Plastic Failure through Environmental Stress Cracking
ESC, “the plastic killer,” is a frequent cause of field failures
Previous Article Next Article
By Jeffrey Jansen
The Madison Group, Madison, Wisconsin, USA
Plastic Failure through Environmental Stress Cracking
ESC, “the plastic killer,” is a frequent cause of field failures
Previous Article Next Article
By Jeffrey Jansen
The Madison Group, Madison, Wisconsin, USA
Plastic Failure through Environmental Stress Cracking
ESC, “the plastic killer,” is a frequent cause of field failures
Previous Article Next Article
By Jeffrey Jansen
The Madison Group, Madison, Wisconsin, USA

Figure 1: Catastrophic failure occurred in a CPVC pipe through ESC associated with contact with an incompatible sound-proofing caulk.
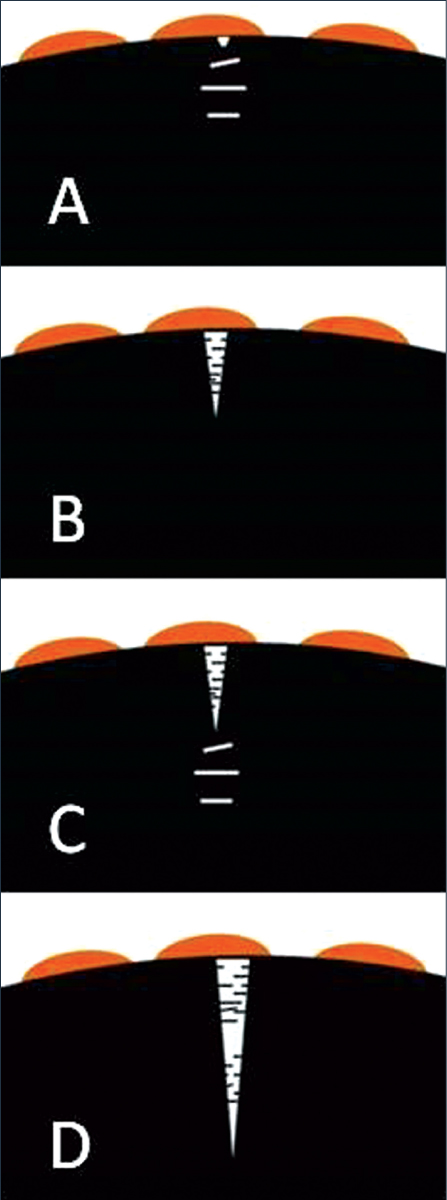
Figure 2: Illustration of the ESC process.
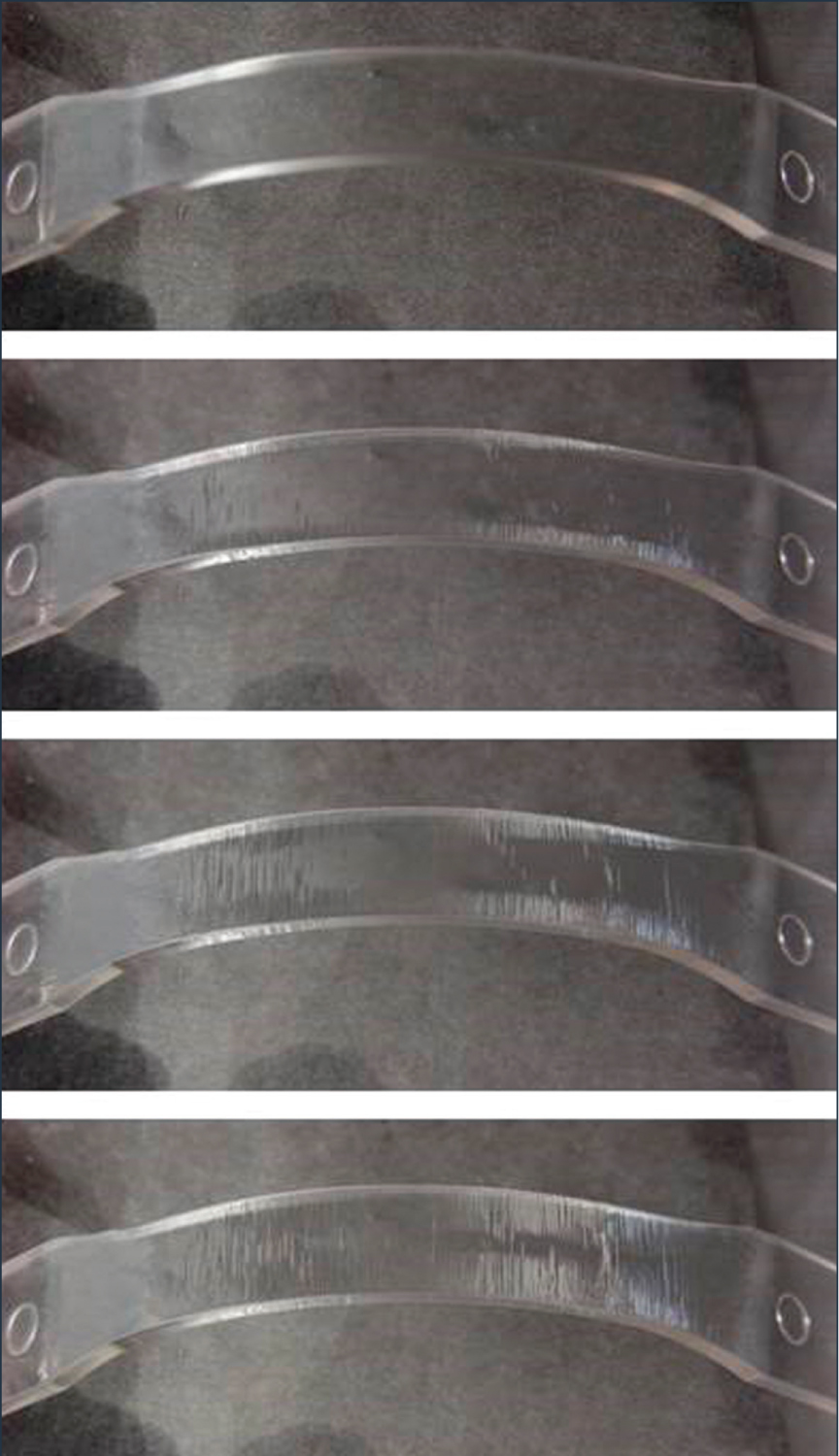
Figure 3: Time-lapse images showing the progression of ESC within a bent strip sample.

Figure 4: Scanning electron micrographs showing a typical ESC fracture surface. At high magnification, radiating bands of opened craze remnants are evident.

Figure 5: A typical latch-handle fracture surface is shown, displaying features associated with multiple crack-initiation sites.

Figure 6: A high concentration of oil was present on the hinge roll pin.
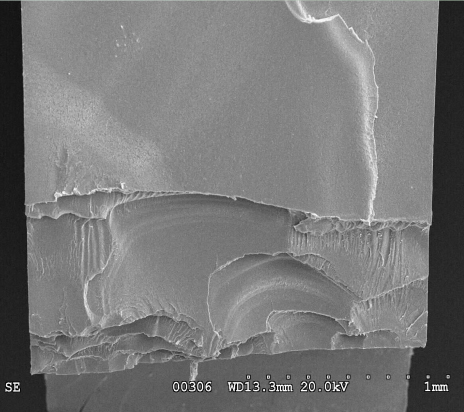
Figure 7: SEM micrograph showing the fractured surface. Multiple cracks initiated along the interior edge of the part wall and coalesced to produce the fracture.
Environmental stress cracking (ESC) is considered a leading cause of plastic part failure. It is the premature embrittlement and subsequent cracking of a plastic due to the simultaneous and synergistic action of stress and contact with a chemical agent. Because of the frequency and severity of ESC failure, it has been nicknamed “the plastic killer” (see, for example, Figure 1).
A principal consideration in the relatively high failure rate associated with environmental stress cracking is the widespread lack of awareness and understanding of the interaction between plastic materials and chemicals, particularly organic-based chemicals, which are so prevalent in manufacturing, commercial, and household settings. Most failures associated with ESC occur through contact with a secondary chemical agent. A secondary chemical agent is one that is not anticipated to contact the molded plastic part throughout its lifecycle.
This is in contrast to a primary chemical agent, where contact is expected, such as gasoline with a small-engine fuel filter, or paint with the tubing in a paint sprayer. Failures connected with primary chemical agents are rare in service because the chemical compatibility is well understood at the design phase. Failures associated with secondary chemical agents are far more common.
ESC Mechanism
A common misconception about environmental stress cracking is that it involves molecular degradation or chemical attack of the plastic material. This is not true. The ESC failure mechanism does not proceed through a chemical reaction between the polymer and the chemical agent. Molecular structure alteration or degradation does not occur. Significantly, in the absence of the chemical the plastic would undergo stress cracking in air, also known as creep rupture, given sufficient time. The chemical simply accelerates the stress cracking. Because of this, environmental stress cracking and creep rupture are parallel failure mechanisms.
As reviewed in the July/August 2015 issue of Plastics Engineering (p. 32), creep is the tendency of a solid material to deform permanently under the influence of constant stress, and occurs as a function of time through extended exposure to levels of stress that are below the yield point of the material. Creep rupture is cracking within a plastic component that occurs as a response to stress that occurs through disentanglement of the polymer chains by overcoming intermolecular forces, such as Van der Waal’s forces and hydrogen bonding, between the polymer chains.
Several factors will accelerate the embrittlement process associated with creep rupture, including an increase in temperature, stress concentration within the part, cyclic loading, fatigue, and contact with specific chemical agents (ESC). In environmental stress cracking, the chemical agent permeates into the molecular structure of the plastic, interfering with the intermolecular forces bonding the polymer chains, allowing accelerated molecular disentanglement. This reduces the energy required for disentanglement to occur. The presence of a moderate environmental stress crack agent can result in an order of magnitude reduction in the time to failure.
An important consideration is that the chemical agent does not permeate substantially into the plastic material, and only the material on the contact surface is affected. The bulk properties of the material, including stiffness and strength, are not affected. Other chemically induced failure mechanisms, such as plasticization and solvation, include further interaction between the chemical agent and the plastic.
Cracks associated with ESC failure generally initiate at localized areas of stress concentration, such as a design corner or notch in the part, a defect, a surface scratch, or a crack. The stress concentration factor associated with these conditions can range from 1.5 to 5 and higher. Under conditions of chemical contact, the chemical agent permeates into the molecular structure of the polymer chains preferentially at the elevated stress field associated with the concentration factors. Very localized plasticization takes place via stress-enhanced fluid absorption.
In response to the stress and facilitated by the plasticizing effect of the chemical, the individual segments of the chains rotate, and become aligned parallel to the direction of the maximum strain. Crazes are formed as planar arrays of fine voids normal to the tensile stress (Figure 2a). The voids are separated by ligaments of highly aligned polymer chains.
Crazes form and grow within the chemically affected zone. Eventually, the crazes rupture to form a crack (Figure 2b). This crack then serves as a stress concentrator, and crazes form and grow within the chemically affected zone in front of the crack, and this process continues (Figures 2c and 2d). Environmental stress cracking can take place through continuous crack growth or via a stepwise progressive mechanism of cracking and arrest, depending on the molecular structure of the plastic, the composition of the chemical agent, the level of stress, and other environmental conditions (Figure 3).
Fracture Surface Features
Fractography is the study of the fracture surfaces of materials, including plastics. Fractographicmethods are routinely used to determine the crack mechanism as part of a failure analysis. The fracture surfaces created through environmental stress cracking have several typical characteristics. ESC failures occur through brittle fracture, even in plastics that would be expected to produce a ductile mechanism. The combination of stress below the yield point of the material and the interference with intermolecular bonding caused by the chemical agent result in a brittle-fracture, slow-crack-growth mechanism.
Generally, ESC results in multiple individual cracks that coalesce into a unified fracture. The crack origins are usually in localized areas of elevated stress within the part that are in direct contact with the environmental stress crack agent. Typically, ESC crack origin areas exhibit a relatively smooth morphology associated with slow crack growth (Figure 4), although, under conditions of relatively high stress and/or contact with aggressive chemical agents, the fracture surface can present more coarse surface features.
The presence of opened craze remnants, often in a series of radiating bands, can be indicative of environmental stress cracking. However, not all ESC failures exhibit these bands, and some creep rupture failures will present these features. In many cases, the final fracture zone will exhibit features associated with mechanical overload. This occurs once the remaining ligament of the fracture surface can the longer withstand the applied load.
In summary, there is not a single fracture morphology that describes every ESC failure. Rather, the fracture surface features are dependent on the combination of material, stress, and chemical agents.
ESC Factors
There are a number of key factors in environmental stress cracking:
Polymer composition: The inherent chemical resistance of a polymeric material is determined by its molecular structure and the functional groups from which it is comprised. In general, amorphous polymers are more susceptible to ESC than semi-crystalline polymers because of their greater free volume. This means that polycarbonate, poly(acrylonitrile-butadiene-styrene), and poly(methyl methacrylate) are inherently less resistant to ESC failure than nylon, polyacetal, and poly(butylene terephthalate).
Chemical agent: The composition of the chemical agent is a primary factor in how aggressive of an ESC agent that substance will be. Some chemicals will affect various plastics, but not others. The interaction between the polymer and chemical is dependent on the affinity between the two. The intermolecular forces within the chemical affect its strength as an ESC agent. Chemicals having moderate hydrogen bonding tend to be the most severe ESC agents. To many types of plastics, the most aggressive ESC agents are organic esters, because of their molecular structure and their relatively prevalent use. Additionally, chemicals with lower molecular weights are generally more aggressive environmental stress crack agents, because of their greater molecular mobility and lower viscosity. Increased chemical concentration will generally result in more rapid or more extensive cracking. However, this is not always the case, and in some situations lower concentrations that result in particular levels of hydrogen bonding or unique solubility parameters can be more aggressive.
Stress level: Elevated levels of stress result in shorter induction times for ESC crack initiation. An important consideration is that the stress on the part is composed of both the externally applied stress and the internal stress associated with forming and assembly. Under many circumstances, environmental stress cracking can occur simply under the molded-in residual stress associated with injection molding and other forming processes.
Identifying incompatibility between a plastic resin and a chemical agent at the design and material selection stage can be the difference in avoiding failure. While chemical compatibility references are available, they are incomplete, and their utility is somewhat limited because of the extremely high number of plastic/chemical combinations. It is more common to find chemical compatibility references related to degradation mechanisms rather than ESC.
A more direct assessment can be made through actual testing. ASTM D543 “Standard Practice for Evaluating the Resistance of Plastics to Chemical Reagents – Practice B – Mechanical Stress/Reagent Exposure” is a common screening method. Here, a rectangular specimen of the plastic being evaluated is bent to a prescribed radius using a mechanical fixture. The applied strain can be determined from the specimen thickness and the radius of the curvature. Chemical contact is maintained over time, and the specimen is examined periodically. The time is noted for crack initiation and catastrophic failure. Normally, the experiment includes testing over a range of strains, with multiple specimens representing each condition. Control samples are also tested, including chemical contact on unstrained specimens and strained specimens with no chemical exposure.
An alternate test method that allows the evaluation of smaller samples or actual molded parts involves the insertion of a metal pin into an undersized machined hole in the specimen. This method is performed in accordance with ISO 22088 “Determination of Resistance to Environmental Stress Cracking (ESC) – Part 4: Ball or Pin Impression Method.”
Case Example
In one case of ESC, a high number of latch handles failed after a relatively short time in service.1 The normal service conditions included periodic actuation of the handles at normal ambient automotive passenger-compartment temperatures. The handles were injection molded from a polycarbonate/poly(acrylonitrile-butadiene-styrene) (PC/ABS) blend. The design utilized an integral metallic roll pin and a spring.
Visual and microscopic examinations of the failed parts revealed significant cracking (Figure 5), which was consistent across all of the handles. The cracks were present within the molded boss that secured the roll pin. When the parts were disassembled, numerous additional, non-catastrophic cracks were apparent at similar locations around the boss hole. The crack surfaces exhibited features characteristic of brittle fracture, and the crack origins were primarily located adjacent to the inner diameter. An oily residue was evident on and adjacent to the fracture surfaces, and on the mating roll pin (Figure 6).
Scanning electron microscopic (SEM) examination of the fracture surface confirmed the presence of multiple crack origins at the inner diameter of the boss hole, at areas that had been in direct contact with the roll pin (Figure 7). The surface morphology within the crack origin areas included craze remnants, suggestive of craze formation as part of the crack initiation mechanism. The fracture surface outside of the origin locations showed features indicative of brittle fracture, including hackle marks and secondary cracking.
Analytical testing of the latch handles produced results characteristic of an unfilled PC/ABS blend. No evidence was found to suggest bulk contamination of the material. Melt flow rate testing of the failed latch handles produced results showing excellent retention of molecular weight, with no indication of molecular degradation.
Analysis of the oily residue found on the fracture surface using spectroscopy produced results indicative of a hydrocarbon oil containing an ester-based additive. The residual oil within the roll pins was also analyzed, and the obtained results produced an excellent match with spectra representing the fracture surface residue. Spectral library searching generated very good matches with commercial fluids for metal processing.
The conclusion of the failure analysis was that the latch handles failed via ESC. The chemical agent responsible for the failure was identified as the fluid used in the forming and processing of the metal roll pin, a hydrocarbon oil containing an organic ester-based additive.
This is a typical example of a secondary chemical agent causing ESC. Ester-based materials are known to be active ESC agents for both ABS and PC resins. The oil had not been properly cleaned from the roll pin, as mandated by the handle manufacturer. This inadequate cleaning was the main factor in the failure of the latch handles. The requisite stress involved in the failure apparently came from the interference fit between the roll pin and the handle boss.
The failure was identified as ESC by the visual, microscopic, and SEM examinations. The telltale features included the irregular but continuous crack pattern, the presence of multiple individual crack-initiation sites, the generally brittle fracture-surface morphology, and the micro-craze remnants within the crack origin locations.
Reference
1. Jansen, J. A. “Environmental Stress Cracking—Examples from the Automotive Industry,” ANTEC Proceedings, SPE, 2005.
About the author… Jeffrey A. Jansen is a senior managing engineer and partner at The Madison Group, an independent plastics engineering and consulting firm. He specializes in failure analysis, material identification and selection, and aging studies for thermoplastic materials. Jansen is a regular contributor to Plastics Engineering’s “Consultant’s Corner” and a regular presenter for SPE’s webinar series, covering a wide range of topics related to plastics failure, material performance, testing, and polymer technology.
Note: Sign up for Jansen’s SPE webinar on ESC coming up on February 11, 2016; see www.4spe.org/Events/webinars.aspx for details.




