Keeping It Clean
Thermoforming in a cleanroom environment presents special challenges—and rewards
Previous Article Next Article
By Michael Tolinski
Keeping It Clean
Thermoforming in a cleanroom environment presents special challenges—and rewards
Previous Article Next Article
By Michael Tolinski
Keeping It Clean
Thermoforming in a cleanroom environment presents special challenges—and rewards
Previous Article Next Article
By Michael Tolinski

Lacerta Group’s cleanroom thermoforming operations are protected from contamination by positive air pressure and curtains.

Lacerta Group’s cleanroom thermoforming operations are protected from contamination by positive air pressure and curtains.
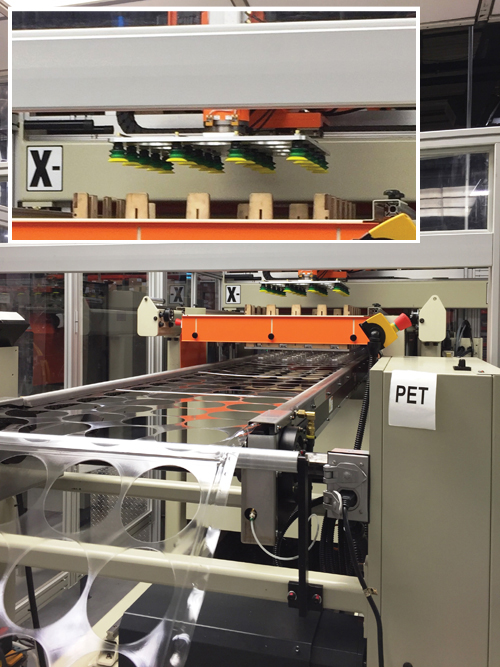
The company’s new robot is fitted with end-of-arm tooling (the green and yellow parts in the magnified image) for handling parts.

Tamper-evident packaging produced by Lacerta Group feature integrated tabs (visible at top of inset photo) which must be ripped to open the container, instead of shrink-film bands or wrap-around labels. These are the company’s new “Fresh n’ Sealed” PET containers.

The company’s thermoformers include Sencorp 2500 systems (photo courtesy of SencorpWhite).

The company’s thermoformers include Sencorp 2500 systems (photo courtesy of SencorpWhite).
The Rest of the Story
“There were signs of instability,” says Ali Lotfi, recalling what Iran was like right before the Iranian Revolution of 1978, when he immigrated to the USA. Soon after arriving, he started and completed work towards an engineering degree from Northeastern University, then worked for BASF before founding Lacerta Group in 1993. Ali’s brother Mory came over a year later, earned undergraduate and graduate engineering degrees at Northeastern, and worked as an R&D engineer for Waters Corp., a maker of analytical lab instruments. Their cousin Mostafa Lotfi likewise has a mechanical engineering background and worked for Polaroid.
Lacerta Group’s path towards thermoforming likewise started with its own uniquely 20th-century reference point: data-storage tape. The company was originally founded to perfect ways of recycling polyester-based magnetic tapes and other storage media that were once commonly used in computers and electronics. Lotfi says the company recovered the polyester, coating, and chromium oxide from waste tape and edge-trim from tape manufacturing. Lacerta partnered with DuPont to develop the operation, which hired people from the Boston inner city as workers.
Ali Lotfi soon saw that they could take the tape polyester “and add value to it”—by making packaging from it. “Based on that, we learned thermoforming.”
The company began buying thermoforming equipment and producing and marketing thermoformed packaging in 1996, moving to its current Mansfield location in 2003. Data storage tape, no longer being made or used much anymore, was no longer the focus—packaging was.
When the economy crashed in 2008, the Lotfis began looking to focus more on the needs of the many medical device manufacturers in eastern Massachusetts, to add to the company’s customer base. This required a class-8 cleanroom environment for thermoforming, and the rest is history.
A family affair: (from left to right) Ali Lotfi, Lacerta president; his cousin Mostafa Lotfi, manufacturing VP; and Ali’s brother Mory, operations VP.
Two days before the New England Patriots football team won the 2015 NFL Super Bowl, Lacerta Group, Inc. opened its doors for a visit from Plastics Engineering. Coincidentally, the company, located in Mansfield, Massachusetts, USA, is only a ten-minute drive from the Patriots’ home stadium.
This packaging thermoformer is on its own kind of winning streak, with its business growing 25% in 2014, plus expected growth of 25% this year and next, says founder and president Ali Lotfi. The company is privately owned by Lotfi and two partners, does nearly $30 million in annual business, and has about 125 employees.
Lacerta specializes in making thermoformed packaging and products with cleanliness requirements. Cleanroom thermoforming has allowed it to expand into wider-ranging work in medical applications, including some complex designs. For example, “Something that’s challenging to make is this package for a catheter,” said Lotfi, pointing to thermoform that’s narrow in width but 67 inches (1.7 m) long. “That’s a pretty long piece for a thermoforming application.”
But food producers are the company’s biggest clients, with food packaging constituting about 50% of its business and medical and consumer products and packaging making up the remainder.
“We run everything in the cleanroom,” Lotfi says. All blisters, trays, clamshells, and other products are formed on ten thermoforming lines in ISO class-8 (U.S. class 100,000) cleanrooms, which provide the level of cleanliness needed for producing medical trays, for instance. And in 2014, the company invested in a 1500-ft2 (140-m2) class-8 hard-walled cleanroom specifically for assembly operations.
A Brief Tour
It’s not easy being clean. Maintaining the thermoforming cleanroom areas requires daily AM and PM cleaning procedures, as well as weekly and monthly cleaning schedules. And workers inside the cleanroom curtains must be covered head to toe with gowns and hair nets (and, when necessary, beard nets).
Outdoor air is pushed through filters into the clean environment, creating positive pressure that prevents the ingress of normal shop air. The filters are checked regularly, as is the whole operation, by periodic visits from third-party inspectors for confirming the cleanroom rating, says Ali Lotfi. Meanwhile, ionized air is used to keep sheet materials free of contaminants.
But how can you be sure it’s always clean enough, especially for medical packaging? Constant part sampling, Lotfi says. Plus, medical products are individually handled and inspected and double-bagged for shipment (not simply stacked and packed). Moreover, the company assigns its more experienced workers to the medical thermoforming lines, he adds.
Having relatively new production equipment helps. The cleanroom’s ten production thermoformers are from SencorpWhite, and all were purchased within the last four years. “I like the robustness of the machines and the accuracy,” says Mostafa Lotfi, vice president of manufacturing. The supplier is based only a short distance away, thus, “The service is also great,” he adds.
Lacerta also just recently installed a robot on one machine, with production end-of-arm tooling that allows automated product inspection as well as part handling and packing.
Quick & Flexible
The chosen thermoformers allow for quick tool changes: in one hour, versus several hours, says Ali Lotfi. Workers go to other work stations during mold changes, he adds—a practice that’s particularly important in this cost-competitive manufacturing region of the USA.
But the flexible cleanroom operation is only a part of the whole. Lacerta designs and builds its own tooling, with a team of six designers using Dassault Systèmes Solidworks for design and CNC Software’s Mastercam for machining programs. Its design group also has at hand a MakerBot 3-D printer, a digital scanner, and three thermoformers devoted to product development and protoyping.
The mold shop has eight CNC machines for cutting aluminum tooling and plug assists, which are used to help the vacuum pull heated sheet into the female mold. The company also builds package-sealing tools for customers’ operations.
Production materials include glycol-modified polyethylene terephthalate (PETG) and high-impact polystyrene for medical applications, and PET and PP for multiple products. Obviously, not all materials process the same way. “We at Lacerta love all thermoformable plastics,” says Mory Lotfi, vice president of operations. But multi-layer/laminated materials can be more challenging and harder to run, he adds, since they “require more control and better design and tooling.”
Recycled-content material is incorporated into some material handling, consumer, and cosmetics packaging products, says Ali Lotfi, but food and medical products require 100% virgin materials. Along with their purity limitations, recycled materials can present package-sealing and color inconsistencies, and the materials often can’t be run as fast as virgin materials in thermoforming, he says.
Some packaging requires the use of distortion-printed rollstock for producing colorful decorated containers. This rollstock can be tricky to use in production to get the right appearance on the package. “We do a lot of printed lids using print registration/distortion technology,” says Mory Lotfi. “There are many variables with much tighter tolerances to keep in mind—both in the design phase and during production.”
Food and Medical Converge
Lately, stricter food-contact safety requirements for packaging are “almost matching up with” medical requirements, observes Ali Lotfi. This could be a challenge for any company in food packaging. “We’ve seen growing emphasis on food safety, cleanliness, and quality requirements” over the last five years, accompanied by much more pressure from customers, he adds.
To this end, the company is implementing SQF Institute Level 2 food safety standards by the middle of the year—this is an industry-wide certification required by major food manufacturers (www.sqfi.com/suppliers/certification-steps). “We are already ISO 9001-2008 certified, so we have already gone through the challenges that a company faces when implementing such certifications,” says Mory Lotfi. “Some aspects of [SQF Level 2] will be new to us, but with proper training and documentation, we should face minimum
challenges.”
Trends and Next Steps
Ali Lotfi is also seeing more requests from customers wanting to transform current injection-molded parts into less-expensive thermoformable designs—like turning a molded PP deli container into a high-clarity formed PET container of the same shape and capability. Tall, deep containers of this type will be challenging to thermoform, he notes.
Another niche the company is moving into is tamper-evident food containers. Here, tabs thermoformed with the container must be ripped in order for the final packaged product to be opened. The company offers this design as a better alternative to shrink-wrap bands or wrap-around labels on PET packages for fresh foods, for example.
Meanwhile, the Lotfis are seeing more business moving south—of the border. Lacerta already has a thermoforming and injection molding operation near Mexico City (for making food and cosmetics packaging), where a lot of customers are located. Ali Lotfi says there’s a “definite possibility” Lacerta will also produce medical packaging in Mexico, because medical customers are moving their operations there.
The company also hopes to continue to provide responsive customer service (with 98.7% of deliveries on time in the last quarter of 2014), flexibility to customers’ needs, innovation with new concepts, and clean products.
