Cancer Chemotherapy Update
Drug Monographs: Ibrutinib and Ramucirumab
Dominic A. Solimando, Jr, MA, FAPhA, FASHP, BCOP, and J. Aubrey Waddell, PharmD, FAPhA, BCOP
Cancer Chemotherapy Update
Drug Monographs: Ibrutinib and Ramucirumab
Dominic A. Solimando, Jr, MA, FAPhA, FASHP, BCOP, and J. Aubrey Waddell, PharmD, FAPhA, BCOP
Cancer Chemotherapy Update
Drug Monographs: Ibrutinib and Ramucirumab
Dominic A. Solimando, Jr, MA, FAPhA, FASHP, BCOP, and J. Aubrey Waddell, PharmD, FAPhA, BCOP
The complexity of cancer chemotherapy requires pharmacists be familiar with the complicated regimens and highly toxic agents used. This column reviews various issues related to preparation, dispensing, and administration of antineoplastic therapy, and the agents, both commercially available and investigational, used to treat malignant diseases. Questions or suggestions for topics should be addressed to Dominic A. Solimando, Jr, President, Oncology Pharmacy Services, Inc., 4201 Wilson Blvd #110-545, Arlington, VA 22203, e-mail: OncRxSvc@comcast.net; or J. Aubrey Waddell, Professor, University of Tennessee College of Pharmacy; Oncology Pharmacist, Pharmacy Department, Blount Memorial Hospital, 907 E. Lamar Alexander Parkway, Maryville, TN 37804, e-mail: waddfour@charter.net.
Name: Ibrutinib
Synonyms: Imbruvica, PCI-32765
The complexity of cancer chemotherapy requires pharmacists be familiar with the complicated regimens and highly toxic agents used. This column reviews various issues related to preparation, dispensing, and administration of antineoplastic therapy, and the agents, both commercially available and investigational, used to treat malignant diseases. Questions or suggestions for topics should be addressed to Dominic A. Solimando, Jr, President, Oncology Pharmacy Services, Inc., 4201 Wilson Blvd #110-545, Arlington, VA 22203, e-mail: OncRxSvc@comcast.net; or J. Aubrey Waddell, Professor, University of Tennessee College of Pharmacy; Oncology Pharmacist, Pharmacy Department, Blount Memorial Hospital, 907 E. Lamar Alexander Parkway, Maryville, TN 37804, e-mail: waddfour@charter.net.
Name: Ibrutinib
Synonyms: Imbruvica, PCI-32765
The complexity of cancer chemotherapy requires pharmacists be familiar with the complicated regimens and highly toxic agents used. This column reviews various issues related to preparation, dispensing, and administration of antineoplastic therapy, and the agents, both commercially available and investigational, used to treat malignant diseases. Questions or suggestions for topics should be addressed to Dominic A. Solimando, Jr, President, Oncology Pharmacy Services, Inc., 4201 Wilson Blvd #110-545, Arlington, VA 22203, e-mail: OncRxSvc@comcast.net; or J. Aubrey Waddell, Professor, University of Tennessee College of Pharmacy; Oncology Pharmacist, Pharmacy Department, Blount Memorial Hospital, 907 E. Lamar Alexander Parkway, Maryville, TN 37804, e-mail: waddfour@charter.net.
Name: Ibrutinib
Synonyms: Imbruvica, PCI-32765
Hosp Pharm 2014;49(8):702–709
2014 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj4908-702




MECHANISM OF ACTION
Ibrutinib is an irreversible inhibitor of the Bruton tyrosine kinase (BTK).1 BTK is a critical factor in B-cell receptor activation.2,3 Inhibition of BTK results in apoptosis, decreased cell proliferation, and B-cell receptor signaling.3-6 Ibrutinib also binds to IL-2-inducible kinase (ITK), inhibiting a signal pathway responsible for cellular activation and proliferation and cytokine release.7
PHARMACOKINETICS
The time to peak concentration (Tmax) occurs at 1 to 2 hours following oral administration; the terminal half-life (T½) is 6 to 9 hours.5,8,9 The mean area under the time versus concentration curve (AUC) is 953 ng•h/mL and 680 ng•h/mL following a 560 mg and 420 mg oral dose, respectively. Administration with food increases the AUC about 2-fold.10
Ibrutinib is highly (97.3%) bound to plasma proteins and has a volume of distribution (Vd) of about 10,000 L.10 The drug is metabolized in the liver, primarily by CYP3A and CYP2D6, to an active metabolite PCI-45227.10 Ibrutinib is eliminated primarily (about 80%) in the feces, with about 1% as unchanged drug. About 10% is excreted in the urine.10
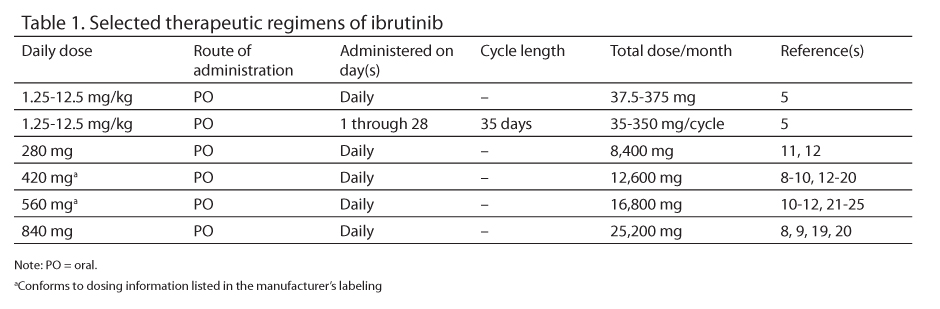
Selected therapeutic regimens of ibrutinib appear in Table 1.
PREPARATION
A. Ibrutinib is available as a 140 mg capsule.
B. The manufacturer recommends that the capsules not be opened, broken, or chewed.10
C. The manufacturer recommends the drug be dispensed in the original container.10
STABILITY
A. Ibrutinib should be stored at controlled room temperature [20°C to 25°C (68°F to 77°F)].
B. Brief (less than 24 hours) exposure to temperatures up to 30°C (86°F) is acceptable.
ADMINISTRATION
Ibrutinib is usually taken once a day, with water.
TOXICITIES
A. Cardiovascular: Edema 29%,19 (grade 1) 26%,19 (grade 2) 1%,19 (grade 1 or 2) 21% to 29%5,8; hypertension 29%,19 (grade 1) 6%,19 (grade 2) 16%,19 (grade 1 or 2) 13%,8 (grade 3) 6%,19 (grade 3 or 4) 5%.8
B. Central Nervous System: Anxiety (grade 1) 16%19; dizziness 26%,19 (grade 1) 23%,19 (grade 1 or 2) 16%,8 (grade 3) 3%,19 (grade 3 or 4) 1%8; headache 19% to 27%,12,19 (grade 1) 10%,19 (grade 2) 6%,19 (grade 1 or 2) 16% to 25%,5,8 (grade 3) 3%,19 (grade 3 or 4) 1%8; insomnia 16%,19 (grade 1) 13%,19 (grade 2) 3%,19 (grade 1 or 2) 11%.5
C. Constitutional: Fatigue 30% to 32%,12,19 (grade 1) 16%,19 (grade 2) 16%,19 (grade 1 or 2)
28% to 38%,5,8 (grade 3) 3%,19 (grade 3 or 4) 4%5,8; pain 13%,19 (grade 1) 3% to 19%,19 (grade 2)
6%,19 (grade 1 or 2) 15% to 63%,5,8 (grade 3) 1%,19 (grade 3 or 4) 2%5; pyrexia (grade 1 or 2) 21% to 22%,5,8 (grade 3 or 4) 2% to 5%.5,8
D. Dermatologic: Alopecia 27%12; rash (grade 1 or 2)
16% to 27%,5,8 (grade 3 or 4) 9%11; contusion (grade 1) 16%,19 (grade 1 or 2) 16%8; erythema (grade 1) 13%19; pruritus (grade 1) 13%19; pruritic rash (grade 1) 13%.19
E. Gastrointestinal: Anorexia/dyspepsia (grade 1 or 2) 30%,5 (grade 3 or 4) 4%5; constipation 27%,12 (grade 1) 23%,19 (grade 1 or 2) 14% to 16%,5,8 (grade 3 or 4) 1%8; diarrhea 30% to 68%,12,19 (grade 1) 45%,19 (grade 1 or 2) 43% to 47%,5,8 (grade 2) 10%,19 (grade 3) 13%,19 (grade 3 or 4) 4%5; dyspepsia 26%,19 (grade 1)
23%,19 (grade 2) 3%19; epistaxis (grade 1) 16%19; gastroesophageal reflux 19%,19 (grade 1) 13%,19 (grade 2) 6%19; nausea 48% to 67%,12,19 (grade 1) 39%,19 (grade 1 or 2) 16%,8 (grade 2) 10%,19 (grade 3 or 4) 1%8; nausea or vomiting (grade 1 or 2) 41%,5 (grade 3 or 4) 2%5; pancreatitis (grade 3 or 4) 9%11; stomatitis 16%,19 (grade 1) 13%,19 (grade 2) 3%19; vomiting 23% to 48%,12,19 (grade 1) 13%,19 (grade 2) 10%,19 (grade 1 or 2) 15%,8 (grade 3 or 4) 1% to 9%.8,11
F. Hematologic: Anemia 16% to 36%,12,19 (grade 1) 6%,19 (grade 2) 10%,19 (grade 3 or 4) 7% to 13%5,12; lymphopenia (grade 3 or 4) 64%11; neutropenia 67%,12 (grade 2) 2%,5(grade 3 or 4) 13% to 44%5,8,11,12; petechiae (grade 1) 16%19; thrombocytopenia 13% to 61%,12,19 (grade 1) 3%,19 (grade 2) 6%,19 (grade 3 or 4) 7% to 18%,5,11,12 (grade 4) 3%.19
G. Hypersensitivity: (grade 3) 2%.5
H. Infection: Cellulitis 13%,19 (grade 1) 6%,19 (grade 2) 6%19; shingles (grade 3 or 4) 9%11; sinusitis 13%,19 (grade 1) 6%,19 (grade 2) 6%,19 (grade 1 or 2) 13%,8 (grade 3 or 4) 5%8; upper respiratory tract 26%,19 (grade 1) 6%,19 (grade 2) 19%,19 (grade 1 or 2) 33%8; urinary tract 23%,19 (grade 2) 19%,19 (grade 3) 3%.19
I. Musculoskeletal: Arthralgia 23%,19 (grade 1) 16%,19 (grade 2) 6%,19 (grade 1 or 2) 16% to 27%5,8; muscle spasms/myalgia 13%,19 (grade 1) 10%,19 (grade 2) 3%,19 (grade 1 or 2) 38%5; muscle spasms (grade 1 or 2) 19%,8 (grade 3 or 4) 1%.8
J. Neurologic: Peripheral sensory neuropathy (grade 1) 13%.19
K. Ophthalmic: Dry eye (grade 1) 13%.19
L. Pulmonary: Cough (grade 1 or 2) 31% to 32%5,8; unspecified respiratory effects (grade 1 or 2) 50%,5 (grade 3 or 4) 7%.5
M. Treatment-Related Mortality: Infections 4%,8 systemic inflammatory response syndrome 1%.8
N. 420 mg daily
- Cardiovascular: Atrial fibrillation 2%,16 edema 11%,15 hypertension (grade 2 or higher) 2%.16
- Central nervous system: Dizziness 11%15; headache 14%,15 (grade 3 or 4) 1%15; subdural hematoma (grade 1) 3%.18
- Constitutional: Fatigue 5% to 47%,15,17,18 (grade 3 or 4) 2% to 10%15,17; night sweats 5%,15 (grade 3 or 4) 1%15; pain 10% to 13%,15,18 (grade 3 or 4) 1%15; pyrexia 24%,15 (grade 3 or 4) 2%.15
- Dermatologic: Bruising (grade 1 or 2) 18%,18 contusion 11%,15 maculopapular rash (grade 3 or 4) 10%.17
- Gastrointestinal: Constipation 15%15; diarrhea 3% to 70%,15,17,18 (grade 2 or higher) 2%,16 (grade 3 or 4) 4%15; mucositis (grade 3) 3%18; nausea 26% to 67%,15,17 (grade 3 or 4) 2%15; stomatitis 11%,15 (grade 2 or higher) 2%,16 (grade 3 or 4) 1%15; vomiting 14%.15
- Hematologic: Anemia 23%,15 (grade 3 or 4) 5%15; epistaxis (grade 2 or higher) 2%16; febrile neutropenia (grade 3 or 4) 7%17; hematoma (grade 2 or higher) 2%16; neutropenia 22%,15 (grade 2 or higher) 19%,16 (grade 3 or 4) 16% to 40%15,17; petechiae 14%15; thrombocytopenia 17%,15 (grade 3 or 4) 6%,15 (grade 2 or higher) 14%,16 (grade 3 or 4) 7%.17
- Infection: Cellulitis (grade 3 or 4) 7%17; herpes zoster (grade 2 or higher) 2%16; pneumonia 10% to 15%,15,18 (grade 3 or 4) 7%15; sinusitis 11%,15 (grade 3 or 4) 1%15; upper respiratory tract 8% to 37%,15,17,18 (grade 3 or 4) 1%15; urinary tract 10%,15 (grade 3 or 4) 4%.15
- Musculoskeletal: Arthralgia 13% to 17%,15,18 (grade 3 or 4) 1%15; muscle spasms 13%15; myalgia 10% to 13%,15,18 (grade 3 or 4) 1%.15
- Neurologic: Peripheral neuropathy 3% to 4%.15,18
- Ophthalmic: Blurred vision 10%.15
- Pulmonary: Cough 19%15; dyspnea 12%,15 (grade 3 or 4) 2%.15
- Treatment-related mortality: Infection 8%,18 respiratory and cardiovascular failure 3%.18
O.560 mg daily
- Cardiovascular: Edema 28%,21 (grade 1) 19%,21 (grade 2) 7%,21 (grade 3) 1%,21 (grade 4) 1%.21
- Constitutional: Fever 18%,21 (grade 1) 13%,21 (grade 2) 5%,21 (grade 3) 1%21; pain 17%,21 (grade 1) 9%,21(grade 2) 3%,21 (grade 3) 6%.21
- Dermatologic: Contusion 17%,21 (grade 1) 15%,21 (grade 2) 2%21; fatigue 41%,21 (grade 1) 20%,21 (grade 2) 17%,21 (grade 3) 5%21; rash 15%, (grade 1) 10%,21 (grade 2) 4%,21 (grade 3) 2%.21
- Gastrointestinal: Anorexia 21%, (grade 1) 10%,21 (grade 2) 9%,21 (grade 3) 2%21; constipation 25%,21 (grade 1) 18%,21 (grade 2) 7%21; diarrhea 50%,21 (grade 1) 32%,21 (grade 2) 12%,21(grade 3) 6%21; nausea 31%,21 (grade 1) 23%,21 (grade 2) 7%21; vomiting (23%, (grade 1) 17%,21 (grade 2) 5%.21
- Hematologic: Neutropenia 18%,21 (grade 1) 1%,21 (grade 2) 1%,21 (grade 3) 6%,21 (grade 4) 10%21; thrombocytopenia 18%,21 (grade 1) 4%,21 (grade 2) 4%,21 (grade 3) 7%,21 (grade 4) 4%.21
- Infection: Upper respiratory tract 23%, (grade 1) 5%,21 (grade 2) 18%.21
- Pulmonary: Cough 18%,21 (grade 1) 12%,21 (grade 2) 6%21; dyspnea 27%,21 (grade 1) 13%,21 (grade 2) 10%,21 (grade 3) 4%.21
- Treatment-related mortality: Dyspnea 1%.21
DOSAGE MODIFICATIONS
A. Hepatic: Moderate hepatic impairment (Child-Pugh B), increased ibrutinib levels are reported. No guidelines for dose modification are available.10
B. Renal10
- Creatinine clearance (Clcr) greater than 25 mL/min, no adjustment required.
- Clcr less than 25 mL/min, no information available.
Name: Ramucirumab
Synonyms: Cyramza, IMC-1121B, LY3009806
MECHANISM OF ACTION
Ramucirumab is a monoclonal antibody that binds to the extracellular domain of vascular endothelial growth factor (VEGF) receptor-2 (VEGFR-2). By binding to VEGFR-2, ramucirumab blocks VEGF signaling, resulting in inhibition of angiogenesis and cell metasteses.26-29
PHARMACOKINETICS
Following an 8 mg/kg infusion given weekly, ramucirumab has a peak concentration (Cmax) of 325 mcg/mL following the initial infusion and 497 mcg/mL following multiple infusions. It has an AUC of 43,824 ng•h/mL and 132,789 ng•h/mL following the initial and multiple infusions, respectively, with a clearance of 0.190 mL/h/kg and 0.067 mL/h/kg following the initial and multiple infusions. The elimination T½ is 123 hours and 318 hours following the initial and multiple infusions, respectively.30
Following a 10 mg/kg infusion weekly, the peak concentration (Cmax) is 406 mcg/mL following the initial infusion and 616 mcg/mL following multiple infusions. The AUC is 40,333 ng•h/mL and 156,840 ng•h/mL following the initial and multiple infusions, respectively, with a clearance of 0.264 mL/h/kg and 0.069 mL/h/kg following the initial and multiple infusions. The elimination T½ is 110 hours and 205 hours following the initial and multiple infusions, respectively.30
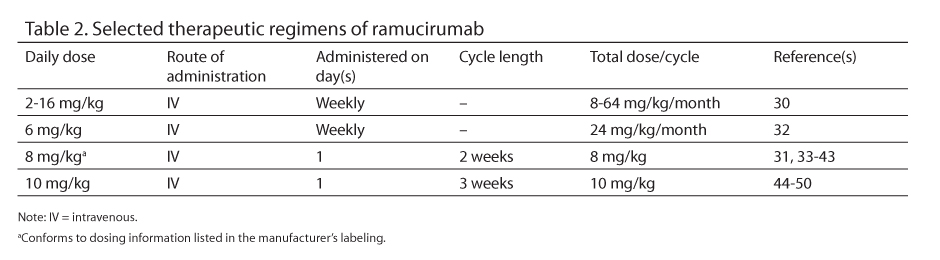
Selected therapeutic regimens of ramucirumab appear in Table 2.
PREPARATION
A. Use ramucirumab injection 10 mg/mL or 50 mg/mL.
B. Dilute the solution in 250 mL 0.9% sodium chloride injection (NS).
STABILITY
Solutions for infusion are stable for 24 hours under refrigeration [2°C to 8°C (36°F to 46°F)] or 4 hours at room temperature [below 25°C (77°F)].
ADMINISTRATION
- Ramucirumab is given as a 1 hour intravenous (IV) infusion.
- The manufacturer recommends that the drug be infused through a protein-sparing 0.22 micron filter.31
TOXICITIES
A. 8 mg/kg
- Cardiovascular: Arterial thromboembolism 2%,33 (grade 3 or 4) 1%33; cardiac failure <1%33; cardiac ischemia/myocardial infarction (grade 4) 3%35; hypertension 16% to 46%,33,34,40 (grade 3) 12%,34 (grade 3 or 4) 7% to 15%,33,40,42,43 (grade 4) 2%34; venous thromboembolism 4%,33 (grade 3 or 4) 1%.33
- Central nervous system: Headache 38%,34 (grade 1 or 2) 23%,35 (grade 3) 2% to 10%.34,39
- Constitutional: Fatigue 36% to 62%,33,34 (grade 1, 2 or 3) 18%,35 (grade 3) 8% to 10%,34,39 (grade 3 or 4) 4% to 7%33,42,43; pain 29%,33 (grade 3 or 4) 5% to 6%.33,42,43
- Endocrine/metabolic: Hyponatremia (grade 3 or 4) 3%.42
- Gastrointestinal: Anorexia 24%,33 (grade 3 or 4) 3%33,42; constipation 15%,33 (grade 3 or 4) less than 1%33; diarrhea 31%,40 (grade 3 or 4) 2%40; dysphagia 11%,33 (grade 3 or 4) 2%33; fistula formation (grade or 4) <1%,33 (grade 4) 2%39; hemorrhage (grade 3) 7%34; nausea 19%,40 (grade 1) 13%34; perforation (grade 3 or 4) <1%,33 (grade 4) 2%39; vomiting 20%,33 (grade 3 or 4) 3%.33
- Hematologic: Anemia 15%,33 (grade 3) 17%,41 (grade 3 or 4) 6% to 9%33,42,43; hemoptysis (grade 2) 3%35; hemorrhage 13%,33 (grade 3 or 4) 3%33; leucopenia (grade 3 or 4) 17%43; neutropenia (grade 3) 33%,41 (grade 3 or 4) 41%,43 (grade 4) 50%41; thrombocytopenia (grade 3) 17%.41
- Hepatic: Ascites 4%.42
- Hypersensitivity: Infusion reactions <1% to 19%.33,40
- Neurologic: Asthenia (grade 3 or 4) 6%.43
- Pulmonary: Dyspnea 9%,33 (grade 3 or 4) 2%.33
- Renal: Proteinuria 3%,33 (grade 2) 3%,35 (grade 3) 33%,41 (grade 3 or 4) <1%.33
- Treatment-related mortality: Cardiopulmonary arrest/myocardial infarction 3% to 4%,35,40 gastrointestinal hemorrhage 2%,34 intestinal perforation 2%.39
B.10 mg/kg
- Cardiovascular: Hypertension 20%,47 (grade 1 or 2) 13%,50 (grade 3 or 4) 10%.47
- Central nervous system: Headache 20% to 38%.47,50
- Constitutional: Fatigue 24% to 50%,47,50 (grade 3 or 4) 2%.47
- Gastrointestinal: Nausea (grade 1 or 2) 50%,50 odynophagia (grade 3) 13%.50
- Hematologic: Anemia 2%47; epistaxis (grade 1 or 2) 13%50; thrombocytopenia 8%,47 (grade 3 or 4) 2%47; febrile neutropenia (grade 3) 13%,50 (grade 4) 9%46; neutropenia (grade 3) 50%.50
- Hypersensitivity: Infusion reactions 14%,47 (grade 3 or 4) 6%.47
- Neurologic: Sensory neuroparthy (grade 1 or 2) 13%.50
- Pulmonary: Pneumothorax (grade 2) 9%,46 (dose limiting) 5%.46
- Renal: Proteinuria 8%,47 (grade 3 or 4) 2%.47
DOSAGE MODIFICATIONS
A. Hepatic: Moderate to severe hepatic impairment (Child-Pugh B or C), use with caution. No specific guidelines are available.31
B. Renal: No information is available.31
REFERENCES
- Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA. 2010;107(29): 13075-13080.
- Chang BY, Huang MM, Francesco M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 ameliorates autoimmune arthritis by inhibition of multiple effector cells. Arthritis Res Ther. 2011;13(4):R115.
- Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011:117(23):6287-6296.
- Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119(5):1182-1189.
- Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31(1):88-94.
- Cheng S, Ma J, Guo A, et al. BTK inhibition targets in vivo CLL proliferation through its effects on B-cell receptor signaling activity. Leukemia. 2014;28(3):649-657.
- Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122(15):2539-2549.
- Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32-42.
- Sukbuntherng J, Jejurkar P, Chan S, et al. Pharmacokinetics (PK) of ibrutinib in patients with chronic lymphocytic leukemia (CLL). Proc Am Soc Clin Oncol. 2013. Abstract 7056. http://meetinglibrary.asco.org/content/112607-132. Accessed May 14, 2014.
- Imbruvica [prescribing information]. Sunnyvale, CA: Pharmacyclics, Inc.; 2014. https://www.imbruvica.com/downloads/Prescribing_Information.pdf. Accessed May 20, 2014.
- Blum KA, Christian B, Flynn JM, et al.A phase I trial of the Bruton’s tyrosine kinase (BTK) inhibitor,ibrutinib
(PCI-32765), in combination with rituximab (R) and bendamustine in patients with relapsed/refractory non-Hodgkin’s lymphoma (NHL). Proc Am Soc Hematol. 2012.Abstract 1643. http://abstracts.hematologylibrary.org/cgi/content/abstract/120/21/1643?maxtoshow=&hits=10&RESULTFORMAT=1&author1=Blum&author2=Christian&title=Ibrutinib&andorexacttitle=and&andorexacttitleabs=and&andorexactfulltext=and&searchid=1&FIRSTINDEX=0&sortspec=relevance&tdate=11/30/2012&resourcetype=HWCIT. Accessed May 14, 2014. - Younes A, Flinn I, Berdeja J, et al. Combining ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP): Updated results from a phase 1b study in treatment-naïve patients with CD20-positive B-cell non-Hodgkin’s lymphoma (NHL). Proc Am Soc Hematol. 2013. Abstract 852. http://bloodjournal.hematologylibrary.org/content/122/21/852.abstract?sid=3fda93c9-ab00-45e4-8ea7-433fded24f1d. Accessed May 15, 2014.
- Farooqui M, Lozier JN, Valdez J, et al. Ibrutinib (PCI 32765) rapidly improves platelet counts in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) patients and has minimal effects on platelet aggregation. Proc Am Soc Hematol. 2012.Abstract 1789. http://abstracts.hematologylibrary.org/cgi/content/abstract/120/21/1789?maxtoshow=&hits=10&RESULTFORMAT=1&author1=Farooqui&author2=Lozier&title=Ibrutinib&andorexacttitle=and&andorexacttitleabs=and&andorexactfulltext=and&searchid=1&FIRSTINDEX=0&sortspec=relevance&tdate=11/30/2012&resourcetype=HWCIT. Accessed May 14, 2014.
- Burger JA, Ghia P, Polliack A, et al. Randomized, multicenter, open-label, phase III study of the BTK inhibitor ibrutinib versus chlorambucil in patients 65 years or older with treatment-naive CLL/SLL (RESONATE-2, PCYC-1115-CA). Proc Am Soc Clin Oncol. 2013. Abstract TPS7130. http://meetinglibrary.asco.org/content/114419-132. Accessed May 14, 2014.
- Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia [published online ahead of print 2014]. New Engl J Med. http://www.nejm.org/doi/pdf/10.1056/NEJMoa1400376. Accessed June 5, 2014.
- Treon SP, Tripsas CK, Yang G, et al. A prospective multicenter study of the Bruton’s tyrosine kinase inhibitor ibrutinib in patients with relapsed or refractory Waldenstrom’s macroglobulinemia.Proc Am Soc Hematol. 2013. Abstract 251. http://bloodjournal.hematologylibrary.org/content/122/21/251.abstract?sid=3fda93c9-ab00-45e4-8ea7-433fded24f1d. Accessed May 15, 2014.
- Brown JR, Barrientos JC, Barr PM, et al. Ibrutinib in combination with bendamustine and rituximab is active and tolerable in patients with relapsed/refractory CLL/SLL: Final results of a phase 1b study.Proc Am Soc Hematol. 2013. Abstract 525. http://bloodjournal.hematologylibrary.org/content/122/21/525.abstract?sid=3fda93c9-ab00-45e4-8ea7-433fded24f1d. Accessed May 15, 2014.
- Burger JA, Keating MJ, Wierda WG, et al. Ibrutinib in combination with rituximab (iR) is well tolerated and induces a high rate of durable remissions in patients with high-risk chronic lymphocytic leukemia (CLL): New, updated results of a phase II trial in 40 patients. Proc Am Soc Hematol. 2013. Abstract 675. http://bloodjournal.hematologylibrary.org/content/122/21/676.abstract?sid=3fda93c9-ab00-45e4-8ea7-433fded24f1d. Accessed May 15, 2014.
- O’Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: An open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15(1):48-58.
- Loury D, Sukbuntherng J, Clow F, et al. Open label evaluation of ECG in patients with chronic lymphocytic leukemia (CLL) receiving ibrutinib monotherapy. Proc Am Soc Clin Oncol. 2013. Abstract 7057. http://meetinglibrary.asco.org/content/112638-132. Accessed May 14, 2014.
- Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507-516.
- Wang L, Martin P, Blum KA, et al. The Bruton’s tyrosine kinase inhibitor PCI-32765 is highly active as single-agent therapy in previously-treated mantle cell lymphoma (MCL): Preliminary results of a phase II trial. Proc Am Soc Hematol. 2011. Abstract 442. http://abstracts.hematologylibrary.org/cgi/content/abstract/118/21/442?maxtoshow=&hits=10&RESULTFORMAT=1&author1=wang&author2=martin&title=PCI-32765&andorexacttitle=and&andorexacttitleabs=and&andorexactfulltext=and&searchid=1&FIRSTINDEX=0&sortspec=relevance&tdate=11/30/2012&resourcetype=HWCIT. Accessed May 16, 2014.
- Wilson WH, Gerecitano JF, Goy A, et al. The Bruton’s tyrosine kinase (BTK) inhibitor, ibrutinib (PCI-32765), has preferential activity in the ABC subtype of relapsed/-refractory de novo diffuse large B-cell lymphoma (DLBCL): Interim results of a multicenter, open-label, phase 2 study. Proc Am Soc Hematol. 2012. Abstract 686. http://abstracts.hematologylibrary.org/cgi/content/abstract/120/21/686?maxtoshow=&hits=10&RESULTFORMAT=1&author1=Wilson&author2=Gerecitano&title=Ibrutinib&andorexacttitle=and&andorexacttitleabs=and&andorexactfulltext=and&searchid=1&FIRSTINDEX=0&sortspec=relevance&tdate=11/30/2012&resourcetype=HWCIT. Accessed May 14, 2014.
- Wang M, Gordon LI, Rule S, et al. A phase III study of ibrutinib in combination with bendamustine and rituximab (BR) in elderly patients with newly diagnosed mantle cell lymphoma (MCL). Proc Am Soc Clin Oncol. 2013. Abstract TPS8613. http://meetinglibrary.asco.org/content/113203-132. Accessed May 14, 2014.
- Salles GA, Gopal AK, Martin P, et al. An open-label phase II study of ibrutinib in patients with refractory follicular lymphoma. Proc Am Soc Clin Oncol. 2013. Abstract TPS8614. http://meetinglibrary.asco.org/content/113208-132. Accessed May 14, 2014.
- Skobe M, Rockwell P, Goldstein N, et al. Halting angiogenesis suppresses carcinoma cell invasion. Nat Med. 1997;3(1):1222-1227.
- Prewett M, Huber J, Li Y, et al. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59(20):5209-5218.
- Bruns CJ, Liu W, Davis DW, et al. Vascular endothelial growth factor is an in vivo survival factor for tumor -endothelium in a murine model of colorectal carcinoma liver metastases. Cancer. 2000;89(3):488-499.
- Sweeney P, Karashima T, Kim SJ, et al. Anti-vascular endothelial growth factor receptor 2 antibody reduces tumorigenicity and metastasis in orthotopic prostate cancer xenografts via induction of endothelial cell apoptosis and reduction of endothelial cell matrix metalloproteinase type 9 production. Clin Cancer Res. 2002;8(8):2714-2724.
- Spratlin JL, Cohen RB, Eadens M, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28(5):780-787.
- Cyramza [prescribing information]. Indianapolis, IN: Eli Lilly & Co., 2014. http://pi.lilly.com/us/cyramza-pi.pdf. Accessed May 20, 2014.
- Hussain M, Rathkopf DE, Liu G, et al. A phase II randomized study of cixutumumab (IMC-A12: CIX) or ramucirumab (IMC‑1121B: RAM) plus mitoxantrone (M) and prednisone (P) in patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC) following disease progression (PD) on docetaxel (DCT) therapy. Proc Am Soc Clin Oncol Genitourinary Cancers Symposium. 2012. Abstract 97.
http://meetinglibrary.asco.org/content/89033-116. Accessed May 15, 2014. - Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31-39.
- Zhu AX, Finn RS, Mulcahy MF, et al. A phase II study of ramucirumab as first-line monotherapy in patients (pts) with advanced hepatocellular carcinoma (HCC). Proc Am Soc Clin Oncol. 2010. Abstract 4083. http://meetinglibrary.asco.org/content/50568-74. Accessed May 15, 2014.
- Garcia JA, Hudes GR, Choueiri TK, et a. Phase II study of IMC-1121B in patients with metastatic renal cancer (mRCC) following VEGFR-2 tyrosine kinase inhibitor (TKI) therapy (IMCL CP12-0605/NCT00515697). Proc Am Soc Clin Oncol Genitourinary Cancers Symposium. 2010. Abstract 326. http://meetinglibrary.asco.org/content/30312-73. Accessed May 19, 2014.
- Wilke H, Cunningham D, Ohtsu A, et al. A randomized, multicenter, double-blind, placebo (PBO)-controlled phase III study of paclitaxel (PTX) with or without ramucirumab (IMC-1121B; RAM) in patients (pts) with metastatic gastric adenocarcinoma, refractory to or progressive after first-line therapy with platinum (PLT) and fluoropyrimidine (FP). Proc Am Soc Clin Oncol. 2012. Abstract TPS4139. http://meetinglibrary.asco.org/content/93982-114. Accessed May 15, 2014.
- Grothey A, Tabernero J, Rougier P, et al. A randomized, double-blind, phase (Ph) III study of the irinotecan-based chemotherapy FOLFIRI plus ramucirumab (RAM) or placebo (PL) in patients (pts) with metastatic colorectal carcinoma (mCRC) progressive during or following first-line therapy with bevacizumab (BEV), oxaliplatin (OXALI), and a fluoropyrimidine (FP) (RAISE) (NCT01183780). Proc Am Soc Clin Oncol. 2012. Abstract TPS3634. http://meetinglibrary.asco.org/content/92144-114. Accessed May 15, 2014.
- Zhu AX, Chau I, Blanc JF, et al. A multicenter, randomized, double-blind, phase III study of ramucirumab (IMC-1121B; RAM) and best supportive care (BSC) versus placebo (PBO) and BSC as second-line treatment in patients (pts) with hepatocellular carcinoma (HCC) following first-line therapy with sorafenib (SOR). Proc Am Soc Clin Oncol. 2012. Abstract TPS4146. http://meetinglibrary.asco.org/content/92970-114. Accessed May 15, 2014.
- Penson RT, Moore KN, Fleming GF, et al. A phase II, -open-label, multicenter study of IMC-1121B (ramucirumab; RAM) monotherapy in the treatment of persistent or recurrent epithelial ovarian (EOC), fallopian tube (FTC), or primary peritoneal (PPC) carcinoma (CP12-0711/NCT00721162). Proc Am Soc Clin Oncol. 2012. Abstract 5012. http://-meetinglibrary.asco.org/content/92977-114. Accessed May 15, 2014.
- Garcia-Carbonero R, Rivera F, Maurel J, et al. A phase II, open-label study evaluating the safety and efficacy of ramucirumab combined with mFOLFOX-6 as first-line therapy in patients (pts) with metastatic colorectal cancer (mCRC): CP12-0709/NCT00862784. Proc Am Soc Clin Oncol -Gastrointestinal Cancers Symposium. 2012. Abstract 533. http://meetinglibrary.asco.org/content/87794-115. Accessed May 15, 2014.
- Yoshino T, Yamazaki K, Yoshida M, et al. A phase (Ph) Ib study of irinotecan (IRI), levofolinate (LV), and 5--fluorouracil (5-FU) (FOLFIRI) plus ramucirumab (RAM; IMC-1121B) drug product in Japanese (JP) patients (pts) with metastatic colorectal carcinoma (mCRC) progressive during or following first-line therapy with bevacizumab (BEV), oxaliplatin (OXALI), and a fluoropyrimidine (FP) (CP12-1029/NCT01286818). Proc Am Soc Clin Oncol Gastrointestinal Cancers Symposium. 2013.Abstract 591. http://meetinglibrary.asco.org/content/106149-133. Accessed May 15, 2014.
- Fuchs CS, Tomasek J, Cho JY, et al. REGARD: A phase III, randomized, double-blinded trial of ramucirumab and best supportive care (BSC) versus placebo and BSC in the treatment of metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma following disease progression on first-line platinum- and/or fluoropyrimidine-containing combination therapy. Proc Am Soc Clin Oncol Gastrointestinal Cancers Symposium. 2013. Abstract LBA5. http://meetinglibrary.asco.org/content/106221-133. Accessed May 15, 2014.
- Wilke H, Van Cutsem E, Oh SC, et al. RAINBOW: A global, phase III, randomized, double-blind study of -ramucirumab plus paclitaxel versus placebo plus paclitaxel in the treatment of metastatic gastroesophageal junction (GEJ) and gastric adenocarcinoma following disease progression on first-line platinum and fluoropyrimidine-containing combination therapy rainbow IMCL CP12-0922 (I4T-IE-JVBE). Proc Am Soc Clin Oncol Gastrointestinal Cancers Symposium. 2014. Abstract LBA7. http://meetinglibrary.asco.org/-content/123363-143. Accessed May 15, 2014.
- Mackey J, Gelmon K, Martin M, et al. TRIO-012: A multicenter, multinational, randomized, double-blind phase III study of IMC-1121B plus docetaxel versus placebo plus docetaxel in previously untreated patients with HER2-negative, unresectable, locally recurrent or metastatic breast cancer. Clin Breast Cancer. 2009;9(4):258-261.
- Garon EB, Cao D, Alexandris E, et al. A randomized, double-blind, phase III study of docetaxel and ramucirumab versus docetaxel and placebo in the treatment of stage IV non-small-cell lung cancer after disease progression after 1 previous platinum-based therapy (REVEL): Treatment -rationale and study design. Clin Lung Cancer. 2012;13(6):505-509.
- Camidge DR, Ballas MS, Dubey S, et al. A phase II, open-label study of ramucirumab (IMC-1121B), an IgG1 fully human monoclonal antibody (MAb) targeting VEGFR-2, in combination with paclitaxel and carboplatin as first-line -therapy in patients (pts) with stage IIIb/IV non-small cell lung cancer (NSCLC). Proc Am Soc Clin Oncol. 2010. Abstract 7588. http://meetinglibrary.asco.org/content/50989-74. Accessed May 15, 2014.
- Carvajal RD, Wong MK, Thompson JA, et al. A phase II randomized study of ramucirumab (IMC-1121B) with or without dacarbazine (DTIC) in patients (pts) with metastatic melanoma (MM). Proc Am Soc Clin Oncol. 2010. Abstract 8519. http://meetinglibrary.asco.org/content/50441-74. Accessed May 15, 2014.
- Vahdat LT, Miller K, Sparano JA, et al. Randomized phase II study of capecitabine with or without ramucirumab (IMC-1121B) or IMC-18F1 in patients with unresectable, locally advanced or metastatic breast cancer (mBC) previously treated with anthracycline and taxane therapy (CP20-0903/NCT01234402). Proc Am Soc Clin Oncol. 2011. Abstract TPS151. http://meetinglibrary.asco.org/content/82467-102. Accessed May 15, 2014.
- Petrylak DP, Chi KN, Vogelzang NJ, et al. -Randomized phase II study of docetaxel with or without ramucirumab (IMC-1121B) or icrucumab (IMC-18F1) in patients with urothelial transitional cell carcinoma (TCC) following progression on first-line platinum-based therapy. Proc Am Soc Clin Oncol. 2012. Abstract TPS4675.
http://meetinglibrary.asco.org/content/95198-114. Accessed May 15, 2014. - Yardley DA, Osborne CRC, Richards PD, et al. Interim safety results of eribulin (E) combined with ramucirumab (RAM) in patients (pts) with advanced metastatic breast cancer (MBC). Proc Am Soc Clin Oncol Breast Cancer Symposium. 2012. Abstract 110. http://meetinglibrary.asco.org/content/102543-125. Accessed May 15, 2014.
