Original Article
Compatibility of Vancomycin and Oxacillin During Simulated Y-Site Delivery
Hilary M. Teibel, PharmD*; Chad A. Knoderer, PharmD*; and Kristen R. Nichols, PharmD*,†
Original Article
Compatibility of Vancomycin and Oxacillin During Simulated Y-Site Delivery
Hilary M. Teibel, PharmD*; Chad A. Knoderer, PharmD*; and Kristen R. Nichols, PharmD*,†
Original Article
Compatibility of Vancomycin and Oxacillin During Simulated Y-Site Delivery
Hilary M. Teibel, PharmD*; Chad A. Knoderer, PharmD*; and Kristen R. Nichols, PharmD*,†
Abstract
Background: Vancomycin and oxacillin may be used together as empiric coverage in patients with proven or suspected Staphylococcus aureus infections. Though vancomycin hydrochloride 20 mg/mL and oxacillin sodium 160 mg/mL are reported to be compatible via Y-site delivery, Y-site compatibility of commonly used concentrations, vancomycin 10 mg/mL and oxacillin 20 mg/mL, has not yet been reported.
Objective: To determine the Y-site compatibility of vancomycin 10 mg/mL and oxacillin 20 mg/mL.
Methods:One vancomycin hydrochloride 1 g vial was reconstituted with 10 mL sterile water for injection (SWFI) and diluted with 90 mL 5% dextrose in water (D5W) in an evacuated intravenous (IV) bag. One oxacillin sodium 2 g vial was reconstituted with 11.5 mL sterile water for injection and diluted with 88 mL sterile water for injection in an evacuated IV bag. Three mL of each vancomycin and oxacillin were mixed in 4 test tubes to simulate Y-site delivery. Spectrometry, pH evaluation, and visual examination were performed for each test tube immediately following mixing and at 30 minutes, 1 hour, and 2 hours after mixing.
Results: Upon visual examination with multiple backgrounds, a white precipitant was immediately evident in the test tubes with vancomycin and oxacillin combined. Spectrometry results strongly supported evidence of precipitation throughout the duration of the experiment.
Conclusion:Vancomycin 10 mg/mL and oxacillin 20 mg/mL were determined to be physically incompatible for Y-site delivery in this study, despite prior evidence that the 2 medications in different concentrations were suitable for Y-site co-administration.
Key Words—compatibility, intravenous, oxacillin, precipitant, vancomycin, Y-site
Hosp Pharm—2015;50:710–713
Abstract
Background: Vancomycin and oxacillin may be used together as empiric coverage in patients with proven or suspected Staphylococcus aureus infections. Though vancomycin hydrochloride 20 mg/mL and oxacillin sodium 160 mg/mL are reported to be compatible via Y-site delivery, Y-site compatibility of commonly used concentrations, vancomycin 10 mg/mL and oxacillin 20 mg/mL, has not yet been reported.
Objective: To determine the Y-site compatibility of vancomycin 10 mg/mL and oxacillin 20 mg/mL.
Methods:One vancomycin hydrochloride 1 g vial was reconstituted with 10 mL sterile water for injection (SWFI) and diluted with 90 mL 5% dextrose in water (D5W) in an evacuated intravenous (IV) bag. One oxacillin sodium 2 g vial was reconstituted with 11.5 mL sterile water for injection and diluted with 88 mL sterile water for injection in an evacuated IV bag. Three mL of each vancomycin and oxacillin were mixed in 4 test tubes to simulate Y-site delivery. Spectrometry, pH evaluation, and visual examination were performed for each test tube immediately following mixing and at 30 minutes, 1 hour, and 2 hours after mixing.
Results: Upon visual examination with multiple backgrounds, a white precipitant was immediately evident in the test tubes with vancomycin and oxacillin combined. Spectrometry results strongly supported evidence of precipitation throughout the duration of the experiment.
Conclusion:Vancomycin 10 mg/mL and oxacillin 20 mg/mL were determined to be physically incompatible for Y-site delivery in this study, despite prior evidence that the 2 medications in different concentrations were suitable for Y-site co-administration.
Key Words—compatibility, intravenous, oxacillin, precipitant, vancomycin, Y-site
Hosp Pharm—2015;50:710–713
Abstract
Background: Vancomycin and oxacillin may be used together as empiric coverage in patients with proven or suspected Staphylococcus aureus infections. Though vancomycin hydrochloride 20 mg/mL and oxacillin sodium 160 mg/mL are reported to be compatible via Y-site delivery, Y-site compatibility of commonly used concentrations, vancomycin 10 mg/mL and oxacillin 20 mg/mL, has not yet been reported.
Objective: To determine the Y-site compatibility of vancomycin 10 mg/mL and oxacillin 20 mg/mL.
Methods:One vancomycin hydrochloride 1 g vial was reconstituted with 10 mL sterile water for injection (SWFI) and diluted with 90 mL 5% dextrose in water (D5W) in an evacuated intravenous (IV) bag. One oxacillin sodium 2 g vial was reconstituted with 11.5 mL sterile water for injection and diluted with 88 mL sterile water for injection in an evacuated IV bag. Three mL of each vancomycin and oxacillin were mixed in 4 test tubes to simulate Y-site delivery. Spectrometry, pH evaluation, and visual examination were performed for each test tube immediately following mixing and at 30 minutes, 1 hour, and 2 hours after mixing.
Results: Upon visual examination with multiple backgrounds, a white precipitant was immediately evident in the test tubes with vancomycin and oxacillin combined. Spectrometry results strongly supported evidence of precipitation throughout the duration of the experiment.
Conclusion:Vancomycin 10 mg/mL and oxacillin 20 mg/mL were determined to be physically incompatible for Y-site delivery in this study, despite prior evidence that the 2 medications in different concentrations were suitable for Y-site co-administration.
Key Words—compatibility, intravenous, oxacillin, precipitant, vancomycin, Y-site
Hosp Pharm—2015;50:710–713
Hosp Pharm 2015;50(8):710–713
2015 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj5008-710


Vancomycin is frequently used in addition to a beta-lactam antimicrobial agent such as oxacillin in the empiric treatment of suspected or proven Staphylococcus aureus infections, including bacteremia, endocarditis, and bone and joint infections. Reduced morbidity and mortality in patients with Staphylococcal bacteremia has been suggested when combination therapy of vancomycin and an antistaphylococcal β-lactam such as oxacillin, nafcillin, or cefazolin are empirically initiated when compared to vancomycin monotherapy.1 This regimen could be de-escalated based on susceptibility data indicating methicillin-susceptible Staphylococcal species.2 An in vitro study has demonstrated synergy of vancomycin and oxacillin for select vancomycin-intermediate S. aureus (VISA) strains, suggesting that this combination may be of some use in those infections.2
Oxacillin and nafcillin are beta-lactam antibiotics, and efficacy is dependent upon sustained drug concentrations. Dose optimization is often achieved by administration via continuous infusion. As pediatric patients may frequently have only one intravenous (IV) access site through which to receive IV medications, compatibility of IV solutions is particularly important when dosing frequencies and infusion strategies are considered. At Riley Hospital for Children, continuous infusions of nafcillin and oxacillin are common in combination with intermittent vancomycin infusions. Nafcillin 10 mg/mL has been demonstrated to be incompatible with vancomycin 2 to 20 mg/mL, so the 2 drugs at the standard concentrations used at Riley Hospital for Children may not be co-infused.3 A prior study determined that vancomycin 20 mg/mL and oxacillin 160 mg/mL were physically compatible, but no published studies have assessed Y-site compatibility of vancomycin 10 mg/mL and oxacillin 20 mg/mL.4 The information from the previous study has been used at Riley Hospital for Children to infer compatibility of the lower concentrations, but recent experience has brought their compatibility into question. The objective of this study was to determine the Y-site compatibility of vancomycin and oxacillin at concentrations used at Riley Hospital for Children.
METHODS
The HEPA-filtered laboratory hood was turned on 30 minutes prior to starting the study. Vancomycin 10 mg/mL and oxacillin 20 mg/mL stock bags were prepared according to the institutional standards at Riley Hospital for Children. One vancomycin hydrochloride 1 g vial (Hospira, lot # 44455DD) was reconstituted with 10 mL sterile water for injection (SWFI) (Hospira) and diluted with 90 mL 5% dextrose in water (D5W) (Baxter) in an empty 150 mL Intravia container (Baxter). One oxacillin sodium 2 g vial (Auromedics, lot # 0C0214034) was reconstituted with 11.5 mL SWFI (Hospira) and diluted with 88 mL SWFI (Hospira) in an empty 150 mL Intravia container (Baxter). Each bag was labeled and given a 24-hour expiration date once the bag spike was inserted. According to manufacturer-provided information, oxacillin in SWFI is stable for 4 days at room temperature and vancomycin in D5W is stable for 24 hours at room temperature.5,6
A Spectronic 20D+ (Thermo Fisher Scientific, Waltham, MA) was used to measure absorbance. It was set to a wavelength of 700 nm and calibrated with deionized water to an absorbance of 0.000. Absorbance of greater than 0.01 was considered evidence of turbidity. The pH meter (Oakton Instruments, Vernon Hills, IL) was calibrated with control solutions at pH of 7 and 4. Three mL of each vancomycin (10 mg/mL) and oxacillin (20 mg/mL) solutions were mixed in 4 test tubes to simulate Y-site delivery. Six mL of each deionized water, D5W, SWFI, vancomycin, and oxacillin were added to separate test tubes for use as control solutions. Spectrometry, pH, and visual examination were performed for each test tube immediately following initial mixing (time 0) and 30 minutes, 1 hour, and 2 hours after mixing. Visual examination was performed for each control and mixture solution with an unaided eye on a white background, unaided eye on a black background, with a 4-cell Maglite on a white background, with a 4-cell Maglite on a black background, through a 4.0 diopter magnifying glass on a white background, and through a 4.0 diopter magnifying glass on a black background.
RESULTS
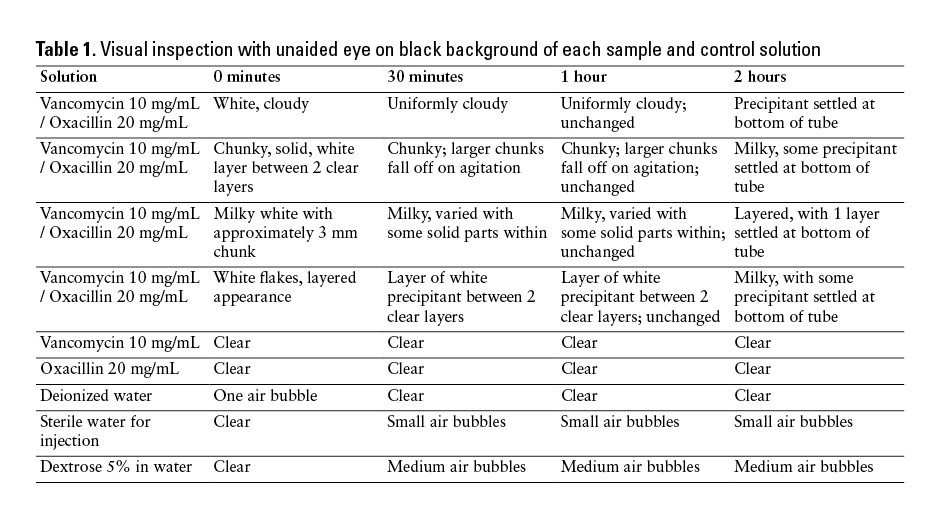
White precipitant was immediately present in the test tubes when the vancomycin and oxacillin solutions were combined (Table 1). The particulate matter was readily visualized with the unaided eye; therefore, results were unchanged upon examination with the magnifying glass or Maglite. Samples ranged from milky solutions to layered solutions with solid flakes of precipitant. Over the 2 hours of visual observation, some of the tubes had white precipitant settle to the bottom of the tube. No visual changes presented in any of the control solutions.
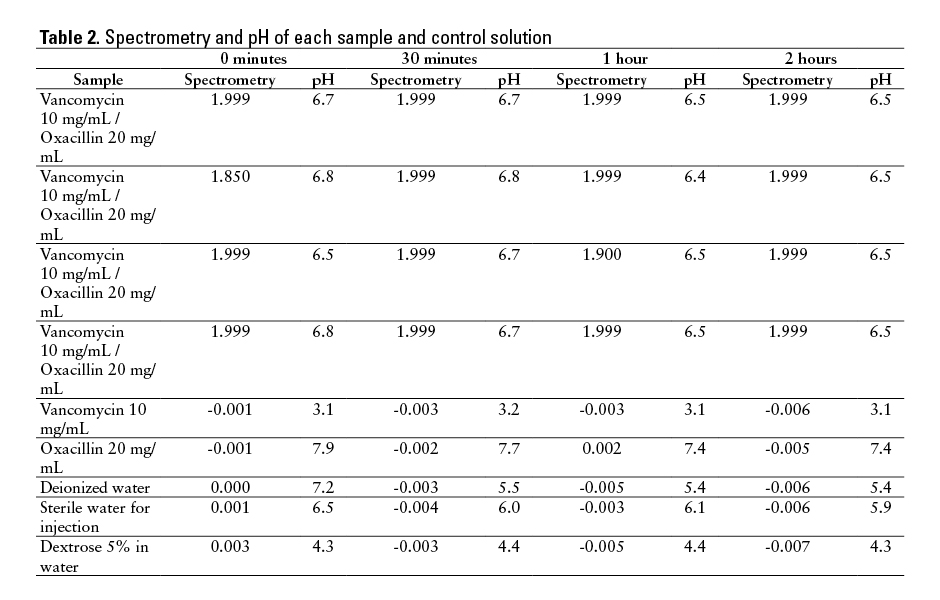
Spectrometry results strongly supported evidence of precipitation in each of the vancomycin and oxacillin mixtures (Table 2). All mixture test tubes demonstrated absorbance greater than 0.01 during each spectrometry reading, providing evidence of turbidity and particulate matter. During 14 of 16 spectrometry testing points, absorbance was 1.999; the other 2 were 1.85 and 1.9. No control solution reached absorbance of greater than 0.01 at any point during the study.
The pH measurement of each sample was stable throughout the 2-hour observation period (Table 2). The oxacillin control sample had a mean pH of 7.6 (range, 7.4-7.9) while the vancomycin control sample had a mean pH of 3.125 (range, 3.1-3.2). When mixed, the vancomycin and oxacillin samples had a mean pH of 6.6 (range, 6.4-6.8).
DISCUSSION
Vancomycin 10 mg/mL and oxacillin 20 mg/mL were determined to be physically incompatible for Y-site delivery in this study, despite prior evidence that the 2 medications in other concentrations were suitable for Y-site co-administration. Trissel used different concentrations of each drug (vancomycin hydrochloride 20 mg/mL and oxacillin sodium 160 mg/mL) but used the same diluents as this study and demonstrated compatibility.4 Our result was surprising given the compatibility of the mixture when higher concentrations were used and the demonstration of concentration-dependent compatibility of vancomycin and beta-lactam antibiotics found by Trissel and colleagues.3 Different manufacturers of vancomycin and oxacillin were used in Trissel’s study (Abbott Laboratories and Apothecon, respectively) than in our study, so differences in compatibility could be a result of inactive ingredients present in the different formulations.
Chemical differences between vancomycin hydrochloride, oxacillin sodium, and their respective diluents may also explain the precipitation when these 2 drugs are mixed. The precipitant may be a result of acid-base reactions due to vancomycin being a hydrochloride salt (average pH 3.125) whereas oxacillin is a sodium salt (average pH 7.6).7 By increasing the pH when the 2 drugs were mixed, the hydrochloride salt may cause vancomycin to precipitate out of the solution. By mixing oxacillin sodium (average pH 7.6) in the D5W in which the vancomycin is diluted, the pH may become too low for oxacillin to remain in solution.
Presence of a precipitant in this study indicates that vancomycin 10 mg/mL and oxacillin 20 mg/mL should not be co-administered via Y-site. Due to inconsistency with prior studies, knowledge of this drug-drug incompatibility may prevent adverse events from occurring in future patients who are being empirically treated with this combination of antibiotics. Unfortunately, this may prevent patients with only one IV access point from empirically receiving a continuous infusion of oxacillin.
ACKNOWLEDGMENTS
The authors report no conflicts of interest.
The authors thank Dr. Hala Fadda, Jo Wagoner, Dr. Emma Lee, Beth Hospadarsky, and Dr. Angela Ockerman for their contributions of supplies and laboratory space.
REFERENCES
- McConeghy KW, Bleasdale SC, Rodvold KA. The empirical combination of vancomycin and a β-lactam for staphylococcal bacteremia. Clin Infect Dis. 2013;57(12):1760-1765.
- Werth BJ, Vidaillac C, Murray KP, et al. Novel combination of vancomycin plus ceftaroline or oxacillin against methacillin-resistant vancomycin-intermediate Staphylococcus aureus (VISA) and heterogenous VISA. Antimicrob Agents Chemother. 2013;57(5):2376-2379.
- Trissel LA, Gilbert DL, Martinez JF. Concentration dependency of vancomycin hydrochloride compatibility with beta-lactam antibiotics during simulated Y-site administration. Hosp Pharm. 1998;33:1515-1522.
- Truven Health Analytics. Trissel’s 2 clinical pharmaceutics database (parenteral compatibility): Vancomycin - oxacillin compatibility detail. www.micromedexsolutions.com/micromedex2.
- Oxacillin sodium [package insert]. Dayton, NJ: AuroMedics Pharma LLC; 2013.
- Vancomycin hydrochloride [package insert]. Lake Forest, IL: Hospira, Inc.; 2014.
- Newton DW. Drug incompatibility chemistry. Am J Health Syst Pharm. 2009;66:348-357.

*Butler University College of Pharmacy and Health Sciences, Indianapolis, Indiana; †Riley Hospital for Children at Indiana University Health, Department of Pharmacy, Indianapolis, Indiana. Corresponding author: Kristen R. Nichols, PharmD, 4600 Sunset Avenue, Indianapolis, IN 46208; phone: 317-948-4239; e-mail: knichols@butler.edu