Solitary Renal Fossa Recurrence of Renal Cell Carcinoma After Nephrectomy
Ji-Jian Chow, MBBS, MA (Cantab),1 Kamran Ahmed, MRCS, PhD,2 Zahoor Fazili, MRCS,3 Mohammed Sheikh, FRCS (Urol),3 Matin Sheriff, FRCS (Urol), FEBU, PhD3
1Department of General Surgery, Queen Elizabeth’s Hospital Woolwich, London, UK; 2MRC Centre for Transplantation, King’s College London, King’s Health Partners, Department of Urology, Guy’s Hospital, London, UK; 3Department of Urology, Medway Maritime Hospital, Kent, UK
Renal cell carcinoma without metastasis responds well to surgical excision but is known to recur postnephrectomy. In a small but significant number of patients this recurrence is not accompanied by metastasis, which is important as these people benefit from further surgery. We examined 20 articles from the current literature to ascertain how best to treat this condition. Surgical management renders better results than conservative or medical therapies. Readily available investigations such as blood tests and computed tomography can help determine the right patients for surgery in an evidence-based fashion. Current findings have allowed us to suggest a protocol for the treatment of solitary renal fossa recurrence of postnephrectomy renal cell carcinoma. There are further opportunities for study in validating our protocol, and in novel renal cell carcinoma treatment strategies that have not been tested on solitary renal fossa recurrences.
[Rev Urol. 2014;16(2):76-82 doi: 10.3909/riu0598]
© 2014 MedReviews®, LLC
Solitary Renal Fossa Recurrence of Renal Cell Carcinoma After Nephrectomy
Ji-Jian Chow, MBBS, MA (Cantab),1 Kamran Ahmed, MRCS, PhD,2 Zahoor Fazili, MRCS,3 Mohammed Sheikh, FRCS (Urol),3 Matin Sheriff, FRCS (Urol), FEBU, PhD3
1Department of General Surgery, Queen Elizabeth’s Hospital Woolwich, London, UK; 2MRC Centre for Transplantation, King’s College London, King’s Health Partners, Department of Urology, Guy’s Hospital, London, UK; 3Department of Urology, Medway Maritime Hospital, Kent, UK
Renal cell carcinoma without metastasis responds well to surgical excision but is known to recur postnephrectomy. In a small but significant number of patients this recurrence is not accompanied by metastasis, which is important as these people benefit from further surgery. We examined 20 articles from the current literature to ascertain how best to treat this condition. Surgical management renders better results than conservative or medical therapies. Readily available investigations such as blood tests and computed tomography can help determine the right patients for surgery in an evidence-based fashion. Current findings have allowed us to suggest a protocol for the treatment of solitary renal fossa recurrence of postnephrectomy renal cell carcinoma. There are further opportunities for study in validating our protocol, and in novel renal cell carcinoma treatment strategies that have not been tested on solitary renal fossa recurrences.
[Rev Urol. 2014;16(2):76-82 doi: 10.3909/riu0598]
© 2014 MedReviews®, LLC
Solitary Renal Fossa Recurrence of Renal Cell Carcinoma After Nephrectomy
Ji-Jian Chow, MBBS, MA (Cantab),1 Kamran Ahmed, MRCS, PhD,2 Zahoor Fazili, MRCS,3 Mohammed Sheikh, FRCS (Urol),3 Matin Sheriff, FRCS (Urol), FEBU, PhD3
1Department of General Surgery, Queen Elizabeth’s Hospital Woolwich, London, UK; 2MRC Centre for Transplantation, King’s College London, King’s Health Partners, Department of Urology, Guy’s Hospital, London, UK; 3Department of Urology, Medway Maritime Hospital, Kent, UK
Renal cell carcinoma without metastasis responds well to surgical excision but is known to recur postnephrectomy. In a small but significant number of patients this recurrence is not accompanied by metastasis, which is important as these people benefit from further surgery. We examined 20 articles from the current literature to ascertain how best to treat this condition. Surgical management renders better results than conservative or medical therapies. Readily available investigations such as blood tests and computed tomography can help determine the right patients for surgery in an evidence-based fashion. Current findings have allowed us to suggest a protocol for the treatment of solitary renal fossa recurrence of postnephrectomy renal cell carcinoma. There are further opportunities for study in validating our protocol, and in novel renal cell carcinoma treatment strategies that have not been tested on solitary renal fossa recurrences.
[Rev Urol. 2014;16(2):76-82 doi: 10.3909/riu0598]
© 2014 MedReviews®, LLC
Key words
Renal cancer • Recurrence • Nephrectomy • Complications • Management
Key words
Renal cancer • Recurrence • Nephrectomy • Complications • Management
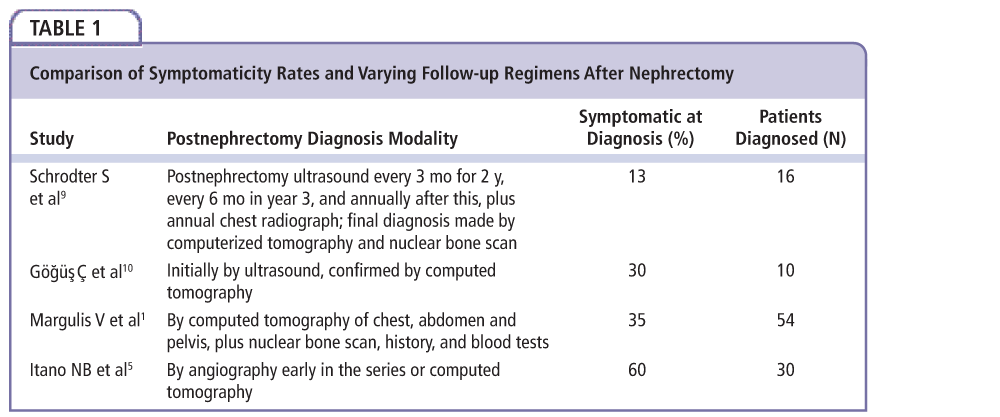

Figure 1. Computed tomography image of a patient with renal fossa recurrence of renal cancer after nephrectomy. Of note is the large mass identifiable in the spleen.

Figure 1. Computed tomography image of a patient with renal fossa recurrence of renal cancer after nephrectomy. Of note is the large mass identifiable in the spleen.

Renal cancer patients should be stratified into risk groups that will guide the intensity of follow-up.
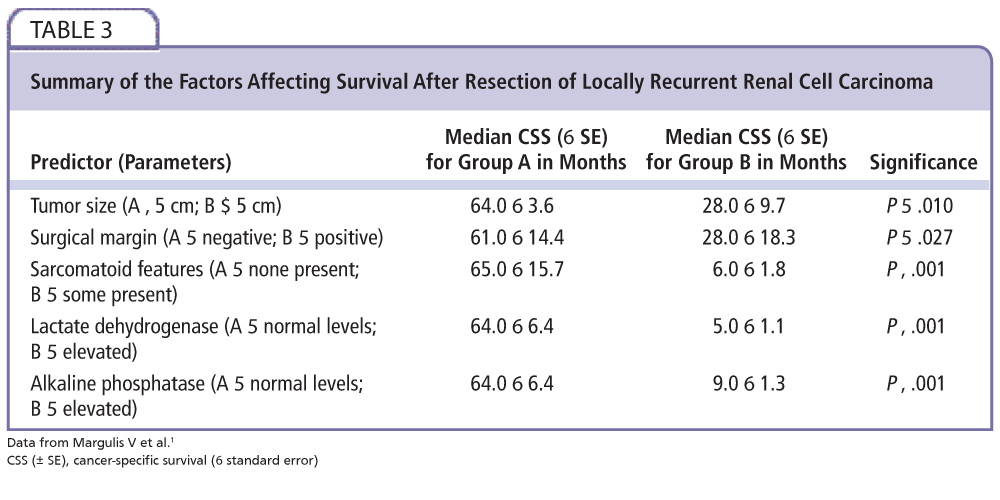

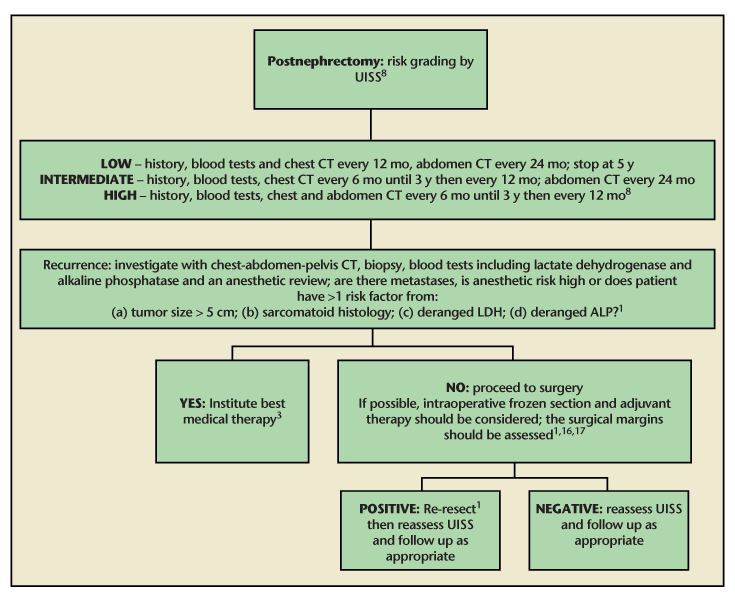
Figure 2. A flowchart detailing our recommended follow-up regimen, from nephrectomy to resection of recurrence and beyond. ALP, alkaline phosphatase; CT, computed tomography; LDH, lactate dehydrogenase; UISS, University of California Integrated Staging System. Data from Margulis et al1; Pertiia and Managadze3; Lam et al8; Sandhu et al17; and Tanguay et al.16

Figure 2. A flowchart detailing our recommended follow-up regimen, from nephrectomy to resection of recurrence and beyond. ALP, alkaline phosphatase; CT, computed tomography; LDH, lactate dehydrogenase; UISS, University of California Integrated Staging System. Data from Margulis et al1; Pertiia and Managadze3; Lam et al8; Sandhu et al17; and Tanguay et al.16


Main Points
• Solitary renal fossa recurrence of renal cancer is a rare but significant event, and these patients respond much better to surgery than do those with metastases.
• Readily available investigations have been shown to be predictive of outcome and therefore useful for selecting patients for surgery.
• Current evidence shows laparoscopic surgical resection should be the mainstay of treatment. However, there is scope for new therapies to be combined into the management of this condition.
Main Points
• Retroperitoneal fibrosis (RPF) is a rare condition characterized by the replacement of normal tissue with fibrosis and/or chronic nonspecific inflammation. In this patientís case, the fibrosis was located in the pelvis.
• Based on clinical findings in this patient, the first course of action was retrograde stent placement, placement of nephrostomy tubes, and bladder decompression.
•The patientís laboratory test results showed a worsening creatinine level and further inflammation after 6 weeks, at which point biopsy was performed, showing cystitis with marked stromal fibrosis, along with increased C-reactive protein, erythrocyte sedimentation rate, and immunoglobulin G4 (IgG4).
• Immunohistochemistry for IgG4 was performed, showing an increase in IgG4-positive plasma cells. The patient started on high-dose corticosteroids, which normalized all inflammatory markers and tissue inflammation (as seen on imaging studies). He currently is without any significantly bothersome urinary symptoms.
• PF can have several etiologies, but in 8% to 10% of cases, the cause is malignancy, which makes biopsy necessary before therapy in all suspected cases.
• Elevated IgG4 levels on a pathology report can indicate an autoimmune origin of this condition. It is unclear whether all cases of RPF are related to IgG4, and whether clinicopathologic characteristics of all cases are similar.
Kidney cancers represent 2% of cancers worldwide; the most common type is renal cell carcinoma. Curative treatment of localized disease is a nephrectomy. Following surgery, recurrence can happen locally with an incidence of 1.61%.1-5 A solitary renal fossa local recurrence is rare but important to distinguish from local recurrence with metastasis, which would not benefit from surgical resection. The 5-year survival postresection of local recurrence for those without metastasis compared with those with metastasis was 62% compared with 0%.4 The kidneys are bordered by the colon, spleen, liver, stomach, and associated neurovascular structures, all of which may be invaded in this form of recurrence; specific morbidity is related to the invasion and subsequent resection of these organs. General morbidity is caused by the surgery itself, with pain, infection, and hemorrhage being major contributors (Figure 1). This article explains predictive factors in recurrence, useful diagnostic modalities, and management, and provides recommendations and highlights opportunities for further study.
Surveillance and Predictive Factors
The most recent guidelines for treatment and follow-up of renal cancer are the 2010 European Association of Urology (EAU) guidelines. They suggest that patients should be stratified into risk groups that will guide the intensity of follow-up. A comparison of the various prognostic scores was undertaken by Cindolo and colleagues,6 who found the Kattan scoring system to be the most accurate, with the UCLA Integrated Staging System (UISS) coming in a close second.6-8 However, the UISS is the only system to stratify the patients into groups, to suggest a follow-up regimen, and to have been externally validated. The UISS classifies patients into low-, intermediate-, and high-risk groups based upon their tumor, lymph node, and metastasis stage, Fuhrman grade, and Eastern Cooperative Oncology Group Performance Status. Local recurrence increases with increasing risk strata, only receiving a brief mention in the low-risk group and representing 14.5% and 25.8% of recurrences in the intermediate- and high-risk groups, respectively.
Although the Kattan scoring system does not provide us with a thorough guide to follow-up, it can be useful in showing us what the risk factors for recurrence are, as the endpoint is recurrence-free survival. Risk factors include pre-nephrectomy systemic symptoms, increasing tumor size, increasing tumor stage, and conventional (rather than papillary or chromophobe) histology.7
Surveillance for early detection is preferable as better outcomes occur for tumors resected in their smaller stages.1 Margulis and colleagues1 found that resected tumors resected < 5 cm yielded a median cancer-specific survival of 64 months, compared with 28 months for tumors > 5 cm. Several studies offer an estimate of symptomaticity,1,5,9,10 summarized in Table 1. The range of results is very wide, but most strikingly, the study by Schrodter and colleagues9 is the only one with a consistent follow-up regimen after nephrectomy and also has the lowest rate of symptomaticity (13%) at diagnosis. This could form the start of a good case for tight, tertiary center–defined follow-up regimens, but with only 16 examples, the evidence is not very strong. However, studies led by Itano5 and Margulis1 provide evidence that symptomaticity at diagnosis does not influence survival (P = .94 in the study by Itano and associates,5 whereas the study from Margulis and associates1 did not provide that statistic).
EAU guidelines suggest that ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI) should be the modalities used in detection and characterization of renal masses. MRI can be used to additionally characterize masses when CT is indeterminate, or when the patient is contrast allergic or pregnant. There is currently no recommendation for positron emission tomography. There is little information on the sensitivities and specificities for each modality in diagnosing renal fossa recurrences. However, Schrodter and colleagues9 noted that 3 of 16 (19%) patients had false-positive CT results proven by surgical exploration. The lesions proved to be two accessory spleens and one scar tissue granuloma from the previous surgery. This may provide a case for radiologically guided percutaneous biopsy before operation. However, caution is needed in large or small tumors as the negative predictive value of biopsies on these subsets can be very poor.11
Management
Management of local recurrence can be conservative, systemic, surgical, or radiotherapeutic; combination treatments have also been tested. Table 2 summarizes the approaches taken by the various literature. From the two studies that compare conservative management and any other treatment, it is clear that survival is improved for some interventions.3,5 Pertiia and Managadze3 showed survival of 27.5 ± 14.9 months in those treated by surgery and 10 ± 8.5 months in conservative management. Itano and colleagues5 showed a 5-year survival rate of 51% on a combination of surgery alone and surgery plus systemic treatment compared with 13% with conservative management.
Systemic treatments alone also do not show promise in the current comparative literature.4,5 Itano and colleagues5 showed 5-year survival of 18% compared with 51% in the surgical group. Bruno and coworkers4 showed 5-year survival of 62% for surgery and 0% for nonsurgical treatments. Median survival was 71.4 months and 9.9 months, respectively. However, in the 7 years between that publication and the writing of this article, several new systemic therapies have been introduced but not tested against surgery; these include sunitinib, temsirolimus and pazopanib, to name a few.12,13 Also, the studies mentioned chose their systemic therapy only groups by unresectability or extensive comorbidity, making the patient groups nonequivalent.
Evidence for combination therapy is limited. Patient numbers are small, and some studies contain both combination and surgical monotherapy without making a comparison between the two.1,5 Master and associates14 showed that intraoperative radiotherapy made no difference to survival. However, Frydenberg and colleagues15 consider perioperative radiotherapy; there is no comparison with surgery so this could still have a role in treatment. Studies by Schrodter and colleagues9 and Tanguay and colleagues16 are at odds about the use of systemic therapy in combination with surgery. The data from Schrodter and associates show no significant difference (P value not provided).9 The study by Tanguay and colleagues shows 75% disease recurrence in the surgery-only group and 50% in the combination group.17 However the combination group had been followed up for a shorter duration than the surgery-only group (range, 3-49 mo vs 62-136 mo). In addition, no comment is made upon the effect on mortality.
Several risk factors for poor outcome are discussed in the article by Margulis and colleagues,1 which can influence management further to the initial resection of local recurrence. They noted that positive surgical margins, recurrent tumor size, sarcomatoid features in the recurrence, and abnormal alkaline phosphatase and lactate dehydrogenase were associated with greater likelihood of cancer-specific death.1 The statistics are summarized in Table 3. Two other studies identify a positive surgical margin as an adverse risk factor.16,17 This provides a few options for further therapy: use of frozen sections to ensure negative margins, intraoperative radiotherapy, or additional techniques already used in localized renal cancer, such as cryotherapy or radiofrequency ablation.18,19 In addition, Margulis and colleagues1 demonstrated no increase in perioperative morbidity or hospital stay for re-resection (P = .265), making this a valid option in positive margin disease.
Within surgery there are also considerations for open and laparoscopic procedures. Bandi and colleagues20 make the strongest case for laparoscopic surgery, finding that conversion to open surgery may have benefitted only one of the five in the series, and even this was unclear. In addition, blood loss, operational time, and recovery were better for the laparoscopic patients than the prior reports on open surgery patients.
With regard to post–local recurrence resection follow-up, no article addresses whether one follow-up regimen is better than any other. Only a few of the authors actually specify their follow-up regimen.1,5,10,14,15,17,20 For a summary of the post–local recurrence resection follow up regimes please refer to Table 4.
Conclusions
Local recurrence without distant metastasis is a rare event. However, early identification and ruling out metastatic disease is important because patients can benefit from surgery, whereas their metastatic counterparts will not. Percutaneous biopsy may well have a role in presurgical diagnosis, as current imaging is not always accurate in this application.
Treatments including surgery outperform conservative and systemic-only therapies in all studies. The results on combination therapies are equivocal, but as many of the studies report that newer targeted systemic therapies are far better tolerated than early forms of immunotherapy, there may be a significant role for combination with surgery. Laparoscopic surgery generally outperforms open in perioperative morbidity with comparable oncologic outcome. Newer destructive energy sources such as radiofrequency and cryotherapy show promise for the future, perhaps as an aid to en bloc resection. The evidence also supports the need for careful resection of the tumor margins to ensure much reduced mortality; this even extends to re-resection as a viable way of improving survival. Surgical therapy with its risks may be more suitable for patients with fewer adverse risk factors for oncologic outcome.
Randomized, controlled, multicenter trials would be ideal to determine relative efficacy of treatments. Also, the trials comparing surgery with systemic therapy often use unresectability and comorbidity to decide treatment allocation, a confounder which future trials may aim to reduce. Further work with respect to new systemic therapies and new destructive energy sources could be carried out focusing on local recurrence resection only, as they are already studied in newly discovered renal cancer. In addition, work to quantify any increase in perioperative morbidity and mortality conferred by surgery, which includes inspection of frozen sections, would also be useful to work out if the benefit of negative tumor margins outweighs the risk of potentially longer surgery. An assessment of the UISS against the natural history of renal fossa recurrences would be invaluable in determining an evidence-based follow-up regimen for these patients. Figure 2 is a flowchart of our recommendations for follow-up and treatment.
The authors report no real or apparent conflicts of interest. ![]()
References
- Margulis V, McDonald M, Tamboli P, et al. Predictors of oncological outcome after resection of locally recurrent renal cell carcinoma. J Urol. 2009; 181:2044-2051.
- Russo P, Jang TL, Pettus JA, et al. Survival rates after resection for localized kidney cancer: 1989 to 2004. Cancer. 2008;113:84-96.
- Pertiia AR, Managadze LG. [Local recurrence of renal cell carcinoma after radical nephrectomy]. Urologiia. 2006;3:17-19.
- Bruno JJ 2nd, Snyder ME, Motzer RJ, Russo P. Renal cell carcinoma local recurrences: impact of surgical treatment and concomitant metastasis on survival. BJU Int. 2006;97: 933-938.
- Itano NB, Blute ML, Spotts B, Zincke H. Outcome of isolated renal cell carcinoma fossa recurrence after nephrectomy. J Urol. 2000;164: 322-325.
- Cindolo L, Patard JJ, Chiodini P, et al. Comparison of predictive accuracy of four prognostic models for nonmetastatic renal cell carcinoma after nephrectomy: a multicenter European study. Cancer. 2005;104:1362-1371.
- Kattan MW, Reuter V, Motzer RJ, et al. A postoperative prognostic nomogram for renal cell carcinoma. J Urol. 2001;166:63-67.
- Lam JS, Shvarts O, Leppert JT, et al. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J Urol. 2005;174:466-472; discussion 472; quiz 801.
- Schrodter S, Hakenberg OW, Manseck A, et al. Outcome of surgical treatment of isolated local recurrence after radical nephrectomy for renal cell carcinoma. J Urol. 2002;167:1630-1633.
- Göğüş Ç, Baltaci S, Bedük Y, et al. Isolated local recurrence of renal cell carcinoma after radical nephrectomy: experience with 10 cases. Urology. 2003;61:926-929.
- Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281-1287.
- Posadas EM, Figlin RA. Systemic therapy in renal cell carcinoma: advancing paradigms. Oncology (Williston Park). 2012;26:290-301.
- Hutson TE. Targeted therapies for the treatment of metastatic renal cell carcinoma: clinical evidence. Oncologist. 2011;16 (suppl 2):14-22.
- Master VA, Gottschalk AR, Kane C, Carroll PR. Management of isolated renal fossa recurrence following radical nephrectomy. J Urol. 2005;174:473-477; discussion 477.
- Frydenberg M, Gunderson L, Hahn G, Fieck J. Preoperative external beam radiotherapy followed by cytoreductive surgery and intraoperative radiotherapy for locally advanced primary or recurrent renal malignancies. J Urol.1994;152:15-21.
- Tanguay S, Pisters LL, Lawrence DD, Dinney CP. Therapy of locally recurrent renal cell carcinoma after nephrectomy. J Urol. 1996;155:26-29.
- Sandhu SS, Symes A, A’Hern R, et al. Surgical excision of isolated renal-bed recurrence after radical nephrectomy for renal cell carcinoma. BJU Int. 2005;95: 522-525.
- Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer. 2008;113:2671-2680.
- Stein RJ, Kaouk JH. Renal cryotherapy: a detailed review including a 5-year follow-up. BJU Int. 2007; 99(5 Pt B):1265-1270.
- Bandi G, Wen CC, Moon TD, Nakada SY. Single center preliminary experience with hand-assisted laparoscopic resection of isolated renal cell carcinoma fossa recurrences. Urology. 2008;71:495-499; discussion 499-500.
- Pereverzev AS, Shchukin DV, Iliukhin IuA. [Local recurrence of renal cell carcinoma after nephrectomy.] Urologiia. 2003;6:14-18.