Undergraduate Modules for Bio-Based Plastics
Educational modules focused on bio-based polymers resulted in good learning outcomes for undergraduate students
Previous Article Next Article
By Carol Barry, Bridgette Budhlall, and Ramaswamy Nagarajan
Department of Plastics Engineering, University of Massachusetts Lowell, Massachusetts, USA
Undergraduate Modules for Bio-Based Plastics
Educational modules focused on bio-based polymers resulted in good learning outcomes for undergraduate students
Previous Article Next Article
By Carol Barry, Bridgette Budhlall, and Ramaswamy Nagarajan
Department of Plastics Engineering, University of Massachusetts Lowell, Massachusetts, USA
Undergraduate Modules for Bio-Based Plastics
Educational modules focused on bio-based polymers resulted in good learning outcomes for undergraduate students
Previous Article Next Article
By Carol Barry, Bridgette Budhlall, and Ramaswamy Nagarajan
Department of Plastics Engineering, University of Massachusetts Lowell, Massachusetts, USA

UMass Lowell’s Mark and Elisia Saab Emerging Technologies and Innovation Center (photo courtesy of UMass Lowell).

UMass Lowell’s Mark and Elisia Saab Emerging Technologies and Innovation Center (photo courtesy of UMass Lowell).
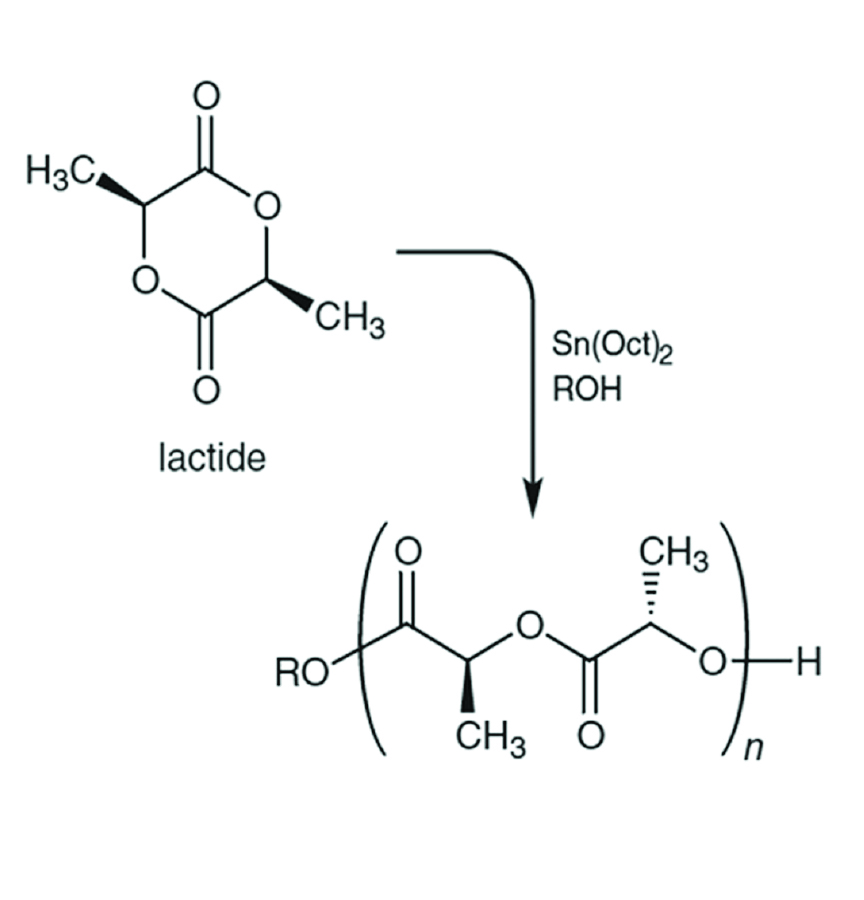
Figure 1: Mechanism of the ring-opening polymerization of L-lactide catalyzed by Sn(Oct)2.
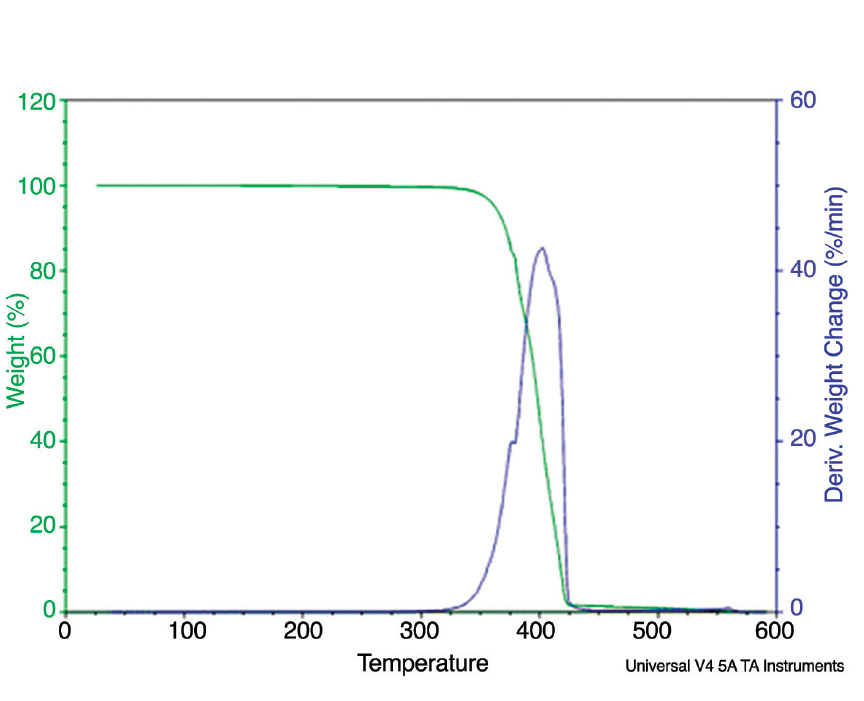
Figure 2: Typical thermogravimetric analysis data for PLA.
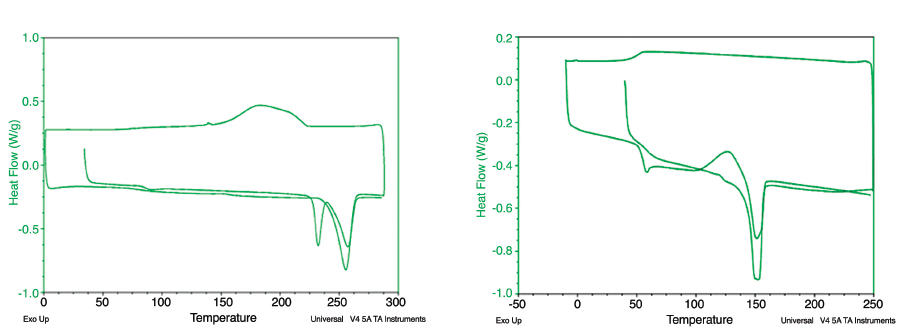
Figure 3: DSC plots of PET (left) and PLA at a heating/cooling rate of 10°C/min.

Figure 3: DSC plots of PET (left) and PLA at a heating/cooling rate of 10°C/min.
Given that plastics significantly improve the quality of life, the use of plastics is projected to increase with the global population. Most of these plastics currently are derived from fossil fuels. Although fossil fuels are expected to last at least 150 years, they have significant negative environmental impacts.
An alternative source for plastics is animal and vegetable matter. These sustainable materials have the potential to replace some of the fossil-fuel-based plastics. Many bio-based plastics also biodegrade, making them attractive for consumer applications. Moreover, the use of biodegradable, bio-based plastics—along with improved recycling systems—can reduce the reviled plastics trash, especially in packaging.
Bio-based and biodegradable polymers, however, create new issues. First, these bioplastics are far behind petroleum-based plastics in terms of performance and processability. Second, the properties of bio-based polymers tend to vary with the source matter, process, location, and year. Third, most bio-based polymers are currently more expensive than their fossil fuel-based counterparts; so non-economic factors must be considered when specifying these materials for a product. Finally, bio-based polymers tend to be overlooked in undergraduate curricula, although they are a growing segment of the plastics resins market.
To address this last issue, educational modules were developed for undergraduate curricula. These modules focused on the synthesis of a biodegradable polymer, characterizing the properties of this polymer and comparing these properties with those of a commonly used petroleum-based polymer, and melt processing a bio-based, biodegradable polymer.
Module 1: Chemical Synthesis of Poly(lactic acid) (PLA) through Ring-Opening Polymerization of L-Lactide
The primary goals of this experiment were to (1) synthesize bio-based and biodegradable poly(lactide) from L-lactide via ring-opening polymerization and (2) develop a better understanding of the ring-opening polymerization mechanism and the role of the initiator and catalyst. For a polymer science laboratory course, the ring-opening polymerization of PLA complemented other polymerization methods, including addition polymerization, solution polycondensation of linear polyesters, interfacial polymerization of linear polyesters (“nylon rope trick”), enzymatic polymerization, and emulsion polymerization. Although enzymatic synthesis of bio-based polymers such as PLA was considered, the long reaction time of the enzymatic synthesis (12 hours) makes it impractical for completion during the laboratory period. Instead, the polymerization was catalyzed using a chemical catalyst (tin(II) 2-ethylhexanoate [Sn(Oct)2]); this reaction can be completed within one hour.
The new module consisted of a 30-minute-long lecture, pre-laboratory activity, and three-hour-long experiment. In the lecture, the students were introduced to biodegradable polymers, biodegradation mechanisms, and the current and potential applications of biodegradable polymers. They also were presented with the fundamentals of the ring-opening polymerization reaction—i.e., the reaction mechanism, the catalyst and initiator, and the need to start with lactide instead of lactic acid for the polymerization reaction.
The polymerization mechanism (Figure 1) is very similar to that of a trans-esterification reaction; the carbonyl of the ester is attached by a nucleophile to form a tetrahedral intermediate. This intermediate can collapse to expel either the nucleophile (the reverse reaction of the first step) or the –OR group in the ring. If the –OR group in the ring is expelled, the ring opens. Ring opening relieves the small amount of ring strain that is present and creates a new alkoxide nucleophile which can attack another lactide monomer, allowing the polymerization to proceed. The tin catalyst acts as a Lewis acid. It coordinates to the ester carbonyl, boosting its electrophilicity.
For the pre-laboratory activity, the students were asked to look up the densities and molecular weights of the reactants and use them to calculate the amount of initiator and catalyst required for the polymerization. During the laboratory, the polymerization reaction was carried out by groups of three or four students under the supervision of a faculty member and teaching assistants. The reaction was performed in a round bottom flask fitted with a circulating water-cooled glass condenser and heated in a constant-temperature oil bath with a stirrer.
The reaction started when 0.035 M Sn(Oct)2 and 0.07 M benzyl alcohol were added into 500 mg L-lactide ((3S)-cis-3,6-dimethyl-1,4-dioxane-2,5-dione). Toluene (3.5 mL) was used as the solvent. The reaction mixture was heated to 108°C and refluxed for one hour while stirring. After one hour, the reaction mixture was removed from the oil bath and allowed to cool to room temperature. The reaction was quenched by adding 0.20-mL 1M HCl (in methanol). Subsequently, 200-mL of heptane was added to the product solution to precipitate the PLA (with cooling using an ice bath). Then solid PLA was obtained by decanting off the extra heptane. The product (i.e., PLA and other solids) was dried in an oven. The students weighed their products and calculated the yield after drying the polymer. The samples were saved for molecular weight determination by gel permeation chromatography and thermal analysis.
Outcomes:This module has been implemented five times for 190 junior-level plastics engineering students. Classroom observations and evaluation data showed that the students were very interested in and enthusiastic about the topic of biodegradable polymers. Students asked many questions during the lectures and laboratory sessions. The experiment itself required learning new concepts and new skills, including refluxing, balancing the reaction temperature and the need to keep the temperature below the boiling point of toluene, and keeping the reactants dry (e.g., using anhydrous toluene) because water affects the reaction kinetics and reduces the molecular weight of the PLA. The students readily accepted these challenges and did quite well during their laboratory sessions. Their 60-80% PLA yields, however, were lower than the expected 85%, and there were issues with low molecular weight PLA and contaminants. Finally, five years of evaluation data indicated that the students had developed a good understanding of (1) ring-opening polymerization, (2) the role of a commonly used tin-based catalyst and initiator in this polymerization system, and (3) the effect of reaction conditions and moisture on the polymerization reaction.
Module 2: Thermal Characterization of a Bio-Based Polymer
The primary goals of this experiment were to (1) study the thermal degradation of PLA and (2) compare it with those of aromatic polyesters and other polymers, as well as to determine (3) the melting and glass transition temperatures of an aromatic polyester (PET) and aliphatic biodegradable polyester (PLA) and (4) the effect of heating and cooling rates on their crystallization behavior (including cold crystallization). Biodegradable polyesters, such as PLA, are hygroscopic and exhibit poor thermo-oxidative stability, especially in the presence of moisture. In addition, the aliphatic structure of PLA changes its transition temperatures and makes it prone to cold crystallization.
The new module consisted of a two 30-minute-long lectures and two three-hour-long experiments. Since the basics of thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) are taught in a separate course, one lecture focused on measurement of moisture content using TGA and interpretation of TGA results, while the second lecture concentrated on measurement of transition temperatures using DSC and interpretation of DSC results.
As presented in Figure 2, typical TGA data for PLA includes the weight loss as a function of temperature and the derivative of weight loss as a function of temperature. The temperature at which a weight loss of 5% was observed (“Td 5%”) can reflect the amount of moisture present in the resin and can be used to assess if the resin is adequately dry before processing.
Figure 3 presents typical DSC curves for polyethylene terephthalate (PET) and PLA. During the first heating cycle, both PET and PLA exhibit glass transition temperatures (baseline shift) and melting temperatures (endothermic peak). The position of the crystallization temperature peak is one of the differences between the PET and PLA curves. At a heating/cooling rate of 10°C/min., the cold crystallization peak is only observed for PLA. A cooling rate of 20°C/min., however, does not provide sufficient time for polymer chains to form ordered domains (crystallites). Therefore, during the heating cycle when the molecules have enough mobility above the glass transition temperature, they tend to crystallize (i.e., exhibit cold crystallization behavior).
For the first experiment, the students performed thermal analysis of both an aromatic polyester (PET) and a bio-based aliphatic polyester (PLA) under air. These polymers had been synthesized by the students in the previous semester, but similar results have been achieved using commercially available PET and PLA. The students recorded the Td 5% and the ultimate decomposition temperature (Td) from the thermogram and the first derivative trace of the thermogram. The thermal stability of PLA was compared with that of PET.
The DSC of PLA samples was performed at different heating and cooling rates (10°C/min. and 20°C/min.). The temperature range was room temperature to temperatures greater than the melting temperatures of the polymers; the latter temperatures had been determined from TGA. The processing history (mechanical and thermal history) usually causes the polymer to have variations in the degree of crystallinity. They are reflected in the first heating cycle. With a 10°C/min. cooling rate, PLA seems to crystallize. During the second heating cycle at a 10°C/min. heating rate, PLA exhibits a clear melting transition. If the melt is cooled at the rate of 20°C/min., however, the polymer does not crystallize. In the subsequent heating cycle, this polymer does not exhibit the melting transition. For the experiment, the students evaluated the effects of heating and cooling rates on the crystallization of the two polymers. In addition, they discussed the impact of partial or lack of crystallization at high cooling rates characteristic of processing PLA.
Outcomes:This module has been implemented four times for 138 junior-level plastics engineering students. Laboratory discussions indicated that the students enjoyed using the TGA and DSC (they were considered more sophisticated than the plastics processing equipment). They had little difficulty in interpreting the results of the analyses and found it extremely interesting that PLA exhibited greater moisture absorption than PET. Course evaluations showed that students had acquired a better understanding of TGA, DSC, and crystallization (including the effects of thermal history and cooling rate) than with previously used experiments. Most students clearly understood how TGA could be used for moisture analysis, and this understanding crept into the processing (manufacturing) course, with students electing to use TGA rather than moisture analyzers or Karl Fischer titration to measure the moisture content of their dried polymers.
Module 3: Processing Sustainable Polymer Nanocomposites
The primary goals of this experiment were to (1) understand the issues when processing of bio-based and biodegradable polymers (PLA) and (2) continue education on the safe handling of nanoparticles (which had been in the curriculum for seven years).
Melt temperatures for PLA are ~170°C to 200°C. Due to presence of ester groups in the polymer chain, PLA has an ability to absorb moisture easily and is sensitive to oxidative degradation during heating. Therefore, drying time and drying temperature are critical to avoid polymer degradation and ensure low moisture content. During processing, PLA also requires low residence times and low shear rates.
The new three-week-long (six-hour-long) experiment consisted of (1) compounding the polymer and nanofillers using a twin screw extruder, (2) injection molding test specimens, and (3) characterizing the mechanical properties of the molded test specimens. Since larger amounts of material were required for this work, this module employed a commercially available PLA and a commercially available montmorillonite clay. Implemented into a junior-level processing laboratory, most students had previous laboratories with the major processing equipment. Basic information about processing bio-based and biodegradable polymers was incorporated into the laboratory manual and pre-laboratory activities for this experiment.
Outcomes: This module has been implemented three times for 93 junior-level plastics engineering students. The students found that there was no need to change extrusion conditions (i.e., barrel temperatures, feed rates, and screw speed) when compounding PLA with 0, 1, 2, 5, and 10% nanoclay; and that the PLA compounded as easily as an impact-modified polystyrene (HIPS) control (during the first offering). The neat PLA was more difficult to mold (than HIPS), but all students obtained suitable processing conditions. Students found that the 5 and 10% clay-filled PLA materials were nearly impossible to mold because the parts tended to become stuck in the mold and/or be so brittle that they fractured upon ejection. Therefore, the clay loadings were reduced to 1 and 2 wt%, which permitted easier molding of the test specimens. Characterizing the mechanical properties was generally successful for the neat PLA and PLA with 1-2 wt% nanoclay test specimens. The PLA samples, however, did not show significant changes in mechanical properties with increases in nanoclay content, whereas the HIPS controls did. Therefore, this experiment was switched to a high-speed twin screw extruder—and the students have evaluated the effect of shear (screw speed) on properties of the PLA nanocomposites. Overall, the students have enjoyed compounding the PLA nanocomposites, but have continued to need assistance when injection molding PLA. Evaluations have shown that they have a greater understanding of the issues associated with processing bio-based and biodegradable polymers.
Conclusions
Educational modules focused on bio-based polymers were successfully developed and implemented for undergraduate curricula. These modules included experiments for (1) the synthesis of a biodegradable polymer, (2) characterizing the properties of this polymer and comparing these properties with those of a commonly used petroleum-based polymer, and (3) melt processing a bio-based, biodegradable polymer. All modules were well-received by and produced significant learning by the undergraduate students. A new module with a three-hour lecture on the basics of bio-based polymers currently is under development.
Acknowledgements
Module development was supported by the National Science Foundation (DUE-1044363). The authors would like to thank Leistritz, Technovel Corp., and Arburg Inc. for the processing equipment, and NatureWorks LLC for the PLA.
Note: This is an updated version of the authors’ SPE ANTEC® Orlando 2015 paper.
