ISMP Medication Error Report Analysis
Decimal Commas Are a Problem
Actiq Is Not for Sore Throats
Dosing Error with Tasigna
Repackaging of Imbruvica Is Approved
Michael R. Cohen, RPh, MS, ScD,* and Judy L. Smetzer, RN, BSN†
ISMP Medication Error Report Analysis
Decimal Commas Are a Problem
Actiq Is Not for Sore Throats
Dosing Error with Tasigna
Repackaging of Imbruvica Is Approved
Michael R. Cohen, RPh, MS, ScD,* and Judy L. Smetzer, RN, BSN†
ISMP Medication Error Report Analysis
Decimal Commas Are a Problem
Actiq Is Not for Sore Throats
Dosing Error with Tasigna
Repackaging of Imbruvica Is Approved
Michael R. Cohen, RPh, MS, ScD,* and Judy L. Smetzer, RN, BSN†
These medication errors have occurred in health care facilities at least once. They will happen again—perhaps where you work. Through education and alertness of personnel and procedural safeguards, they can be avoided. You should consider publishing accounts of errors in your newsletters and/or presenting them at your inservice training programs.
Your assistance is required to continue this feature. The reports described here were received through the Institute for Safe Medication Practices (ISMP) Medication Errors Reporting Program. Any reports published by ISMP will be anonymous. Comments are also invited; the writers’ names will be published if desired. ISMP may be contacted at the address shown below.
Errors, close calls, or hazardous conditions may be reported directly to ISMP through the ISMP Web site (www.ismp.org), by calling 800-FAIL-SAFE, or via e-mail at ismpinfo@ismp.org. ISMP guarantees the confidentiality and security of the information received and respects reporters’ wishes as to the level of detail included in publications.
These medication errors have occurred in health care facilities at least once. They will happen again—perhaps where you work. Through education and alertness of personnel and procedural safeguards, they can be avoided. You should consider publishing accounts of errors in your newsletters and/or presenting them at your inservice training programs.
Your assistance is required to continue this feature. The reports described here were received through the Institute for Safe Medication Practices (ISMP) Medication Errors Reporting Program. Any reports published by ISMP will be anonymous. Comments are also invited; the writers’ names will be published if desired. ISMP may be contacted at the address shown below.
Errors, close calls, or hazardous conditions may be reported directly to ISMP through the ISMP Web site (www.ismp.org), by calling 800-FAIL-SAFE, or via e-mail at ismpinfo@ismp.org. ISMP guarantees the confidentiality and security of the information received and respects reporters’ wishes as to the level of detail included in publications.
These medication errors have occurred in health care facilities at least once. They will happen again—perhaps where you work. Through education and alertness of personnel and procedural safeguards, they can be avoided. You should consider publishing accounts of errors in your newsletters and/or presenting them at your inservice training programs.
Your assistance is required to continue this feature. The reports described here were received through the Institute for Safe Medication Practices (ISMP) Medication Errors Reporting Program. Any reports published by ISMP will be anonymous. Comments are also invited; the writers’ names will be published if desired. ISMP may be contacted at the address shown below.
Errors, close calls, or hazardous conditions may be reported directly to ISMP through the ISMP Web site (www.ismp.org), by calling 800-FAIL-SAFE, or via e-mail at ismpinfo@ismp.org. ISMP guarantees the confidentiality and security of the information received and respects reporters’ wishes as to the level of detail included in publications.
Hosp Pharm 2014;49(8):689–691
2014 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj4908-689


DECIMAL COMMAS ARE A PROBLEM
Granix (tbo-filgrastim) was approved in 2012 by the US Food and Drug Administration (FDA). It has recently become available for use as a leukocyte growth factor. A hospital pharmacist reported that he noticed something unusual while evaluating samples of the product as a possible formulary replacement for Neupogen (filgrastim), a similar leukocyte growth factor. Both the outer carton and the peel-away prefilled syringe wrappers refer to the syringe volume using the period as a decimal point (eg, 300 mcg per 0.5 mL). However, increments on the barrel of the syringe use commas (eg, 0,5 mL) to separate the integers (Figure 1).
Decimals are marked with commas in Europe and many other countries. Granix is manufactured in Lithuania and is an Israeli product distributed by Teva Pharmaceuticals USA. One would not think that a comma would pose much of a problem. However, the decimal comma is unfamiliar to US health professionals, and its use has caused problems in the past, so it should not be permitted in the US. For example, if a comma is the decimal marker in a drug database, some computer systems may not be able to calculate properly.
We have also heard of instances in which a period key produced a comma whenever it was struck. Thus, an order for a patient-controlled analgesia dose of morphine 0.5 mg IV would print on labels and reports as “morphine, 5 mg IV” if a leading zero was omitted (a dangerous practice by itself). An error occurred when such a label was misread as “morphine, 5 mg.” We imagine the same type of error could occur if someone types or writes a comma instead of a decimal point after seeing it on a syringe barrel. Also, in handwritten orders, the comma can look like the number 1, allowing “morphine, 5 mg” to look like “morphine 15 mg.”
To prevent these issues, we have asked the FDA to look into the use of a comma as a decimal with Granix, and we have let Teva know of our concern.
ACTIQ IS NOT FOR SORE THROATS
A hospital reported 3 events in which a provider attempted to order Actiq (fentanylcitrate oral transmucosal lozenge) to treat a sore throat, mistaking the powerful opioid as a typical throat lozenge.
In 2 cases, the pharmacist identified the error and contacted the provider. In the third case, the provider caught his own error.
In each of these cases, the patients did not have other opioids on their profiles or a history of opioid use. Use of this product in opioid-naïve patients could have resulted in serious harm.
The hospital where the events occurred is considering possible information technology (IT) changes. The hospital is also implementing safeguards to -prevent further occurrences, including limitations on prescribing to pain management specialists, anesthesiologists, hematologists, oncologists, palliative care, and hospice providers. Strict limitations on prescribing, dispensing, and distributing this drug are required by the Transmucosal Immediate Release -Fentanyl (TIRF) Risk Evaluation and Mitigation Strategy (REMS), an FDA-required program designed to ensure informed risk-benefit decisions before initiating treatment and appropriate use while patients are treated. Before enrollment in the TIRF REMS Access program, prescribers and pharmacists must review an education program (http://www.tirfremsaccess.com/TirfUI/rems/pdf/education-and-ka.pdf), successfully complete a knowledge assessment with a score of 100%, and sign acknowledgment statements on the enrollment form.
The hospital where these events happened is also implementing an automatic medication order form that includes criteria for use of the product that cannot be bypassed without completing the form. Due to the risk of fatal respiratory depression, Actiq is contraindicated in opioid-naïve patients.
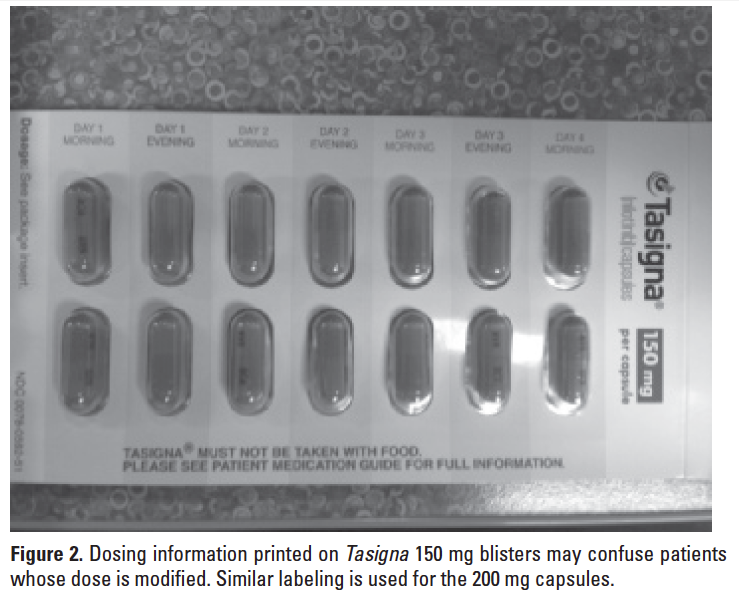
DOSING ERROR WITH TASIGNA
Patient confusion may lead to dosing errors with Tasigna (nilotinib) due to preprinted dosing instructions on the manufacturer’s packaging that reflect only the usual recommended starting doses. A patient with chronic myeloid leukemia (CML) was prescribed Tasigna 150 mg with instructions to take 2 capsules (300 mg) twice daily, the recommended starting dose for newly diagnosed CML. Product labeling for Tasigna includes a boxed warning about QT prolongation and sudden death. After several weeks on this new medication, the patient developed QT prolongation.
The patient’s physician followed the manufacturer’s label recommendations that state if clinically significant moderate or severe nonhematologic toxicity develops, providers should withhold the medication then resume at 400 mg once daily when the toxicity has resolved. The patient was instructed to hold the medication for 2 weeks and then undergo further clinical evaluation.
After the QT prolongation resolved, a new Tasigna prescription was provided for a reduced dose of 400 mg once daily. The patient filled the prescription, and the pharmacy provided 200 mg capsules in a blister pack similar to the 150 mg capsules, with instructions to take 2 capsules (400 mg) daily.
At a follow-up visit after the reduced dose was ordered, an EKG again showed QT prolongation. During this visit, it came to light that Tasigna capsules are packaged in a 2-part blister pack of 28 capsules, each with preprinted instructions to take 2 pills every morning and 2 pills every evening. This caused the patient to be confused about what dose to take. Even though the prescription label attached to the outside of the Tasigna blister packs instructed the patient to take two 200 mg capsules daily, the patient took 2 capsules twice a day as listed on the blister packs where morning and evening doses are listed as day # morning and day # evening (see Figure 2). So, instead of decreasing the dose from a total of 600 mg daily to 400 mg daily, as per the manufacturer’s labeling, the patient accidentally increased the dose to 800 mg daily (400 mg twice daily). Fortunately, the error was identified during the office visit and it was quickly corrected.
We have informed both the FDA and Novartis about this incident and asked them to completely remove the dosing instructions from the blister -packages.
REPACKAGING OF IMBRUVICA IS APPROVED
Imbruvica (ibrutinib) was recently approved by the FDA for the treatment of mantle cell lymphoma (MCL) and chronic lymphocytic leukemia (CLL) in patients who have had at least one prior therapy. The drug is available only through specialty pharmacies. This new oral chemotherapy medication has shown very encouraging results for patients who previously had limited therapeutic options. Its use will likely continue to rise, but there has been some confusion involving Imbruvica dosing and packaging.
First, dosing depends on the specific condition being treated. A dose of 420 mg (3 capsules) once daily is given to patients who have CLL. For patients with MCL, 560 mg (4 capsules) is given once daily. However, there have been 2 reported instances where a dose of only 420 mg was prescribed for a patient with MCL. If this drug is being prescribed by any oncology practitioners at your facilities, surveillance is needed in case dose clarification is required.
Another issue is with Imbruvica packaging. The drug is available only in 140 mg capsules in bottles of 90 (for CLL patients) or 120 (for MCL patients). Current labeling states that the medication must be “dispensed in original package.” This would be a problem for patients taking a moderate CYP3A4 inhibitor (eg, atazanavir, fluconazole, darunavir, erythromycin, diltiazem, aprepitant, crizotinib, imatinib, verapamil, ciprofloxacin, grapefruit products), as the labeling calls for a dose reduction to just one 140 mg capsule daily (30 capsules a month). (The drug should not be given at all if a strong CYP3A4 inhibitor is being used.) Patients requiring a dose reduction would be forced to purchase a 3-month supply (90 capsules) rather than a 1-month supply (30 capsules) of the medication.
In addition to increasing drug costs, this could result in patients taking more than the intended dose. When we asked the FDA for clarification, a representative told us that the product must be retained in its original package until dispensing. The FDA indicated there is no problem repackaging Imbruvica in smaller amounts at the time of dispensing. ![]()
*President, Institute for Safe Medication Practices, 200 Lakeside Drive, Suite 200, Horsham, PA 19044; phone: 215-947-7797; fax: 215-914-1492; e-mail: mcohen@ismp.org; Web site: www.ismp.org. †Vice President, Institute for Safe Medication Practices, Horsham, Pennsylvania.