; 42(6), 1027-1028
2 S.S. Krishnan, “Detection of Gunshot Residue: Present Status”, in “Forensic Science Handbook”, R. Saferstein, Ed., Prentice Hall, NJ, 1982, pp.139-183
3 R. D. Koons, J. Forensic Sci. 1998; 43(4), 748 – 754
4 US Environmental Protection Agency, EPA Method 200.8, Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma Mass Spectrometry, Version 5.4, 1994
5 E. Bakowska, HP Application Brief, September 1998, Publ.No. (23) 5968- 1953E
This application bulletin demonstrates feasibility of concept. Additional development and/or validation may be required for routine use.