Adenocarcinoma of the Urethra With Mucinous Features
Arthi Satyanarayan, BA,1 Lucas Redd, MD,2 Anthony Dyer, MD,3 Andrew Wright, MD,3 Jonathan Walker, MD3
1The University of Arizona College of Medicine, Tucson, AZ; 2Department of Pathology, The University of Arizona Medical Center, Tucson, AZ; 3Division of Urology, The University of Arizona Medical Center, Tucson, AZ
Primary adenocarcinoma of the female urethra is a rare malignancy. Previous studies hypothesize multiple origins, including periurethral glands or intestinal metaplasia. We report a case of a 60-year-old white woman with adenocarcinoma of the urethra who initially presented with obstructive voiding complaints secondary to a urethral mass. Wide local excision revealed invasive adenocarcinoma of the urethra with mucinous features. There was intestinal metaplasia adjacent to the tumor, as well as separate identification of intestinal metaplasia along the urethra. Ultimately, the patient underwent radical cystectomy with ileal conduit urinary diversion with no evidence of recurrence, indicating the role of early identification and surgical intervention for such cases.
[Rev Urol. 2015; 17(1):38-41 doi: W.3909/riu0622]
© 2015 MedReviews ®, LLC
Adenocarcinoma of the Urethra With Mucinous Features
Arthi Satyanarayan, BA,1 Lucas Redd, MD,2 Anthony Dyer, MD,3 Andrew Wright, MD,3 Jonathan Walker, MD3
1The University of Arizona College of Medicine, Tucson, AZ; 2Department of Pathology, The University of Arizona Medical Center, Tucson, AZ; 3Division of Urology, The University of Arizona Medical Center, Tucson, AZ
Primary adenocarcinoma of the female urethra is a rare malignancy. Previous studies hypothesize multiple origins, including periurethral glands or intestinal metaplasia. We report a case of a 60-year-old white woman with adenocarcinoma of the urethra who initially presented with obstructive voiding complaints secondary to a urethral mass. Wide local excision revealed invasive adenocarcinoma of the urethra with mucinous features. There was intestinal metaplasia adjacent to the tumor, as well as separate identification of intestinal metaplasia along the urethra. Ultimately, the patient underwent radical cystectomy with ileal conduit urinary diversion with no evidence of recurrence, indicating the role of early identification and surgical intervention for such cases.
[Rev Urol. 2015; 17(1):38-41 doi: W.3909/riu0622]
© 2015 MedReviews ®, LLC
Adenocarcinoma of the Urethra With Mucinous Features
Arthi Satyanarayan, BA,1 Lucas Redd, MD,2 Anthony Dyer, MD,3 Andrew Wright, MD,3 Jonathan Walker, MD3
1The University of Arizona College of Medicine, Tucson, AZ; 2Department of Pathology, The University of Arizona Medical Center, Tucson, AZ; 3Division of Urology, The University of Arizona Medical Center, Tucson, AZ
Primary adenocarcinoma of the female urethra is a rare malignancy. Previous studies hypothesize multiple origins, including periurethral glands or intestinal metaplasia. We report a case of a 60-year-old white woman with adenocarcinoma of the urethra who initially presented with obstructive voiding complaints secondary to a urethral mass. Wide local excision revealed invasive adenocarcinoma of the urethra with mucinous features. There was intestinal metaplasia adjacent to the tumor, as well as separate identification of intestinal metaplasia along the urethra. Ultimately, the patient underwent radical cystectomy with ileal conduit urinary diversion with no evidence of recurrence, indicating the role of early identification and surgical intervention for such cases.
[Rev Urol. 2015; 17(1):38-41 doi: W.3909/riu0622]
© 2015 MedReviews ®, LLC
Key words
Urethral adenocarcinoma • Urethra • Skene gland • Genital tract tumor • Radical cystectomy
Key words
Urethral adenocarcinoma • Urethra • Skene gland • Genital tract tumor • Radical cystectomy
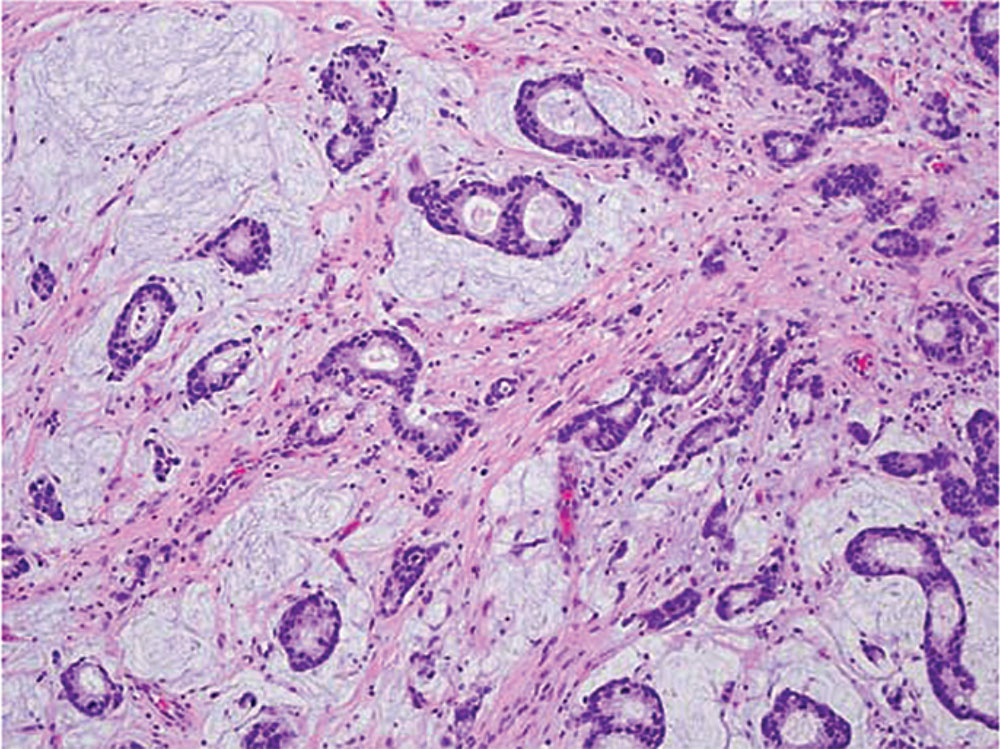
Figure 1. Invasive adenocarcinoma with mucinous features (original magnification, × 200). Some areas contained adenocarcinoma glands floating in pools of mucin, which prompted the diagnosis of adenocarcinoma with mucinous features.

Figure 1. Invasive adenocarcinoma with mucinous features (original magnification, × 200). Some areas contained adenocarcinoma glands floating in pools of mucin, which prompted the diagnosis of adenocarcinoma with mucinous features.
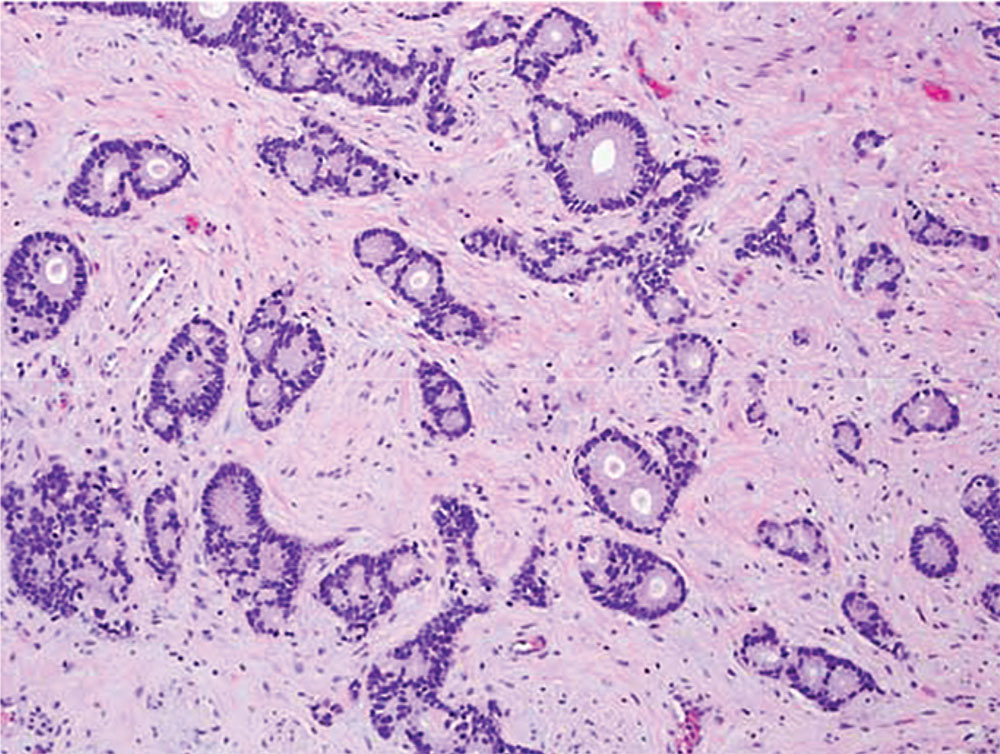
Figure 2. Invasive adenocarcinoma with mucinous features (original magnification, × 200). The typical columnar type adenocarcinoma can be seen infiltrating the subepithelial connective tissue with the tumor cells forming both individual glands and clusters of glands.
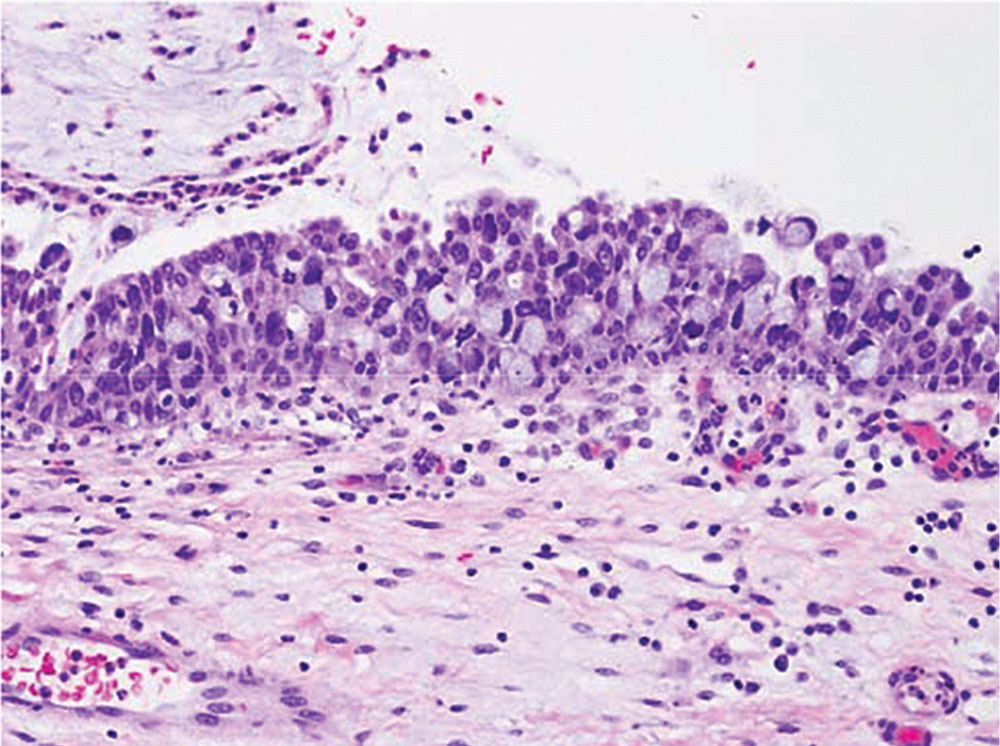
Figure 3. Mucinous metaplasia (original magnification, × 400). In sections adjacent to the tumor, metaplastic mucin-filled goblet cells can be seen intermixed with the normal urothelium.

Figure 3. Mucinous metaplasia (original magnification, × 400). In sections adjacent to the tumor, metaplastic mucin-filled goblet cells can be seen intermixed with the normal urothelium.
It is possible that primary adenocarcinoma of the urethra has distinct subtypes that develop through multiple pathways, with variation in immunohistochemical staining patterns and associated histologic findings … supporting this theory.
Main Points
• Primary urethral adenocarcinoma in women is a rare malignancy of unclear origin; it is divided into two primary histologic subtypes: clear cell and columnar/mucinous. Clear-cell adenocarcinoma is rare and may histologically resemble genital tract tumors in women.
• One origin of primary urethral adenocarcinoma is thought to be the periurethral Skene glands. An alternative theory of origin includes development from the intestinal metaplasia; cases of the tumor arising out of urethritis glandularis have been reported.
• Tumors typically spread through local extension and can ulcerate to the skin and vulva. Due to the short length of the female urethra, local spread tends to be more destructive. Lymphatic spread is uncommon; hematogenous spread can also occur to the lung, liver, bone, and brain.
• Magnetic resonance imaging can be used for tumor identification and staging; however, biopsy is required for a definitive diagnosis. For advanced disease, combination chemotherapy, radiation, and surgery have been described in the literature.
Main Points
• Primary urethral adenocarcinoma in women is a rare malignancy of unclear origin; it is divided into two primary histologic subtypes: clear cell and columnar/mucinous. Clear-cell adenocarcinoma is rare and may histologically resemble genital tract tumors in women.
• One origin of primary urethral adenocarcinoma is thought to be the periurethral Skene glands. An alternative theory of origin includes development from the intestinal metaplasia; cases of the tumor arising out of urethritis glandularis have been reported.
• Tumors typically spread through local extension and can ulcerate to the skin and vulva. Due to the short length of the female urethra, local spread tends to be more destructive. Lymphatic spread is uncommon; hematogenous spread can also occur to the lung, liver, bone, and brain.
• Magnetic resonance imaging can be used for tumor identification and staging; however, biopsy is required for a definitive diagnosis. For advanced disease, combination chemotherapy, radiation, and surgery have been described in the literature.
Carcinoma of the urethra in women is an uncommon malignancy, accounting for I approximately 0.02% of cancers in women.1,2 Of the urethral cancers, 70% are squamous cell, 20% are transitional cell, and 10% are adenocarcinoma.1 Primary urethral adenocarcinoma in women is a rare malignancy of unclear origin. It has been divided into two primary histologic subtypes: clear cell and columnar/mucinous (“intestinal”).3 The histologic appearance of columnar/mucinous-type adenocarcinoma is similar to colonic and endocervical malignancies.4 However, clear-cell adenocarcinoma is rare and may histologically resemble genital tract tumors in women.2,4 Previous studies have alluded to the origin of urethral adenocarcinoma in the periurethral Skene glands, as these tumors were known to stain positive for prostate-specific antigen (PSA).5,6 Other studies have proposed a different pathway after noting that chronic irritation of the urethral mucosa can lead to metaplasia into intestinal tissue, or urethritis glan-dularis.6 Although a PSA-negative adenocarcinoma does not necessarily rule out a periurethral Skene gland origin, tumors arising in this urethritis glandularis pathway are typically PSA negative.7,8 Clear-cell carcinoma has been postulated to originate from a third pathway based on a different morphology and staining pattern. With regard to location, proximal tumors are typically the adenocarcinoma type and have a poorer prognosis than distal squamous cell tumors.4 Urethral adenocarcinoma typically presents with vague symptoms leading to discovery of more advanced tumors at the time of presentation. Delayed presentation has made standardization of treatment difficult to determine in the majority of patients.2
Case Report
A 60-year-old woman presented with obstructive voiding symptoms. Physical examination revealed a 1.0 × 1.5-cm distal urethral mass. The mass was well circumscribed, and firm in appearance. She underwent an open excision of the mass and cystoscopy at an outside institution, which revealed no mucosal bladder lesions. The original pathology report favored a gastrointestinal primary adenocarcinoma but also discussed the possibility of an uncommon primary urethral tumor. Cytology at the time of surgery was also positive for adenocarcinoma.
Postoperatively she developed vaginal spotting and new-onset gross hematuria. She transferred her care to our tertiary care center. Physical examination revealed no residual mass. Positron emission tomography and computed tomography scan results were negative for metastatic disease. She underwent repeat cystoscopy and distal urethrectomy Pathology revealed invasive adenocarcinoma with mucinous features (Figures 1 and 2) with negative margins in the urethral mass excision. The tumor stained positive for cytokeratin (CK) 20 and CDX2 and negative for CK7. The residual tumor was 0.6 cm in size. No lymphovascular invasion was identified. An adjacent focus of mucinous metaplasia in the distal urethra (Figure 3) was also present, suggesting that the tumor developed from the metaplastic cells. Cystoscopy was unremarkable and cytology showed only atypical cells.
After extensive counseling, the patient desired radical cystectomy and the creation of ileal conduit urinary diversion. Within the residual proximal urethra, a microscopic focus of adenocarcinoma was found. An additional separate focus of intestinal metaplasia was found in the more distal residual urethra. No evidence of malignancy or intestinal metaplasia was identified within the bladder or lymph nodes. She was disease-free at 9-month follow-up.
Discussion
Primary urethral carcinoma in women is a rare diagnosis, accounting for 0.003% of malignant neoplasms of the female genitourinary tract.7 In the United States, the incidence of urethral cancer is approximately 1.5 per 1 million women.2 One origin of primary urethral adenocarcinoma is thought to be the periurethral Skene glands.1 An alternative theory of origin includes development from the intestinal metaplasia, as cases of the tumor arising out of urethritis glandularis have been reported.8 Our case is an example representative of the latter theory of origin. It is possible that primary adenocarcinoma of the urethra has distinct subtypes that develop through multiple pathways,3 with variation in immunohistochemical staining patterns and associated histologic findings (adjacent intestinal metaplasia or Skene glands with cytologic atypia) supporting this theory. Columnar-type adenocarcinoma is most often composed of colonic-type glandular epithelium and may contain a mucinous component characterized by areas that exhibit abundant extracellular mucin, as in this case. A previous case report of urethral adenocarcinoma reported that intestinal metaplasia can occur in women from age 2 to age 78 years.8 Intestinal metaplasia in older women can occur secondary to chronic inflammation, or mechanical or chemical injury, and appears positive on CDX2 staining.8 Primary urethral adenocarcinoma can resemble adenocarcinoma at other sites, such as the colon or pancreas, on hematoxylin and eosin-stained sections.
Patients can present with a variety of symptoms, including dysuria, urinary frequency, and a palpable mass, which account for 50% of presenting symptoms. Interestingly, gross hematuria is a less common presenting symptom.9 Tumors typically spread through local extension and can ulcerate to the skin and vulva. Due to the short length of the female urethra, local spread tends to be more destructive.2 Lymphatic spread is uncommon, but one third of patients have palpable nodes at the time of presentation. Hematogenous spread can also occur to the lung, liver, bone, and brain.1 Studies indicate that magnetic resonance imaging can be used for tumor identification and staging; however, biopsy is required for a definitive diagnosis.10,11 Our patient presents a challenging management scenario. She had normal postoperative physical examination results and imaging studies, yet she had persistent microscopic invasive adenocarcinoma in the residual urethra taken during the cystectomy. The lack of reliable monitoring options creates a management dilemma with regard to continuing local therapies or proceeding with more invasive surgical options.
For advanced disease, combination chemotherapy, radiation, and surgery have been described in the literature.12 Using early surgical intervention, we hope to have avoided any adjuvant therapy for our patient in the future. ![]()
References
- Wang X, Bai P, Su H, et al. Management of primary adenocarcinoma of the female urethra: report of two cases and review of the literature. Oncol Lett. 2012;4:951-954.
- Scantling D, Ross C, Jaffe J. Primary clear cell adenocarcinoma of a urethral diverticulum treated with multidisciplinary robotic anterior pelvic exenteration. Case Rep Med. 2013;2013:387591.
- Dodson MK, Cliby WA, Pettavel PP, et al. Female urethral adenocarcinoma: evidence for more than one tissue of origin? Gynecol Oncol. 1995;59: 352-357.
- Mehra R, Vats P, Kalyana-Sundaram S, et al. Primary urethral clear-cell adenocarcinoma: comprehensive analysis by surgical pathology, cytopathology, and next-generation sequencing. Am J Pathol. 2014;184:584-591.
- Han JY, Kim KH, Kim L, et al. Cytologic findings of clear cell adenocarcinoma of the urethra: a case report. Korean J Pathol. 2012;46:210-214.
- Murphy DP, Pantuck AJ, Amenta PS, et al. Female urethral adenocarcinoma: immunohistochemical evidence of more than 1 tissue of origin. J Urol. 1999;161:1881-1884.
- Reis LO, Billis A, Ferreira FT, et al. Female urethral carcinoma: evidences to origin from Skene’s glands. Urol Oncol. 2011;29:218-223.
- Hale CS, Huang H, Melamed J, et al. Urethral adenocarcinoma associated with intestinal-type metaplasia, case report and literature review. Int J Clin Exp Pathol. 2013;6:1665-1670.
- DiMarco DS, DiMarco CS, Zincke H, et al. Surgical treatment for local control of female urethral carcinoma. Urol Oncol. 2004;22:404-409.
- Karnes RJ, Breau RH, Lightner DJ. Surgery for urethral cancer. Urol Clin North Am. 2010;37: 445-457.
- Ouzaid I, Hermieu JF, Dominique S, et al. Management of adenocarcinoma of the female urethra: case report and brief review. Can J Urol. 2010;17: 5404-5407.
- Wein AJ, Kavoussi LR, Novick AC, et al. Campbell- Walsh Urology. 10th ed. Philadelphia, PA: Saunders Elsevier; 2011.