Comparing Thermoplastic Elastomers and Thermoset Rubber
Each class of material is well suited for its range of applications, but there are key differences
Previous Article Next Article
By Jeffrey Jansen
The Madison Group, Madison, Wisconsin, USA
Comparing Thermoplastic Elastomers and Thermoset Rubber
Each class of material is well suited for its range of applications, but there are key differences
Previous Article Next Article
By Jeffrey Jansen
The Madison Group, Madison, Wisconsin, USA
Comparing Thermoplastic Elastomers and Thermoset Rubber
Each class of material is well suited for its range of applications, but there are key differences
Previous Article Next Article
By Jeffrey Jansen
The Madison Group, Madison, Wisconsin, USA
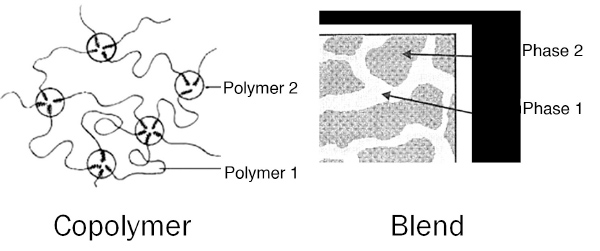
Figure 1: Basic structural comparison of a TPE copolymer and TPE blend.
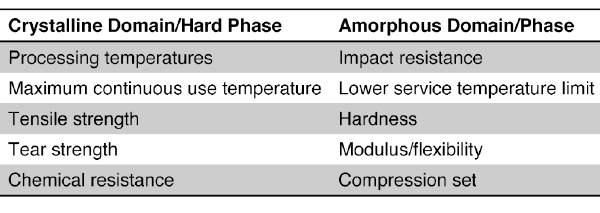
Table 1: Key Properties Closely Associated with Crystalline or Amorphous Domains of TPEs
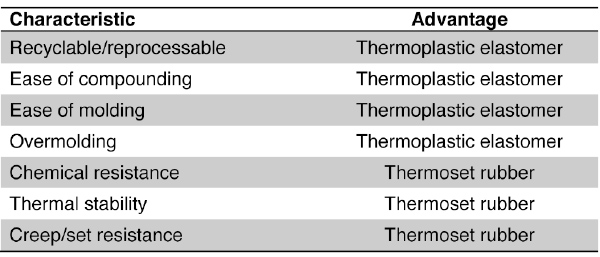
Table 2: Property Comparison of TPEs and Thermoset Rubber

Figure 2: Basic applications of TPEs (overmolded toothbrush handle) and thermoset rubber (tires).
Thermoplastic elastomers (TPE) are an important class of materials and are used in a wide variety of applications. In general, TPEs encompass multiple different types of polymeric material structures, and are noted for being soft and flexible like thermoset rubber, while being melt-processable and reprocessable like thermoplastics.
TPEs have been defined by the International Institute of Synthetic Rubber Producers as:
“Polymers, polymer blends or compounds which, above their melt temperatures, exhibit thermoplastic character that enables them to be shaped into fabricated articles and which, within their design temperature range, possess elastomeric behavior without cross-linking during fabrication. This process is reversible and the product can be reprocessed and remolded.”1
TPE Structure
Essentially, TPEs are block copolymers or a physical mix of polymers which exhibit simultaneous thermoplastic and elastomeric properties. As a family, TPEs encompass a special group of polymeric materials that undergo a high level of elastic deformation without crosslinking. They exhibit characteristics of both thermoplastics and thermoset rubber simultaneously.
Structurally, TPEs are divided into two distinct categories: blends and block copolymers (Figure 1). Regardless of whether the TPE is a blend or a block copolymer, the polymer system has crystalline and amorphous domains. For blends this is achieved by a mechanical mixture of semi-crystalline and amorphous polymers. Alternately, block copolymers are composed of discrete blocks of crystalline and amorphous domains within the same polymer chain.
This duality of structure accounts for the unique properties of TPEs. The crystalline domains, known as the hard block, have an orderly, locked structure that gives the material thermoplastic properties. The relatively tightly packed crystalline phase and the associated intermolecular forces holding the chains together produce a molecular structure that somewhat mimics a crosslinked configuration within the material.
The amorphous domains, known as the soft block, have a disordered structure that imparts an elastomeric character to the material. This is due to the greater free volume within the molecular structure, which allows more molecular movement. The functionality of the hard blocks and soft blocks is similar between both blended and block copolymer TPEs.
Block copolymer-based TPEs are based upon polymers that have hard blocks and soft blocks along the backbone of the polymer chain. As a bulk response, solidification from the melt state results in coalescence of the crystalline domains into hard blocks, resulting in characteristic thermoplastic behavior. Conversely, the amorphous domains form elastomeric bridges, otherwise known as tie molecules, representing soft blocks that give an elastomeric behavior.
TPEs produced through the blending of materials are commonly based upon amorphous domains (the elastomeric phase) within hard domains (the thermoplastic phase)—although the reverse can also be true, with some materials consisting of hard domains in a soft elastomeric base polymer.
Regardless of whether the TPE material is a copolymer or a blend, the hard block will have a melting point, or less commonly a glass transition temperature, well above room temperature. Accordingly, the soft block will have a glass transition temperature, or less frequently a melting point, well below room temperature.
Specific properties can be obtained and tailored by selective combination of the structure and ratios of individual hard phases and soft phases. While both the hard and soft phases contribute to the overall physical and mechanical properties of a TPE, some key properties may be more closely associated with one domain or the other. Some of the key properties associated with the individual phases are indicated in Table 1.
Kinds of TPEs and Rubbers
There are six generic classes of commercial TPEs:
- styrenic block copolymers (SBC): block copolymers and terpolymers of styrene and butadiene;
- polyolefin blends (TPO): blends of polypropylene and un-crosslinked poly(ethylene propylene diene monomer) (EPDM) rubber;
- elastomeric alloys (TPV): blends of polypropylene and vulcanized (cross-linked) EPDM rubber;
- thermoplastic polyurethanes (TPU): linear segmented block copolymers formed by the reaction of diisocyanates with short-chain diols and diisocyanates with long-chain diols;
- thermoplastic copolyesters (COPE): copolymers of bifunctional aromatic polyesters with ether linkages; and
- thermoplastic polyamides (PEPA): copolymers obtained by polycondensation of a carboxylic acid polyamide with an alcohol-terminated polyether.
In contrast to thermoplastic elastomers, thermoset rubbers are single-phase materials, without the dual hard and soft phases. Rubber materials are natural or synthetic polymer macromolecules, and can be polymerized as homopolymers or random copolymers/terpolymers.
The structure of rubber is amorphous, exclusive of crystalline domains. Because of this, rubber materials undergo a glass transition, but no melting point. By definition, thermoset rubber materials have a glass transition temperature below room temperature. This is in contrast with thermoset plastic materials, which have a glass transition temperature above ambient conditions.
In addition to the intermolecular bonds holding the polymer chains together, similar to that of TPEs, rubber materials have non-reversible covalent chemical bonds binding the individual chains together. These chemical bonds, or crosslinks, are formed during molding through a process known as vulcanization, alternatively known as crosslinking or curing.
There are a wide variety of types of thermoset rubber materials, with the following representing some of the most common, with their common abbreviations:
- natural rubber (NR);
- polyisoprene (IR);
- polychloroprene (CR);
- styrene butadiene rubber (SBR);
- nitrile butadiene rubber (NBR);
- ethylene propylene diene monomer rubber (EPDM);
- butyl rubber (IIR);
- polybutadiene (BR);
- epichlorohydrin (ECO);
- fluorinated hydrocarbon (FKM); and
- silicone rubber (Q).
The crosslinking process within thermoset rubber is a chemical reaction that takes place at a relatively high temperature during the molding process. The most common crosslinking agents are sulfur, sulfur-containing chemicals, and peroxides.
Both thermoplastic elastomers and thermoset rubber materials get their principal properties from the base polymer. However, both types of materials contain formulation additives that modify and enhance the final properties of the compounds. These additives commonly include reinforcing fillers, non-reinforcing fillers, plasticizers, stabilizers and anti-degradants, process aids, and many types of specific performance enhancers. Thermoset rubber compounds also contain curatives, as well as cure activators and accelerators to enhance the crosslinking process.
Property Comparison
On a basic level, thermoplastic elastomers exhibit some of the characteristics of thermoset rubber, but above their melt or softening temperatures, are melt processable like thermoplastics. This allows TPEs to be reprocessed and remolded. In the viewpoint of those familiar with thermoplastics, TPEs allow for ease of fabrication and design flexibility not afforded by thermoset rubber.
Compared with other thermoplastic materials, TPEs offer advantages in properties including:
- softness: low hardness;
- flexibility: low modulus;
- impact strength;
- tear and abrasion resistance;
- fatigue resistance;
- desirable compression set;
- broad use-temperature range;
- chemical resistance; and
- low specific gravity.
However, thermoset rubber compounds offer distinct performance advantages over TPEs due to their crosslinked structure. Absolute properties will be highly dependent on the exact compounds being compared. A general property comparison is presented in Table 2, illustrated also by Figure 2.
As indicated in Table 2, the advantages of TPEs are predominately in the area of processing, while thermoset rubber typically is superior in regards to performance properties, especially those related to temperature, chemical contact, and sustained stress. In all fairness, the advantages indicated in ease of compounding and ease of molding are based upon one’s viewpoint. For those familiar with working with thermoset rubber compounds, a difference of opinion may be held.
Both types of materials, thermoplastic elastomers and thermoset rubbers, are diverse classes of polymeric materials offering a wide range of properties. A comparison shows that the inherent properties are dependent on the different structures comprising the two sets of materials, as well as formulation additives.
The best material for a particular application will depend on many parameters, including the design of the component and the service conditions. Designers of articles and assemblies should be well familiar with both thermoplastic elastomers and thermoset rubbers in order to choose the most appropriate material to ensure the best chance for product success.
Reference
1. See also: J. A. Brydson, Thermoplastic Elastomers: Properties and Applications, Rapra Review Reports, vol. 7, no. 9, 1995, p. 3.
About the author… Jeffrey A. Jansen is a senior managing engineer and partner at The Madison Group, an independent plastics engineering and consulting firm. He specializes in failure analysis, material identification and selection, and aging studies for thermoplastic materials. Jansen is a regular contributor to Plastics Engineering’s “Consultant’s Corner” and a regular presenter for SPE’s webinar series, covering a wide range of topics related to plastics failure, material performance, testing, and polymer technology.
Note: Jeff Jansen will be offering two upcoming SPE webinars related to elastomers: “Basic Rubber Technology,” on May 12, and “Thermoplastic Elastomers,” on Nov. 10, 2016. Register at www.4spe.org/Events/webinars.aspx.
