Moldmaking Beyond the Tooling
NyproMold addresses medical molders’ needs in multiple ways
Previous Article Next Article
By Michael Tolinski
Moldmaking Beyond the Tooling
NyproMold addresses medical molders’ needs in multiple ways
Previous Article Next Article
By Michael Tolinski
Moldmaking Beyond the Tooling
NyproMold addresses medical molders’ needs in multiple ways
Previous Article Next Article
By Michael Tolinski

NyproMold senior tool maker Jim Boudreau (left) and business unit director Ghassan Aswad with a two-shot stack mold for a personal care product. Top: An overview of NyproMold’s operation (all photos in this article courtesy of NyproMold).

NyproMold senior tool maker Jim Boudreau (left) and business unit director Ghassan Aswad with a two-shot stack mold for a personal care product. Top: An overview of NyproMold’s operation (all photos in this article courtesy of NyproMold).

A development mold, built in less than 12 days.

A press setup used for testing customer molds.

NyproMold’s CT scanner.
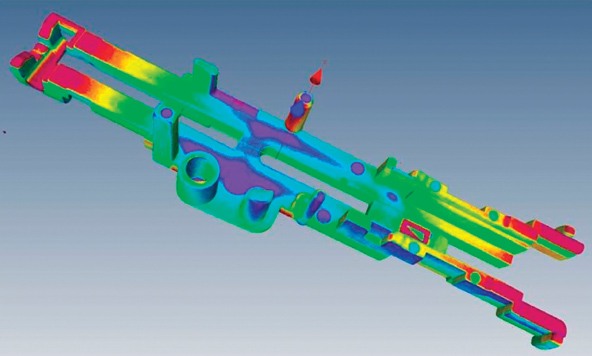
A representative part scan.
For makers of injection molds, it’s not really just about the mold—it’s about the parts produced by the mold (the mold being literally just a tool). Parts from the mold must meet customer expectations, with low cycle times and consistent quality, and—especially for parts used in healthcare—they must satisfy stringent inspection and documentation requirements.
Of course this all requires a high-quality precision mold. But large, successful mold manufacturers must address other customer needs in terms of global manufacturing requirements, multiple volume levels, part and mold development, and process verification. This was evident during a visit to NyproMold in eastern Massachusetts in February.
Global Focus
“High-precision, high-complexity, multi-volume-scale programs, engineered with data-driven solutions, that launch globally,” is how NyproMold’s business unit director Ghassan Aswad sums up his company’s specialization. Its medical mold customer base is composed of large pharmaceutical firms that have chosen the company to develop programs “and take them from conception to fruition faster,” he adds.
Its customers’ healthcare parts include mainly high-volume disposable parts for drug-delivery and diagnostics and other medical devices, some of them produced with very high-cavitation stack molds of 144 cavities or more. “We primarily focus on a part footprint that’s 6 by 6 by 6 inches” (15 × 15 × 15 cm), maximum, says Aswad. The devices and parts include inhalers, diabetes injection pens, small gears, and caps.
The company supports global launches of products that have to be released simultaneously, shipping multiple identical tools for the same part to different parts of the world. Aswad says customers are willing to pay the premium to ship even large molds to overseas locations, which he says typically lack the mold development talent and skills needed for these kinds of programs. The company exports roughly 10-15% of its tools, and “we fly out our team globally to support these operations.”
NyproMold’s design team also helps some customers find the most cost-effective resin for the job. Because of the high cost of some engineering resins a customer might specify, Aswad says “we step back, re-evaluate … and try to find a more comparable material,” that still fits the application, driven by the customer’s cost-cutting goals.
Thus a mold design might allow a high-end polyetherimide grade to be replaced by a polycarbonate that’s one-tenth as expensive. Or designers might be able “to put the part on a diet,” offering a design that eliminates 10-20% of its mass, which can add up to major savings over the years, and eventually “pays for the tool itself,” he says.
Clean Molds for Clean Molding
For healthcare products, the main mold material is typically 420 stainless steel, plus other materials with high heat conductivity. To make the tooling as robust as possible, wear-resistant coatings are also used, since some molds must be able to create hundreds of millions of parts.
Overall, “It’s an automatic one-one”—a minimum guarantee from the company that the mold can produce one million parts over one year, though Aswad says molds producing 5 million parts are not unheard of in the packaging industry.
“That an awful lot of whacks, at 300 to 500 tons per whack,” adds Al Cotton, Nypro’s longtime director of communications.
For cleanroom molding, customers don’t want to use any hydraulics, “and they don’t want to use grease because they don’t want any foreign matter touching the parts,” says Aswad. There are certain FDA-approved greases or alternative lubricants that can be used, for example, as a possible solution for some cases.
Other tooling capabilities include in-mold closing and labeling, multi-shot molding, and vertical rotating stacks. “Medical customers are trending toward multi-shot [molding], but they’re primarily using it for sealing, so they can make their device water-proof,” Aswad explains. For example, an overmolded TPE seal might be integrated to protect drugs inside a device.
Medical product manufacturing is notorious for its testing and documentation requirements. It’s more than just a hassle for medical mold manufacturing. Whereas a packaging mold can be produced and debugged and shipped quickly, “in medical we’ve got to run an extensive DOE [design of experiments],” says Aswad. They must test for multiple variables, pushing the tool to its limits, then “break the tool down, inspect it, … and put it back together and into the press for ‘round two’ and the same exact testing.”
But there is some justification for all this, since something like flash on a drug-delivery part could prevent insulin flowing to a user, for example. Al Cotton puts it succinctly: “When you’re designing for a healthcare product, you make a mistake and people can die; that’s not going to happen on a package for baby wipes.”
The Development Team
But what if a molder customer asks: “I need just a few hundred-thousand parts; can you help us out?”
In response to questions like these, NyproMold focuses on programs for customers who need minor production quantities of parts, but can’t get the needed functionality or volumes from a rough prototype mold. With its development team, “What we build is not a Class 101 tool,” Aswad says, but rather a one- or multi-cavity stand-alone mold with the cooling and durability to provide a volume of parts. (A molder-customer might at first think it needs only a few hundred parts, and then finds it really needs a mold that can produce over 100,000.)
And since the mold is designed with the cooling patterns and gating styles of a high-cavitation mold, the “production-minded” development mold also allows customers to learn lessons from molding a part with one cavity before scaling up to a 144-cavity tool.
The company’s development team can be used in other ways. In one case, Aswad says a customer asked, “‘What can we do with the same exact footprint in the press we currently have to increase volumes?’” NyproMold built prototypes and found a way of reducing a 13-second cycle time down to 9 seconds, says Aswad, “helping them produce 42 million more parts per year—with the same equipment [footprint] and the same cavitation.”
The Shop Floor
In the machining shop (which is climate-controlled, since only a few degrees of changing temperature can throw off machining tolerances), “Everything’s driven off of the CAD system,” Aswad says. CNC five-axis milling, electrical discharge machining, dimensional verification, and other steps of moldmaking are all linked to ensure consistency in multiple identical tools made over time.
A program might call for something like 16 identical 32-cavity molds, for example, shipped to one location or multiple locations. And each standard mold may contain over 2,000 individual components, up to as many as 5,000-6,000 components for a more complex two-shot core-back mold.
Ghassan Aswad is hesitant to talk about the company’s choices in machining centers—for a reason. “That’s one thing we do a lot of R&D on”—the process of selecting a new CNC mill. New machining centers are run for six months to show they can hold required tolerances, and when one doesn’t pass, its delivery is not accepted.
But it’s a volume business too, with tons of expensive metal being cut each day. Looking at a rack of raw mold-material stock, Aswad says, “I know how well we’re doing, based on how full this is. The more steel we have on this rack, the better.”
DOEs and CT Scans
NyproMold validates parts and debugs new molds in its technical center in the most realistic way possible—even to the extent of bringing customers’ actual injection molding presses into the tech center for mold trials. And given the relatively large high-cavitation molds produced, the presses are by no means small.
For customers whose machines can’t be brought in, the company runs mold trials on presses that are as close as possible in brand, type, and tonnage. For instance, a JSW press is used for molds bound for Asia since that’s a typical brand used there, says Aswad. The molds ship only after the multiple trials and DOEs required for medical parts.
The tech center also has a molding press in a cleanroom for developing molds for sensitive parts in a realistic production environment.
For validating parts that come out of the molds, NyproMold uses a technology more commonly found in hospitals: CT scanning (otherwise known as 3-D X-ray computed tomography). Marketed as a division of the company called 3D ProScan, the company’s CT scanner can develop a fine 3-D model of a part’s external and internal features and dimensions. However, unlike with the hospital scanning of patients, this industrial scanning moves the part as it’s scanned,
Metrology technician Ryan Bergeron says that the CT scanner can be used for measuring a part’s wall thickness and other dimensions, checking for voids and warpage, part-to-part comparisons, reverse engineering, and analysis of a part’s assembly (such as an inhaler with multiple internal gears). The part, mounted in simple polystyrene foam (transparent to X-rays), rotates 360° in the scanner as the full 3-D picture is created scan-by-scan.
The coordinate measuring machine (CMM) in the lab, however, is still essential. It’s more cost-effective and faster than the scanner for measuring large batches; it just doesn’t supply the thousands of data points of the CT scanner, adds Bergeron.
The CT scanning “has been a huge differentiator” for the company, says Ghassan Aswad. “We’ve developed it as a business of its own” for customers wanting to “break down” their assemblies non-destructively.



