Phase Behavior of Polyamide 6/612 Blends
Previous Article Next Article
By Ying Shi
A. Schulman Inc., Akron, Ohio, USA
Phase Behavior of Polyamide 6/612 Blends
Previous Article Next Article
By Ying Shi
A. Schulman Inc., Akron, Ohio, USA
Phase Behavior of Polyamide 6/612 Blends
Previous Article Next Article
By Ying Shi
A. Schulman Inc., Akron, Ohio, USA

Table 1: Material Characterization and Properties
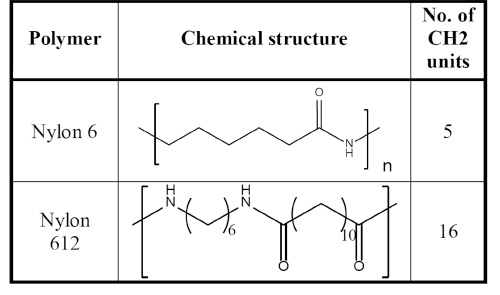
Table 2: Chemical Structure and Number of Methyl Units of Nylon 6 and Nylon 612

Table 3: Solubility Parameter Calculation of Nylon 6 and Nylon 612

Figure 1: DSC curves of nylon 6, nylon 612, and their blends.

Table 4: Thermal Transitions of Nylon 6, Nylon 612, and Their Blends
Note: This is an abridged version of the author’s ANTEC®Indianapolis 2016 technical paper. The paper’s full version, including a theory section and additional results, will be available at and after ANTEC. See www.antec.ws for the current technical agenda at the conference.
Blending or alloying polymers is a relatively simple way to develop materials with a full set of desired properties, such as a combination of strength and solvent resistance. In some cases, a blend costs less than the pure polymer products. For example, a nylon-6/PP blend offers the benefits of low moisture absorption and reduced density.1 Low moisture absorption provides part designers with more predicable physical properties and part dimensions. Reduced density results in an overall lower amount of material usage.
Polyamide (PA), commonly known as nylon, is one of the largest engineering thermoplastics materials used in automotive, electronics, and packaging applications. The nylon family consists of aliphatic nylon 6, nylon 66, nylon 610, nylon 12, nylon 46, nylon 612, and aromatic nylons. Among these, aliphatic nylon 6 and nylon 66 are the most common ones.
Compared to nylon 66, nylon 612 has lower modulus, higher elongation, lower strength, and a lower melting point. However, nylon 612 absorbs half as much water as nylon 66. The difference is the number of methyl units between the two carbonyl groups. As the methyl units increase in length, the molecules appear to be more like polyethylene. Moisture absorption is decreased due to reduced polarity with further separation of the polar amide groups. Therefore, the properties of nylon 612 are much more consistent and predictable when the end application is exposed to ambient humid conditions.
Even though nylon 612 has advantages of dimensional stability and toughness, it is much more expensive than nylon 6 and nylon 66. Blending nylon 6 or nylon 66 with nylon 612 will yield a product with reduced moisture absorption, enhanced toughness, and relatively low cost. In this paper, we chose to study the phase behavior of a nylon 6 and nylon 612 blend because their melting temperature are closer and easier for melt processing.
Common aliphatic nylon blends have been studied on various occasions. Verma and coworkers investigated nylon 6 and nylon 66 blends with particular emphasis on small amounts of one polymer in the other.2 Significant property improvements were achieved.
Ong and coworkers studied dynamic mechanical properties of some aliphatic nylon blends, including nylon 6, nylon 66, nylon 11, nylon 12, nylon 612, and nylon 610.3 They concluded that all the studied pairs were compatible except nylon 6 and nylon 12. This phenomenon was observed by Kyotani earlier,4 who reported solvent cast blends of nylon 6 and nylon 12 formed individual crystals, suggesting phase separation of the two polymers.
Ellis examined several aliphatic nylon blends, including nylon 66 and nylon 612, using an enthalpy recovery measurement technique.5 The conclusion was that phase separation was the dominant behavior in these blends.
This paper focuses on the binary blending of nylon 6 and nylon 612. The phase behavior was studied with thermo-analytical techniques.
Materials
Commercial grades nylon 6 and nylon 612 raw resin were used. Their properties are summarized in Table 1. The two nylons studied in this work have similar relative viscosity, which was measured in 96% sulfuric acid using a solution viscometer according to standard ISO 307. The melting temperatures listed in Table 1 were reported from the resin manufacturer, and they were close to each other.
Water absorption was measured by immersing the test specimens in deionized water for 24 hours at room temperature, and weight changes were recorded. Nylon 612 absorbed much less water than nylon 6 (about 1/4). Table 2 shows the chemical structures of the two polymers and number of methyl units in each of the nylons.
Experiments
Five blends with various compositions of nylon 6 and nylon 612 were produced using a twin rolling mixing chamber on a Brabender mixer. The premixed pellets were blended at 10°C above the higher melting temperature for 15 minutes. The blends were removed immediately and allowed to cool to room temperature.
Thermal analysis was performed by using a TA Instruments Q2000 differential scanning calorimeter (DSC) at a heating rate of 20°C/min. The glass transition temperature Tg was defined as the temperature at the half-height of the heat capacity change, and melting temperature Tm was defined as the maximum rate of melting, i.e., the peak temperature of the melting endotherm.
Since both nylon 6 and nylon 612 are semi-crystalline materials, it is necessary to allow the crystals to establish equilibrium. This was achieved by melting the sample and isothermal holding at this temperature for two minutes during the heating cycle.
Results and Discussion
Solubility of nylon 6 and nylon 612
The close similarities of chemical structure and physical properties of aliphatic nylon 6 and nylon 612 have led people to believe they are miscible. In order for two polymers to form a homogeneous or miscible blend, a favorable interaction has to exist between them. This interaction often results in the formation of an attractive association between chemical functionalities or species contained in the polymer molecules.
The degree of interaction between polymers can be numerically estimated using solubility parameter, which is also a good indication of solubility between polymers. Two polymers with similar solubility values are likely to be miscible. The solubility parameter can be calculated as the square root of the cohesive energy density6:
![]()
where Ecoh (J/mol) is cohesive energy and V (cm3/mol) is molar volume of each repeat unit of the polymer.
The cohesive energies can be added together to obtain the total group cohesive energy. The values of cohesive energy of the repeating units can be readily obtained from literature. Table 3 summarizes the cohesive energy and mole volume of repeating units of nylon 6 and nylon 612, as well as the calculated solubility parameters. The values are close but not the same. These two polymers should be miscible, without considering intramolecular hydrogen bonding.
A single glass transition intermediate between those of the pure components is one of the criterions determining miscibility. Additional phenomena such as the incremental change in heat capacity (ΔCp) at Tg detects immiscibility. All of these thermal transition behaviors were evaluated in this investigation to determine the miscibility between nylon 6 and nylon 612.
Figure 1 shows DSC second heating curves of nylon 6, nylon 612, and their blends. The blends were denoted as volume fractions; for example, 20/80 means nylon 6’s volume fraction is 20% in this blend and the rest is nylon 612. Table 4 summarizes thermal transition temperatures, heat capacity of the glass transition, and heat of fusion of melting. A single Tg was observed for all the blends.
The glass transition temperatures of the pure components were measured as 54°C for nylon 6 and 46°C for nylon 612. The Tgs of these two polymers are very close, and there is only 8°C separation, which makes it difficult to determine the phase behavior. The glass transition of nylon 612 was broad (Figure 1) and showed very small change of heat capacity. Nylon 6 showed a higher glass transition and larger change of heat capacity. The difference is due to higher polarity of nylon 6 and consequently higher degree of hydrogen bonding between polymer chains.
The Tgs of the blends were intermediate between nylon 6 and nylon 612, except some of the blends showed even lower Tg than that of nylon 612. The heat capacity values of the blends ranged in between the pure components too. When nylon 6 is the dominant component in the blend, the changes of heat of capacity were rather small. However, no incremental changes in heat capacity were detected. One can at least conclude that the blends prepared in this study were compatible.
The melting temperature of nylon 6 was slightly higher than nylon 612, which is due to nylon 6’s polar nature forming more compact crystals. The melting temperatures of blends were slightly lower than both of the pure components. This melting-point-depression phenomena indicates miscibility between the two polymers. Nylon 6 were more crystalline than nylon 612, which showed about 30 J/g less heat of fusion. As nylon 6 content increased in the blends, heat of fusion values of the blends increased (Table 4).
Concerning the glass transition temperatures and melting points of the blends vs. volume fraction of nylon 6, there is no clear trend of the changes of these two temperatures as a function of blend composition. Nylon 6 has the highest Tg and Tm. At around a volume fraction of 0.4~0.6 range, the lowest Tg and Tm occurred.
Estimation of interaction parameter (χ)
The interaction parameter χ can be evaluated by the analysis of melting point depression of a polymer by a second miscible amorphous polymer. This is a well-known method developed by Flory.6 The simplified equation can be expressed as:
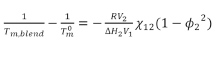
Where Tm,blend and Tm0 are the equilibrium melting points of the blend and the crystallizable component in the pure state; R is the gas constant; ΔH is the heat of fusion per mole of repeat unit; V is the molar volume of the respective component; φ2 is the volume fraction of component 2 in the blends; and χ12 is interaction parameter of the blends.
All five blends had negative interaction parameters, even though they were very small and close to 0. The negative interaction parameters suggested these blends were miscible. In addition, χ decreased as the nylon 612 content increased, which suggests the blends were more miscible when nylon 612 was the dominant component.
Conclusions
Nylon 6 and nylon 612 blends were prepared by a melt processing method and characterized using thermal analysis techniques. The results showed a single glass transition of the blends and melting temperature depressions, which suggested compatible blends were obtained. The interaction parameters were calculated using the melting point depression theory. Negative interaction parameters were obtained for all blends. This concludes that the blends prepared in this study were miscible.
Acknowledgement
The author would like to thank Bryan Hildenbrand for his excellent blends preparation and thermal characterization work.
References
1. Y. Shi, “Compatibilization Improvement of Nylon 6/Propylene Blends,” ANTEC 2014, Las Vegas, Nevada, USA, April 28-30, 2014.
2. Verma, A., Deopura, B.L., and Sengupta, A.K., J. Appl. Polym. Sci., 31,747, 1986.
3. Ong, E.S., Kim, Y., and Williams, H.L., J. Appl. Polym. Sci., 31, 367, 1986.
4. Kyotani, M., J. Macromol. Sci.-Phys., B21, 219, 1982.
5. Ellis, T. S., Polymer, 33, 1469, 1992.
6. Flory, P. J., Principles of Polymer Chemistry,Cornell University Press: Ithaca, New York, 1953.

