Water-Soluble Free Radical Addition Polymerizations: Polyacrylamides
Acrylamide can form high-molecular-weight polymers and react with a variety of co-monomers
Previous Article Next Article
By Wesley L. Whipple and Hua Zheng
Water-Soluble Free Radical Addition Polymerizations: Polyacrylamides
Acrylamide can form high-molecular-weight polymers and react with a variety of co-monomers
Previous Article Next Article
By Wesley L. Whipple and Hua Zheng
Water-Soluble Free Radical Addition Polymerizations: Polyacrylamides
Acrylamide can form high-molecular-weight polymers and react with a variety of co-monomers
Previous Article Next Article
By Wesley L. Whipple and Hua Zheng
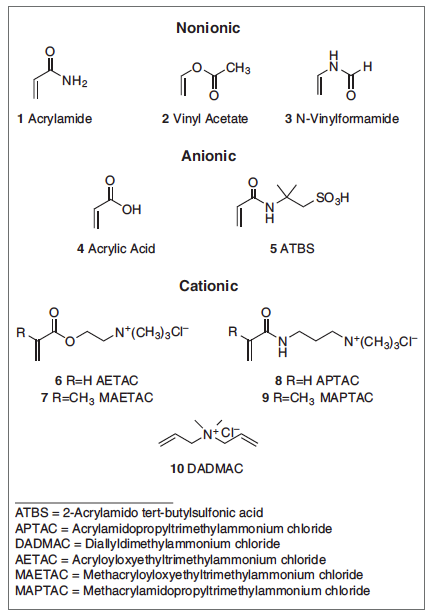
Figure 1: Common nonionic, anionic, and cationic monomers used in the synthesis of water-soluble polymers.
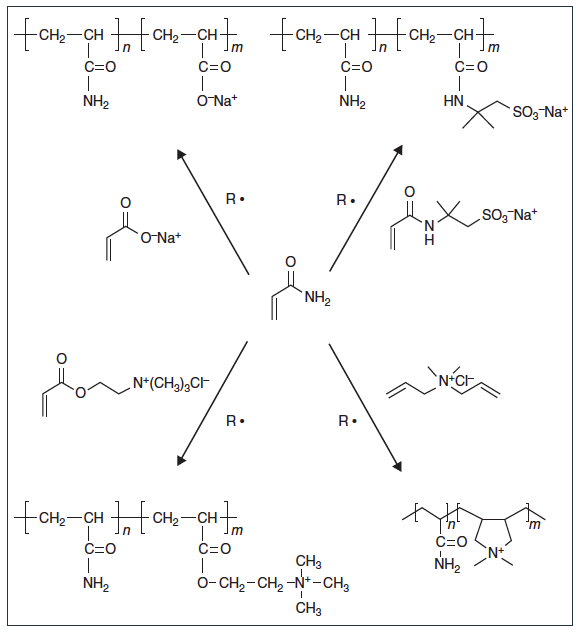
Figure 2: Synthesis scheme for the preparation of copolymers of acrylamide with sodium acrylate, sodium ATBS, AETAC, and DADMAC.
"Polyacrylamides are formed from the radical chain polymerization of acrylamide with cationic or anionic monomers… a highly exothermic reaction."
Note:
Below is an excerpt from Chapter 19 of the book Monitoring Polymerization Reactions: From Fundamentals to Applications, ed. by Wayne F. Reed and Alina M. Alb (2014, John Wiley & Sons).
In 2009, an estimated four billion metric tons of natural, semisynthetic, and synthetic water-soluble polymers were consumed globally for use in the production of food, clean water, and energy; and for personal care, pharmaceutical, and industrial applications.1 Synthetic water-soluble polymers synthesized by free-radical addition polymerization methods account for half of this volume. They include polyacrylamides, polyacrylates, polydiallyldimethylammonium chloride (polyDADMAC), polyvinyl alcohol, and polyvinylpyrrolidones. Depending on the target applications, these polymers can have different molecular weights, charge, and architecture.
Polyacrylamides: Overview
Several reviews that cover the synthesis, characterization, and application of polyacrylamides are available.6-9 Acrylamide has the unique ability to form high-molecular weight polymers (> 1 million Da), to react with a variety of co-monomers to produce cationic, anionic, nonionic, amphoteric, or zwitterionic polymers, and to produce specialized polymers available through derivatization. Monomers commonly used in the synthesis of water-soluble polymers of commercial interest are shown in Figure 1.
Polyacrylamides of high commercial interest are copolymers of acrylamide (1, in Figure 1) with sodium or ammonium salts of acrylic acid (4) or 2-acrylamido tert-butylsulfonic acid (ATBS) (5) to produce anionic polymers or with acryloyloxyethyltrimethylammonium chloride (AETAC, 6), to produce cationic polymers. Although not produced in large volume, copolymers of acrylamide and DADMAC (10) are used to produce cationic polymers for use in papermaking applications.
Polyacrylamides with a variety of charge densities (obtained by varying monomer ratios), molecular weights, and architecture (linear, branched, and structured) are available commercially in dry and liquid form. Branched and structured polyacrylamides are obtained by including multifunctional monomers in the polymerization mix or through cross-linking following polymerization.10, 11
Polyacrylamides are produced commercially in an aqueous environment by free radical polymerization, which follows the classical vinyl polymerization model with initiation, propagation, and termination processes. In this model, the propagation/termination ratio (kp/kt)½ and chain transfer to monomer, polymer, initiator, and other small molecules impact the molecular weight of the formed polymer. High molecular-weight polyacrylamides are possible because of the high (kp/kt)½ ratio for acrylamide and the low chain-transfer activity to monomer and polymer in an aqueous environment. Polymer molecular weights can be lowered and customized through the use of chain-transfer agents. Initiation with water and oil-soluble azo compounds, redox couples, peroxides, or photochemical initiators is common.6, 12-14
Cationic Polyacrylamides
Common cationic polyacrylamides produced industrially include copolymers of acrylamide (1) with AETAC (6), MAETAC (7), MAPTAC (9), or DADMAC (10) (Figures 1 and 2). Among them, acrylamide/AETAC copolymers are the most common due to the relatively low AETAC monomer cost and similar reactivity with acrylamide (r1(AM) = 0.61, r2(AETAC) = 0.47) producing copolymers with a roughly uniform sequence distribution of co-monomers.
In general, cationic methacrylates and methacrylamides are more reactive than their acrylate and acrylamide counterparts. In batch copolymerizations of acrylamide with MAETAC (r1(AM) = 0.25, r2(MAETAC) = 1.71) or MAPTAC (r1(AM) = 0.57, r2(MAPTAC) = 1.13), the more reactive MAETAC and MAPTAC monomers react faster with their own monomers than with acrylamide. This leads to compositional drift and often poor performance.6
There are more significant differences in the reactivity ratios of AM and DADMAC ((r1(AM) = 6.4, r2(DADMAC) = 0.06).14 DADMAC (10) is the least-expensive cationic monomer. The molecular weight of DADMAC-containing polymers is limited due to the high chain transfer activity from the DADMAC allylic moiety. The low molecular weight, compositional heterogeneity, and branching have limited the use of acrylamide/DADMAC copolymers to predominantly just a few papermaking applications (coated broke, pitch and stickies control).
Semibatch strategies have been employed where the more reactive monomer is withheld from the initial reaction mixture and fed into the reactor during polymerization to obtain copolymers with similar co-monomer sequence distributions.15, 16 Extensive reactivity ratio data of acrylamide with cationic and anionic monomers under various conditions are summarized elsewhere.6, 7, 14
Hydrolytic stability and pH have to be considered when selecting a cationic monomer. Cationic acrylate esters are susceptible to base hydrolysis above pH 6, resulting in the loss of cationic charge on the polymer. The rate of hydrolysis is concentration- and temperature-dependent. In contrast, cationic acrylamide copolymers containing amide monomers such as APTAC and MAPTAC are reasonably stable up to a pH of 9-10. Acrylamide/MAPTAC copolymers and acrylamide/MAPTAC/acrylate terpolymers can be found as conditioners and deposit aids in hair-care formulations.
Anionic Polyacrylamides
Common commercial anionic polyacrylamides include copolymers of acrylamide with sodium and ammonium salts of acrylic acid (4) and ATBS (5) (in Figure 2). Criteria for polymer selection include cost, molecular weight, polymer solubility, application pH, and salt tolerance. Acrylic acid is a rather inexpensive monomer and yields very high molecular weight acrylamide/acrylate copolymers. Sodium or ammonium acrylates are typically formed prior to polymerization by reaction of acrylic acid with sodium hydroxide or ammonia.
Acrylamide/acrylate copolymers have poor solubility at low pH (acrylic acid pKa = 4.3) and have poor salt tolerance. The high molecular weights available for anionic flocculants render them useful in mining and papermaking applications. In contrast, acrylamide/Na-ATBS copolymers have good solubility, maintain their charge at low pH (pKa ATBS = 1.7), and have a high tolerance for salt (including many divalent cations). These features render them useful for oil field applications and as flocculants in phosphate production. Reactivity ratios of acrylamide with acrylic acid salts vary with pH. At pH 4 r1(AM) = 0.57, r2(acrylic acid) = 0.32, and at pH 8 r1(AM) = 0.12, r2(acrylic acid) = 0.63. It was reported that a random copolymer is formed at a pH of about 5.7
Derivatized Polyacrylamides
A variety of commercially useful polyacrylamides obtained via the derivatization of polyacrylamide are reported. These include the formation of anionic polyacrylamide through hydrolysis,17 the synthesis of sulfomethylated derivatives from the reaction of polyacrylamide with formaldehyde and sodium bisulfate,18 the formation of aminomethylated polyacrylamide from the reaction of polyacrylamide with formaldehyde and dimethylamine (Mannich reaction),19 and the generation of various derivatives by transamidation reactions including hydroxamated polyacrylamides used as a flocculant in the Bayer process.20, 21
Fluorescent polyacrylamide or polyacrylate derivatives, useful for monitoring polymer concentration in industrial applications, are obtained by the incorporation of a small amount of fluorescent monomer into the polymer backbone.22
Polyacrylamide Manufacturing Process
Liquid polyacrylamides are available as solutions, inverse (water-in-oil) emulsions, or dispersions. Dry polyacrylamides are available as powders from a dried gel or as beads from a water-in-oil suspension process. Polyacrylamides are formed from the radical chain polymerization of acrylamide with cationic or anionic monomers, which is a highly exothermic reaction (Figure 2).7
Reactions may be run isothermally or adiabatically. In an isothermal process, the rate of heat generation can be controlled by adjusting the initiator concentration, reaction temperature, or initiator feed rate. If the heat generation exceeds the rate at which heat can be removed from the reactor, a runaway reaction will result, which could be potentially dangerous in large-scale reactions. For an adiabatic process, temperature rise can be calculated for given monomer concentrations and the final temperature estimated based on the initial temperature. Formula adjustments can be made to avoid exceeding the pressure rating of the reaction equipment.6
References
- Will R.K. Water-soluble polymers. In: Specialty Chemicals Strategies for Success. Volume 16. Menlo Park: SRI International; 2010.
- Frayne C. Chemical treatments and programs for cooling water. In: Cooling Water Treatment – Principles and Practice. New York: Chemical Publishing Company Inc.; 1999. p. 137-176. Available at www.knovel.com/web/portal/browse/display?_ EXT_KNOVEL_DISPLAY_bookid = 3074&VerticalID=0. Accessed 2012 Feb 25.
- Bolto B., Gregory J. Organic polyelectrolytes in water treatment. Water Res 2007; 41: 2301-2324.
- Jackson L.A. Applications of cationic polymers in water treatment. In: Amjad Z., editor. Science and Technology of Industrial Water Treatment. Boca Raton: CRC Press; 2010. p. 465-479.
- Heitner H.I. Flocculating agents. In: Kroschwitz, J.I., Howe- Grant, M., editors. Kirk-Othmer Encyclopedia of Chemical Technology. New York: John Wiley and Sons; 2004. Available at http: // onlinelibrary.wiley.com/doi/10.1002/0471238961.0612150308050920.a01.pub2/full. Accessed 2012 Feb 25.
- Huang S.Y., Lipp D.W., Farinato R.S. Acrylamide polymers. In: Mark H.F., editor. Encyclopedia of Polymer Science and Technology; 2001. New York: John Wiley & Sons Inc. Available at http: // dx.doi.org/10.1002/0471440264.pst004. Accessed 2012 Feb 25.
- Buchholz F.L. Polyacrylamides and poly(acrylic acids). In: Elvers B., Hawkins S., Schulz G., editors. Ullmann’s Encyclopedia of Industrial Chemistry. 5th ed. Volume A21. Weinheim: VCH Publishers, Inc.; 1992. p. 143-156.
- Caulfield M.J., Qiao G.G., Solomon D.H. Some aspects of the properties and degradation of polyacrylamides. Chem Rev 2002; 102: 3067-3083.
- Hunkeler D.J., Hernandez-Barajas J. Inverse-emulsion/suspension polymerization. In: Salamone JC, ed. Polymeric Materials Encyclopedia. Volume 5. Boca Raton: CRC Press; 1996. p. 3322-3334.
- Flesher P., Farrar D., Field J.R. Flocculation processes. European patent 202780. 1986.
- Neff R.E., Pellon J.J., Ryles R.G. High performance polymer flocculating agents. European patent 374458. 1988.
- Sarac A.S. Redox polymerization. Prog Polym Sci 1999; 24: 1149-1204.
- Hunkeler D., Hamielec A.E., Baade W. Mechanism, kinetics, and modeling of the inverse-microsuspension homopolymerization of acrylamide. Polymer 1989; 30: 127-142.
- Baade W., Hunkeler D., Hamielec A.E. Copolymerization of acrylamide with cationic monomers in solution and inversemicrosuspension. J Appl Poly Sci 1989; 38: 185-201.
- Hernandez-Barajas J., Hunkeler D.J. Inverse-emulsion copolymerization of acrylamide and quaternary ammonium cationic monomers with block copolymeric surfactants: copolymer composition control using batch and semi-batch techniques. Polymer 1997; 38: 449-458.
- Wong Shing JB, Hurlock J.R., Maltesh C., Nagarajan R. Papermaking process utilizing hydrophilic dispersion polymers of diallyldimethyl ammonium chloride and acrylamide as retention and drainage aids. US patent 6071379. 2000.
- Santini J.J., Yankie N.A. Stabilized polyacrylamide emulsions and methods of making same. US patent 5548020. 1996.
- Fong D.W., Kowalski D.J. Sulfomethylamide-containing polymers. US patent 5120797. 1992.
- Ryan M., Pawlowska L. Quaternary mannich polymer microemulsion (QMM) with rapid standard viscosity (SV) development. US patent 5789472. 1998.
- Domb A.J., Langer R.S., Cravalho E.G., Golomb G., Mathiowitz E., Laurencin C.T. Method of making hydroxamic acid polymers from primary amide polymers. US patent 5128420. 1992.
- Rothenberg A., Flieg G., Cole R. Reduction of impurities in Bayer process alumina trihydrate. US patent 5665244. 1997.
- Murray P.G., Whipple W.L. Fluorescent water-soluble polymers. US patent 6344531. 2002.
Note:
For information about Monitoring Polymerization Reactions: From Fundamentals to Applications, go to www.wiley.com/WileyCDA/.
