Formulary Drug Reviews
Ledipasvir/Sofosbuvir
Dennis J. Cada, PharmD, FASHP, FASCP (Editor)*; Danial E. Baker, PharmD, FASHP, FASCP†; and Ross Jason Bindler, PharmD‡
Formulary Drug Reviews
Ledipasvir/Sofosbuvir
Dennis J. Cada, PharmD, FASHP, FASCP (Editor)*; Danial E. Baker, PharmD, FASHP, FASCP†; and Ross Jason Bindler, PharmD‡
Formulary Drug Reviews
Ledipasvir/Sofosbuvir
Dennis J. Cada, PharmD, FASHP, FASCP (Editor)*; Danial E. Baker, PharmD, FASHP, FASCP†; and Ross Jason Bindler, PharmD‡
Each month, subscribers to The Formulary Monograph Service receive 5 to 6 well-documented monographs on drugs that are newly released or are in late phase 3 trials. The monographs are targeted to Pharmacy & Therapeutics Committees. Subscribers also receive monthly 1-page summary monographs on agents that are useful for agendas and pharmacy/nursing in-services. A comprehensive target drug utilization evaluation/medication use evaluation (DUE/MUE) is also provided each month. With a subscription, the monographs are sent in print and are also available on-line. Monographs can be customized to meet the needs of a facility. A drug class review is now published monthly with The Formulary Monograph Service. Through the cooperation of The Formulary, Hospital Pharmacy publishes selected reviews in this column. For more information about The Formulary Monograph Service, call The Formulary at 800-322-4349. The March 2015 monograph topics are paritaprevir, ritonavir, ombitasvir, and dasabuvir; meningococcal B bivalent recombinant vaccine; alemtuzumab; ceftolozane/tazobactam; and peramivir solution. The Safety MUE is on peramivir solution.
Generic Name: Ledipasvir/Sofosbuvir
Proprietary Name: Harvoni (Gilead Sciences Inc)
Approval Rating: 1P
Therapeutic Class: Nonstructural protein 5B polymerase inhibitors; oral nonstructural protein 5A inhibitors
Similar Drugs: Boceprevir, ribavirin, simeprevir, sofosbuvir
Sound- or Look-Alike Names: None
Each month, subscribers to The Formulary Monograph Service receive 5 to 6 well-documented monographs on drugs that are newly released or are in late phase 3 trials. The monographs are targeted to Pharmacy & Therapeutics Committees. Subscribers also receive monthly 1-page summary monographs on agents that are useful for agendas and pharmacy/nursing in-services. A comprehensive target drug utilization evaluation/medication use evaluation (DUE/MUE) is also provided each month. With a subscription, the monographs are sent in print and are also available on-line. Monographs can be customized to meet the needs of a facility. A drug class review is now published monthly with The Formulary Monograph Service. Through the cooperation of The Formulary, Hospital Pharmacy publishes selected reviews in this column. For more information about The Formulary Monograph Service, call The Formulary at 800-322-4349. The March 2015 monograph topics are paritaprevir, ritonavir, ombitasvir, and dasabuvir; meningococcal B bivalent recombinant vaccine; alemtuzumab; ceftolozane/tazobactam; and peramivir solution. The Safety MUE is on peramivir solution.
Generic Name: Ledipasvir/Sofosbuvir
Proprietary Name: Harvoni (Gilead Sciences Inc)
Approval Rating: 1P
Therapeutic Class: Nonstructural protein 5B polymerase inhibitors; oral nonstructural protein 5A inhibitors
Similar Drugs: Boceprevir, ribavirin, simeprevir, sofosbuvir
Sound- or Look-Alike Names: None
Each month, subscribers to The Formulary Monograph Service receive 5 to 6 well-documented monographs on drugs that are newly released or are in late phase 3 trials. The monographs are targeted to Pharmacy & Therapeutics Committees. Subscribers also receive monthly 1-page summary monographs on agents that are useful for agendas and pharmacy/nursing in-services. A comprehensive target drug utilization evaluation/medication use evaluation (DUE/MUE) is also provided each month. With a subscription, the monographs are sent in print and are also available on-line. Monographs can be customized to meet the needs of a facility. A drug class review is now published monthly with The Formulary Monograph Service. Through the cooperation of The Formulary, Hospital Pharmacy publishes selected reviews in this column. For more information about The Formulary Monograph Service, call The Formulary at 800-322-4349. The March 2015 monograph topics are paritaprevir, ritonavir, ombitasvir, and dasabuvir; meningococcal B bivalent recombinant vaccine; alemtuzumab; ceftolozane/tazobactam; and peramivir solution. The Safety MUE is on peramivir solution.
Generic Name: Ledipasvir/Sofosbuvir
Proprietary Name: Harvoni (Gilead Sciences Inc)
Approval Rating: 1P
Therapeutic Class: Nonstructural protein 5B polymerase inhibitors; oral nonstructural protein 5A inhibitors
Similar Drugs: Boceprevir, ribavirin, simeprevir, sofosbuvir
Sound- or Look-Alike Names: None
Hosp Pharm 2015;50(3):224-234
2015 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj5003-224


INDICATIONS
Ledipasvir/sofosbuvir is approved for the treatment of chronic hepatitis C virus (HCV) genotype 1 infections in adults.1,2 Ledipasvir/sofosbuvir has been evaluated with and without ribavirin for 8, 12, or 24 weeks in treatment-naive and treatment-experienced patients with chronic HCV genotype 1 infections.3-6 It is also being evaluated for the treatment of HCV genotypes 2, 3, 4, 5, and 6.7 Ledipasvir has been studied in combination with the nonstructur-al protei-n (NS) 3 protease inhibitor vedroprevir (GS-9451), the imidazopyridine inhibitor tegobuvir (GS-9190), and ribavirin in adults infected with HCV genotype 1 who were treatment naive.8 It is also being studied in combination with vedroprevir, ribavirin, and peginterferon for 6 to 24 weeks in both treatment-naive and treatment-experienced adults infected with HCV genotype 1.9,10
In 2013, approximately 3.2 million individuals in the United States were living with a chronic HCV infection, but only 1.8 million have been diagnosed. Of these patients, 1 to 1.2 million (32% to 38%) were referred for care and 220,000 to 360,000 (7% to 11%) were treated.11 HCV genotype 1a and genotype 1b account for up to 73% of all HCV infections and are considered the more difficult forms of the hepatitis virus to treat.12 Historically, most of the drug regimens for the treatment of HCV genotype 1 have included an interferon product and ribavirin with or without another antiviral agent.
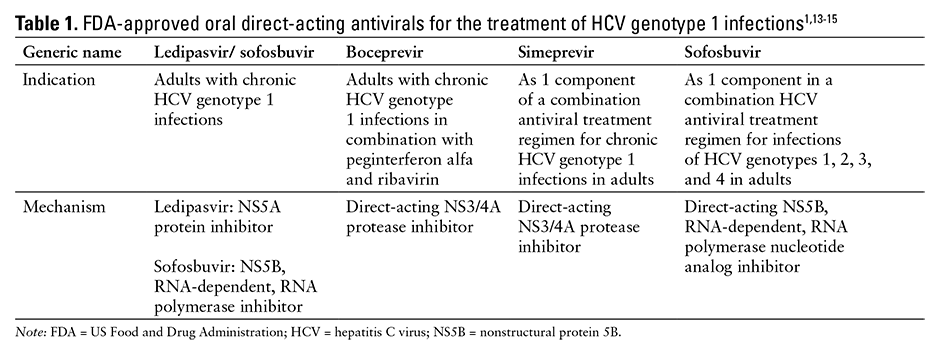
See Table 1 for a comparison of the approved oral direct-acting antivirals for the treatment of HCV genotype 1 infections.1,13-15
CLINICAL PHARMACOLOGY
Ledipasvir is a unsymmetric benzimidazole-difluorofluorene-imidazole with an azabicyclic ring system showing highly potent inhibitory activity against the NS5A of HCV genotype 1.1,16 In a dose-ranging study, ledipasvir administration resulted in rapid reduction of up to a 3.3 log10 units/mL HCV RNA from baseline.17 Sofosbuvir is a nucleotide analog inhibitor of HCV NS5B polymera-se.1
In vitro resistance modeling shows decreased susceptibility to ledipasvir against HCV with Q30E and Y93H mutations in the genotype 1a strain and Y93H in genotype 1b strain.18 Those mutations along with M28T, Q30R, L31M, and Y93C mutations were found to have reduced susceptibility to ledipasvir, although all mutants tested remained fully susceptible to other classes of direct-acting antiretrovirals, such as the protease inhibitors used for HCV, interferon alfa, and ribavirin.18 When ledipasvir was examined in HCV genotype 3, there were indications that multiple strains had reduced susceptibility to the medication’s antiviral effects.19
Sofosbuvir is a prodrug that has an active metabolite with a direct-acting antiviral agent that inhibits HCV NS5B RNA-dependent polymerase, a vital component in viral replication. The active metabolite of sofosbuvir, uridine analog triphosphate (GS-461203), has activity against HCV genotyp-es 1, 2, 3, 4, and 6, acting as a chain terminator.1,20-23 GS-461203 may also show efficacy against genotype 5a.1 GS-461203 does not inhibit human DNA polymerase or human or mitochondrial RNA polymerase.1
PHARMACOKINETICS
Ledipasvir pharmacokinetics are best characterized by a 2-compartment model, with first-order absorption and elimination.24 Maximal concentra-tions were achieved 4 to 6 hours after oral administrati-on and displayed time-independent, nearly linear pharmacokinetics.1,17 Ledipasvir has an extended plasma half-life between 37 and 45 hours in healthy individuals and 22 and 50 hours in those with HCV genotype 1 infections.16,17,24 Half the maximal effective concentration for ledipasvir against HCV genotype 1a, traditionally the more difficult strain to clear, is 21 nM, which was readily obtained by oral doses of 3 to 100 mg/day.16 The volume of distribution for the central compartment is 299 L and the peripheral compartment is 620 L.24 Changes related to age, body weight, sex, race, cirrhosis, ribavirin use, disease status, and concomitant medications appeared not to have any impact on ledipasvir exposure.24 Ledipasvir is minimally metabolized, less than 2%, and mainly excreted through the feces, with renal excretion playing a limited role.25
The peak plasma concentration (Cmax) of sofosbuvir occurred at 0.8 to 1 hours after oral administration,1 whereas the Cmax of sofosbuvir’s inactive GS-331007 metabolite occurred within 4 hours.1 Sofosbuvir undergoes approximately 61% to 65% protein binding that is independent of concentration at tested concentrations between 1 to 20 mcg/mL. In contrast, GS-331007 exhibits minimal protein binding.1
Sofosbuvir is extensively metabolized in the liver through a variety of mechanisms. This metabolic process requires human cathepsin A or carboxylesterase 1 to catalyze the hydrolysis of the carboxyl ester moiety. Additionally, the histidine triad nucleotide-binding protein 1 facilitates phosphoramidate cleavage, and the pyrimidine nucleotide biosynthesis pathway facilitates phosphorylati-on. This process yields an active nucleoside analog triphosphate of sofosbuvir, GS-461203. The dephosphorylation of GS-461203 leads to production of a metabolite that has no anti-HCV activity in vitro, GS-331007. Sofosbuvir accounts for 4% of drug-related material systemic exposure, whereas GS-331007 accounts for 90%.1
Sofosbuvir is mainly cleared through the renal pathway, with 80% of a 400 mg dose recovered in the urine. Most of the drug recovered in the urine is GS-331007 (78%) and sofosbuvir (3.5%). Less prominent pathways of sofosbuvir elimination are through the feces (14%) and expired air (2.5%). The median terminal half-life of sofosbuvir is 0.5 hours. The GS-331007 metabolite has a median terminal half-life of 27 hours.1
Compared with a normal estimated glomerular filtration rate (GFR) (greater than 80 mL/min/1.73 m2), the area under the curve (AUC) of sofosbuvir and GS-331007 increased with increasing renal impairment (from mild and moderate to severe renal failure). A hemodialysis period of 4 hours removed approximately 18% of a given dose. No dosing adjustment is required for patients with mild and moderate renal impairment, and no dose recommendation can be made for patients who have severe renal impairment or require dialysis.1
In comparison with normal hepatic function, sofosbuvir and GS-331007 both had an increased AUC. Cirrhosis did not have any clinically relevant effects. Dosing adjustments are not required for patients with mild, moderate, or severe hepatic impairment. Ethnic background, gender, and age did not appear to have clinically relevant effects on sofosbuvir pharmacokinetics, and changes associated with the pharmacokinetics of sofosbuvir in pediatric patients have not been determined.1
Food does not appear to influence the pharmacokinetics of ledipasvir. Ledipasvir was tested in the absence of food, with a moderate-fat meal, and with a high-fat, high-calorie meal; there were no observed differences.26 Sofosbuvir can be taken without regard to food because administration after a high-fat meal did not alter its AUC, Cmax, or the exposure of GS-331007 compared with fasting states.1 Ledipasvir/sofosbuvir can be administered without regard to food.1
COMPARATIVE EFFICACY
Indication: Chronic Infection with Hepatitis C Virus Genotype 1a and 1b in Combination with Sofosbuvir with or without Ribavirin
Guidelines
Guideline: Infectious Disease Society of America (IDSA) and American Association for the Study of Liver Diseases (AASLD) up-to-date guidance for the treatment of HCV infection
Reference: IDSA and AASLD, 201427
Comments: Traditional treatment for HCV genotype 1 was interferon with a weight-based dosage of ribavirin for 12 months. Newer regimens have focused on the use of sofosbuvir with a weight-based dosage of ribavirin plus weekly peginterferon alfa subcutaneous injections for 12 weeks regardless of subtype. In patients with HCV genotype 1b or infections caused by HCV genotype 1a with no Q80K polymorphism at baseline, the regimen has included simeprevir for 12 weeks plus a weight-based dosage of ribavirin and weekly subcutaneous peginterferon alfa injections for 24 weeks. Some patients, especially those who showed drug intolerance or were ineligible for interferon therapy, were treated with sofosbuvir and simeprevir with or without weight-based ribavirin.
Guideline: Chronic HCV infection: Treatment considerations from the Department of Veterans Affairs National Hepatitis C Resource Center Program and the Office of Public Health
Reference: Department of Veterans Affairs National Hepatitis C Resource Center Program, 201428
Comments: The recommended first-line regimen for all patients infected with HCV genotype 1 who are interferon eligible is sofosbuvir, weight-based ribavirin, and weekly subcutaneous injections of peginterferon alfa, all for 12 weeks. An alternative regimen for treatment-naive patients who are interferon eligible and infected with HCV genotype 1b or the HCV genotype 1a strain without the Q80K mutation, regardless of cirrhotic status, is simeprevir for 12 weeks and weight-based ribavirin plus weekly subcutaneous injections of peginterferon alfa for 24 weeks. The alternative regimen for treatment-experienced patients infected with HCV genotype 1b or the HCV genotype 1a strain without the Q80K mutation (considered relapsers) is simeprevir for 12 weeks and weight-based ribavi-ri-n plus weekly peginterferon for 24 weeks; for partial or null responders, simeprevir for 12 weeks and peginterferon alfa plus weight-based ribavirin for 48 weeks is an alternative regimen. Sofosbuvir and simeprevir with or without weight-based ribavirin for 12 weeks is considered an alternative regimen for null responders infected with HCV genotype 1b or genotype 1a strain without the Q80K mutation only. Treatment-naive interferon ineligible/intolerant patients with HCV genotype 1 infections without cirrhosis should receive sofosbuvir and weight-based ribavirin for 24 weeks; treatment-naive patients with cirrhosis, as well as treatment-experienced patients with or without cirrhosis, who are interferon ineligible/intolerant should receive sofosbuvir, simeprevir, and weight-based ribavirin for 12 weeks.
Studies
Drug: Ledipasvir and Sofosbuvir with or without Ribavirin vs Sofosbuvir, GS-9669, and Ribavirin
Reference: Gane EJ, et al, 2014 (ELECTRON trial)29
Study Design: Randomized, open-label, phase 2 study conducted at 2 centers in New Zealand
Study Funding: Gilead
Patients: One hundred thirteen patients with chronic HCV genotype 1 infections were enrolled in the trial; 75 were treatment naive and 38 were considered null responders to previous therapy. Half (19 patients) of the 38 patients classified as previous null responders were considered to have compensated cirrhosis. All patients were 18 years and older and had no evidence of concurrent hepatitis B virus (HBV) and/or HIV infections. HCV genotype 1a was the causative virus in 83% of the patients.
Intervention: Treatment-naive patients were randomized to receive ledipasvir 90 mg and sofosbuvir 400 mg/day with a twice-daily, weight-based ribavirin for either 6 or 12 weeks, or sofosbuvir 400 mg and GS-9669 500 mg/day plus weight-based ribavirin twice daily for 12 weeks. Previous null responders without cirrhosis received both ledipasvir 90 mg and sofosbuvir 400 mg daily with weight-based ribavirin twice daily or sofosbuvir 400 mg and GS-9669 500 mg/day with weight-based ribavirin twice daily for 12 weeks in both groups. Finally, null responders who were considered to have cirrhosis received ledipasvir 90 mg/sofosbuvir 400 mg with or without weight-based ribavirin twice daily for 12 weeks.
Results
Primary Endpoint(s)
- Sustained virologic response 12 weeks after the end of therapy (SVR12) in the intent-to-treat (ITT) population:
- One hundred percent of the 25 treatment-naive patients who received ledipasvir and sofosbuvir plus ribavirin for 12 weeks achieved SVR12, whereas only 68% of the 25 administered the same combination for 6 weeks achieved SVR12; 23 patients (92%) receiving sofosbuvir, GS-9669, and ribavirin for 12 weeks achieved SVR12.
- All previous null responders without cirrhosis achieved SVR12 regardless of whether they received ledipasvir and sofosbuvir plus ribavirin or sofosbuvir, GS-9669, and ribavirin for 12 weeks of therapy.
- Of the previous null responders with cirrhosis, all patients randomized to receive ledipasvir and sofosbuvir with ribavirin achieved SVR12, whereas only 7 of the 10 patients (70%) randomized to ledipasvir and sofosbuvir achieved a SVR12.
Secondary Endpoint(s)
- On-treatment virologic response in the ITT population:
- Viral suppression after the start of treatment was observed in all groups. By week 6 of the treatment period, all patients had HCV RNA levels lower than the limit of detection (15 units/mL), which were sustained until the end of treatment.
- Virologic failure during the treatment period or during follow-up:
- No participants had virologic failure while receiving study medication.
- A total of 13 patients had virologic failure during follow-up; 12 patients experienced virologic relapse, whereas 1 patient was reinfected with the HCV genotype 3a strain, not the original genotype 1 strain.
Comments: The majority of patients in this study achieved SVR12, but it was a small sample size overall. This arm of the ELECTRON trial was a continuation of the original ELECTRON trial used in the approval of sofosbuvir and was mainly executed to determine the effectiveness of 2 diffe-rent regimens for varying lengths of time in 3 different HCV genotype 1–infected populations. Study participants included treatment-naive patients, those considered nonresponders to previous treatments, and patients with cirrhosis. This study also utilized the lowest HCV RNA detection level of 15 units/mL; all other trials used 25 units/mL as the lower limit of detection.
Limitations: No sample size calculation was performed in the study design, and the results were not statistically evaluated. This study was conducted with a small study size at only 2 centers within New Zealand and included only patients who were infected with the genotype 1 strain of HCV.
Drug: Ledipasvir and Sofosbuvir with or without Ribavirin
Reference: Lawitz E, et al, 2014 (LONESTAR trial)6
Study Design: Randomized, open-label, phase 2 study at 1 site in San Antonio, Texas
Study Funding: Gilead
Patients: One hundred patients older than 18 years with chronic HCV genotype 1 infections were enrolled in the study. Cohort A (n = 60) consisted of treatment-naive individuals randomized to 1 of 3 treatment groups. Cohort B (n = 40) was made up of patients who had previously failed a protease inhibitor–containing regimen; these patients were randomized to 1 of 2 treatment arms. In cohort B, 55% of patients (n = 22) had compensated cirrhosis; however, patients with hepatic decompensation were excluded from study enrollment. Subjects with a body mass index of less than 18 kg/m2 and/or a coinfection with HBV, HIV, or both were excluded.
Intervention: In cohort A, subjects were randomized to receive ledipasvir 90 mg plus sofosbuvir 400 mg daily for 8 or 12 weeks, or ledipasvir 90 mg and sofosbuvir 400 mg daily plus twice daily weight-based ribavirin all for 8 weeks. The subjects in cohort B were randomized to ledipasvir 90 mg and sofosbuvir 400 mg daily with or without twice-daily, weight-based ribavirin all for 12 weeks.
Results
Primary Endpoint(s)
- Proportion of patients achieving a SVR12 in the ITT study population:
- Ninety-seven of the 100 patients achieved SVR12 in this study, with no subjects experiencing virological failure or breakthrough. One patient in cohort A receiving ledipasvir and sofosbuvir daily for 8 weeks had virologica-l relapse, and 1 subject in cohort B receiving ledipasvir and sofosbuvir daily for 12 weeks also experienced relapse. One patient in cohort A receiving daily ledipasvir and sofosbuvir for 12 weeks was lost to follow-up; this was counted as a failure to achieve SVR12.
Comments: All patients achieved a viral RNA below the lower limit of quantification by week 8 of treatment. No statistical differences in SVR12 were noted between groups receiving twice-daily weight-based ribavirin and those that did not. Patients included in this trial had all 3 variants of interleukin (IL) 28B; in previous studies, those with CT or TT changes to this gene had shown a reduced response to interferon-containing regimens compared with those with the standard CC gene. Both of the 2 documented patients who did not achieve SVR12 were infected with a HCV genotype 1a strain.
Limitations: Only 1 study site in the United States was utilized in this study, and it had an overall small population size; the investigators considered this study to be exploratory and did not power it to allow for comparisons between the groups. There was no in-depth discussion of what the previous failed treatment regimen consisted of, either boceprevir or telaprevir, or the length of previous therapy. This study only included patients who were infected with the genotype 1 strain of HCV. Finally, the lower limit of detection for this study was 25 units/mL, compared with 15 units/mL in the ELECTRON trial.
Reference: Afdhal N, et al, 2014 (ION-1 trial)4
Study Design: Randomized, open-label, multicenter, multinational phase 3 study
Study Funding: Gilead
Patients: The ION-1 study included 865 treatment-naive patients who were chronically infected with HCV, specifically the genotype 1 strain. To participate in the trial, subjects had to be older than 18 years and in good overall health besides the HCV infection. Patients were randomized to 1 of 4 groups and stratified by the presence or absence of compensated cirrhosis and by the specific viral subtype causing the infection, genotype 1a or genotype 1b; 15.7% of the study population had cirrhosis and 67.2% of patients were infected with HCV genotype 1a. The use of drugs and alcohol as well as any history of significant medical conditions were key exclusion criteria.
Intervention: Patients were randomized 1:1:1:1 to receive ledipasvir 90 mg and sofosbuvir 400 mg daily with or without weight-based ribavirin twice daily for 12 or 24 weeks.
Results
Primary Endpoint(s)
- SVR12 in the randomized study population receiving treatment:
- In the group receiving ledipasvir and sofosbuvir daily without ribavirin for 12 weeks, SVR12 was achieved in 99% of patients (95% confidence interval [CI], 96% to 100%); there was only 1 confirmed case of viral relapse within this group and 2 patients were lost to follow-up.
- In patients who received ledipasvir and sofosbuvir daily without ribavirin for 24 weeks, SVR12 was achieved in 98% of patients who completed treatment (95% CI, 95% to 99%); there was 1 confirmed case of virological failure during treatment and 1 case of viral relapse; 1 patient withdrew consent and 2 patients were lost to follow-up.
- SVR12 occurred in 97% of patients receiving ledipasvir and sofosbuvir once daily with weight-based ribavirin twice daily for 12 weeks (95% CI, 94% to 99%). There were no known cases of virologic failure during treatment or viral relapse in this group; 4 patients were lost to follow-up and 2 withdrew consent.
- Of patients who received 24 weeks of once-daily ledipasvir and sofosbuvir with twice-daily weight-based ribavirin, 99% achieved SVR12 (95% CI, 97% to 100%). There were no known virological failures or cases of viral relapse. Two patients were lost to follow-up.
Secondary Endpoint(s)
- All 4 treatment groups were superior to the historical rate of 60% for SVR (P < .001).
- The SVR12 in the 4 treatment groups ranged from 97% to 99% in patients with HCV genotype 1a infection, 97% to 99% in patients with a non-CC IL28B allele, 94% to 100% in patients with cirrhosis, and 91% to 100% among Black patients.
- Discontinuation rates secondary to adverse reactions were 2% in the group treated without ribavirin and 3% in the group treated with ribavirin.
Comments: This trial showed a rapid response to treatment; by week 2 of treatment, over 80% of patients in all groups had an HCV RNA level below the 25 units/mL limit of detection, and by week 4, nearly 100% of all groups had achieved viral RNA below the limit of detection. This response was continued throughout the remainder of the trial. Also, this trial contained patients with all 3 variants of the IL28B gene, CC, CT, and TT. In the 2 reported cases of virologic relapse, both patients had NS5A-resistant strains of HCV detected at time of relapse. The ION-1 trial also included patients who were diagnosed with compensated cirrhosis.
Limitations: The study population of ION-1 only included patients who had not been previously treated for their HCV genotype 1 infections; patients who had previously been treated for HCV infections were not included. There was no active control group in this study, but each study arm was compared with an adjusted historic response rate based on 3 telaprevir and boceprevir studies. Also, only a small number of the total population (136 of the 865 patients studied [15.7%]) had compensated cirrhosis. The lower limit of detection was set at 25 units/mL compared with the 15 units/mL used in the ELECTRON trial.
Reference: Afdhal N, et al, 2014 (ION-2 trial)3
Study Design: Randomized, open-label, phase 3 study at 64 sites within the United States only
Study Funding: Gilead
Patients: Patients in the ION-2 study included those with chronic HCV infection caused by either the genotype 1a or genotype 1b strains. This study only included individuals who had not previously obtained an SVR after treatment with peginterferon alfa and ribavirin with or without a protease inhibitor. Twenty percent of the study population had cirrhosis; all 3 genotypes of IL-28B variants were included, but the majority (88%) had the non-CC IL28B genotype and 52% had been previously treated with a protease inhibitor. Participants were 18 years and older and had previously failed HCV treatment; patients could not have discontinued prior treatment due to an adverse reaction related to therapy with a previous medication.
Intervention: Participants were randomized 1:1:1:1 to receive ledipasvir 90 mg plus sofosbuvir 400 mg daily with or without a twice-daily regimen of weight-based ribavirin for 12 or 24 weeks.
Results
Primary Endpoint(s)
- The primary endpoint was SVR12 in randomized patients who received treatment.
- In subjects taking only ledipasvir and sofosbuvir daily for 12 weeks, 93.6% (95% CI, 87% to 97%) achieved an SVR12. A total of 7 patients in this group had virologic relapse.
- Of patients taking ledipasvir and sofosbuvir daily for 24 weeks, the SVR12 was 99.1% (95% CI, 95% to 100%). The single patient in this group who did not achieve an SVR12 withdrew consent at posttreatment week 4; an SVR was maintained until that point.
- Of patients taking ledipasvir and sofosbuvir with ribavirin for 12 weeks, 96.4% achieved an SVR12 (95% CI, 91% to 99%). There were 4 reported cases of viral relapse.
- Of patients who received ledipasvir and sofosbuvir with ribavirin for 24 weeks, 99.1% achieved an SVR12 (95% CI, 95% to 100%). There were no reported cases of viral relapse in this arm of the study.
Secondary Endpoint(s)
- All treatment groups were superior (P < .001) to the adjusted historical response rate of 25%.
- SVR at 24 weeks after treatment was 100% in patients with SVR12.
Endpoint(s)
- Virologic breakthrough during the study treatment period in those who were randomized and received treatment. There was 1 reported case of virologic breakthrough during the study treatment period; when the patient’s medication levels were tested, the concentrations of ledipasvir and the active metabolite of sofosbuvir, GS-331007, were near or below the limit of quantification.
Comments: This study included only patients previously treated for HCV infections who were treatment failures. It also included patients who had compensated cirrhosis and patients with all 3 genotypes of IL28B. As seen in the other studies with ledipasvir, the antiviral response to all regimens occurred quickly, and there was only 1 case of virologic breakthrough during treatment. There was a difference noted between patients with compensated cirrhosis receiving 24 weeks versus 12 weeks of therapy; the number needed to treat was calculated to be 8. All 11 patients with reported viral relapse had measurable levels of NS5A-resistant HCV strains at the time of testing.
Limitations: Overall this trial had a relatively small study size, only about half the size of the ION-1 trial. The study designers did not enroll any treatment-naive patients. The lower limit of detection was 25 units/mL. The study excluded any patient who could not finish their previous treatment because of a medication-induced adverse event, which could have selected those patients less likely to have an adverse event in this trial. Only subjects infected with HCV genotype 1 were included in the study.
Reference: Kwodley KV, et al, 2014 (ION-3 trial)5
Study Design: Randomized, open-label, multicenter phase 3 study
Study Funding: Gilead
Patients: The ION-3 trial included 647 patients 18 years and older who were chronically infected by a genotype 1a or genotype 1b strain of HCV and had previously been untreated. Of the study population, 19% were Black and 6% were Hispanic. Patients with cirrhosis were excluded from the trial. All 3 genotypes of IL28B were included. HCV genotype 1a infection was present in 80% of patients and non-CC IL28B genotype was present in approximately 74%.
Intervention: Eligible patients were randomized 1:1:1. Group 1 received ledipasvir 90 mg and sofosbuvir 400 mg daily with a twice-daily weight-based regimen of ribavirin for 8 weeks. The other 2 groups were administered ledipasvir 90 mg and sofosbuvir 400 mg every day without ribavirin for 8 or 12 weeks.
Results
Primary Endpoint(s)
- The primary endpoint was SVR12 in randomized patients and received 1 dose of study medications.
- In patients receiving ledipasvir and sofosbuvir daily for 8 weeks, the SVR12 was 93.9% (95% CI, 90% to 97%). A total of 11 patients had virologic relapse and 1 patient was lost to follow-up and 1 patient withdrew consent.
- Of patients receiving ledipasvir and sofosbuvir with ribavirin for 8 weeks, 93.1% achieved SVR12 (95% CI, 89% to 96%). Nine patients had confirmed cases of virologic relapse, 5 patients were lost to follow-up, and 1 patient withdrew consent.
- Of patients receiving ledipasvir and sofosbuvir for 12 weeks, 95.4% achieved SVR12 (95% CI, 92% to 98%). A total of 3 patients had confirmed cases of virologic relapse and 7 were lost to follow-up.
Secondary Endpoint(s)
- All 3 drug regimens were superior to the adjusted historical response rate of 60%, based on 3 studies with telaprevir and boceprevir (P < .001).
- Only the 8-week regimen of ledipasvir and sofosbuvir was deemed noninferior to the other treatment regimens in this study (ledipasvir and sofosbuvir with ribavirin for 8 weeks or ledipasvir and sofosbuvir daily for 12 weeks).
- Subgroup analysis for patients with characteristics of poor response to previously available drug therapy (eg, patients with non-CC IL28B genotype, patients with HCV genotype 1a infection, patients with high baseline viral load, and Black patients) had a response rate similar to those without these characteristics.
Comments: The lower limit of detection was set at 25 units/mL. There were no reported cases of virologic failure during the duration of treatment in this trial. There were no differences in SVR12 between those with wild-type IL28B, CC, versus those with the traditionally more difficult to treat genotypes CT or TT. Of the 23 confirmed relapsers, 15 had NS5A strains detected at the time of viral relapse.
Limitations: Smaller study size than in the ION-1 trial. The study did not include patients who had previously been treated for HCV or those who had cirrhosis. Only patients infected with HCV genotype 1 were included in the study.
CONTRAINDICATIONS, WARNINGS, AND PRECAUTIONS
Contraindications
The product labeling contains no identified contraindications to the use of ledipasvir/sofosbuvir.1
Any hypersensitivity to ledipasvir or sofosbuvir or any of the tablet components (colloidal silicon dioxide, copovidone, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, FD&C yellow #6/sunset yellow FCF aluminum lake, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide) should be considered a contraindication to the use of ledipasvir and sofosbuvir.
Warnings and Precautions
The product labeling states use with other products containing sofosbuvir is not recommended.1 Ledipasvir/sofosbuvir is classified as Pregnancy Category B; no adequate and well-controlled studies have been conducted, but no effects on fetal development were observed in rats and rabbits.1
It is not known if ledipasvir or sofosbuvir are excreted in human breast milk.1
Safety and effectiveness have not been established in pediatric patients.1
ADVERSE REACTIONS
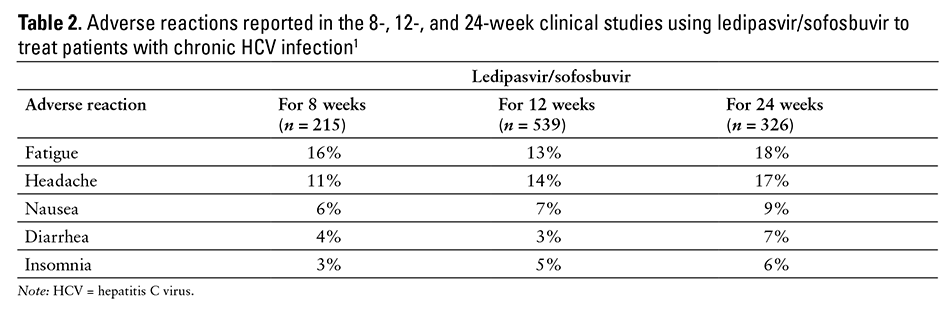
The most commonly reported adverse reactions are fatigue (13% to 18%) and headache (11% to 17%)1,3,4,5,6,29 (see Table 2). Discontinuation of ledipasvir/sofosbuvir therapy due to adverse effects was low in the clinical trials (1% or less).1
The common adverse events observed in clinical trials of ledipasvir and sofosbuvir include fatigue, headache, nausea, insomnia, and diarrhea.1,3,4,5,6,29 Other reported adverse effects included rash, upper respiratory tract infections, bronchitis, irritability, dizziness, muscle spasm, anemia, dry skin, cough, back pain, changes in appetite, and difficulty breathing.1,3,4,5,6,29
No clinically meaningful changes were observed in the QT/QTc interval in healthy volunteers who were given supratherapeutic doses of ledipasvir (120 mg orally twice daily for 10 days).30
Elevations in bilirubin greater than 1.5 times the upper limit of normal (ULN) occurred in 3% or less of patients and transient, asymptomatic lipase elevations (>3 times ULN) were observed in 3% or less of patients treated for up to 24 weeks with ledipasvir and sofosbuvir in the clinical trials.1
DRUG INTERACTIONS
Ledipasvir and sofosbuvir are substrates for the drug transporters P-glycoprotein (P-gp) and breast cancer resistance protein. Use with P-gp inducers (eg, rifampin, St. John’s wort) may alter the concentrations of ledipasvir and sofosbuvir. Therefore, use of P-gp inducers in patients requiring treatment with ledipasvir/sofosbuvir is not recommended.1 Ledipasvir is an inhibitor of P-gp and breast cancer resistance protein and, in theory, may increase intestinal absorption of coadministered substrates for these transporters; however, no documented drug-drug interactions based on this mechanism have been identified.1
Ledipasvir’s solubility is pH dependent (practically insoluble across the pH range of 3 to 7.5 and slightly soluble below pH 2.3) and decreases as the pH increases; therefore, drugs that alter gastric pH may affect the adsorption of ledipasvir. Administration with antacids (eg, aluminum and magnesium hydroxide) can decrease the absorption of ledipasvir; therefore, time of administration of the 2 products should be separated by at least 4 hours.1 When ledipasvir/sofosbuvir was coadministered with famotidine, there was a 20% or less reduction in ledipasvir’s peak serum concentrations without a change to its overall AUC, but staggered famotidine administration had no impact.26 Coadministration of omeprazole with ledipasvir and sofosbuvir resulted in a 4% decrease in ledipasvir’s AUC and 11% reduction in the Cmax, but had no impact on sofosbuvir’s AUC or Cmax.26
Ledipasvir/sofosbuvir can increase the serum levels of digoxin. Routine monitoring of digoxin levels is recommended if coadministration of digoxin and ledipasvir/sofosbuvir is necessary.1
Administration with enzyme-inducing anticonvulsants (eg, carbamazepine, phenytoin, phenobarbital, oxcarbazepine), antimycobacterials (eg, rifabutin, rifampin, rifapentine), or herbals (eg, St. John’s wort) may result in lower levels of ledipasvir and sofosbuvir; therefore, coadministration of these agents with ledipasvir/sofosbuvir is not recommended.1
Coadministration with tenofovir-containing HIV antiretroviral regimens may result in increased tenofovir serum levels.1 Administration with tipranavir/ritonavir may decrease the serum levels of ledipasvir/sofosbuvir.1 Coadministration of any of these agents should be done with caution or avoided.
Coadministration with simeprevir is not recommended. The levels of ledipasvir/simeprevir may be increased.1
Administration with rosuvastatin may increase the concentration of rosuvastatin and increase the risk of myopathy, including rhabdomyolysis; therefore, concurrent treatment with ledipasvir/sofosbuvir and rosuvastatin is not recommended.1
Ledipasvir alone was studied with raltegravir, with no meaningful changes in the pharmacokinetics of ledipasvir or raltegravir.31 Ledipasvir/sofosbuvir was then studied with the combinations of either efavirenz/emtricitabine/tenofovir or rilpivirine/tenofovir.31 There was a 34% reduction in exposure of ledipasvir when administered with efavirenz/emtricitabine/tenofovir and up to a 2.6-fold increase in overall tenofovir exposure when administered with concurrent ledipasvir/sofosbuvir.31
RECOMMENDED MONITORING
No specific monitoring has been recommended for ledipasvir/sofosbuvir therapy.
DOSING
The approved dose for the treatment of chronic HCV genotype 1 infection in adults is 1 tablet (ledipasvir 90 mg/sofosbuvir 400 mg) orally once daily with or without food. The treatment duration is dependent on the baseline status of the patient; treatment-naive patients with or without cirrhosis require 12 weeks, treatment-experienced patients (ie, individuals who have failed treatment with either peginterferon alfa and ribavirin or an HCV protease inhibitor, peginterferon alfa, and ribavirin) without cirrhosis require 12 weeks, and treatment-experienced patients (ie, individuals who have failed treatment with either peginterferon alfa and ribavirin or an HCV protease inhibitor, peginterferon alfa, and ribavirin) with cirrhosis require 24 weeks.1
It may be possible to initiate and maintain therapy at the standard adult dosage of ledipasvir/sofosbuvir for only 8 weeks in treatment-naive patients without cirrhosis whose pretreatment HCV RNA levels were less than 6 million units per mL.1
Patients with mild to moderate renal impairment or mild, moderate, or severe hepatic impairment (Child-Pugh class A, B, or C) can be treated with the recommended adult dose of ledipasvir/sofosbuvir. However, there is no dosing recommendation for patients with severe renal impairment (estimated GFR of less than 30 mL/min/1.73 m2) or end-stage renal disease; the metabolite of sofosbuvir has the potential to accumulate in these patients when standard adult dosing is utilized.1
PRODUCT AVAILABILITY
Ledipasvir/sofosbuvir was approved by the US Food and Drug Administration (FDA) on October 10, 2014. It is available as a fixed-dose combination tablet containing ledipasvir 90 mg/sofosbuvir 400 mg.2 The tablets are supplied in bottles containing 28 tablets. The product should be stored at room temperature below 30°C (86°F). The manufacturer recommends that the product only be dispensed in the original container.1
DRUG SAFETY/RISK EVALUATION AND MITIGATION STRATEGY (REMS)
No REMS is required for ledipasvir/sofosbuvir.2
CONCLUSION
Ledipasvir/sofosbuvir is a new medication approved for the treatment of chronic HCV infections caused by genotype 1 strains. High rates of SVR12 were achieved with ledipasvir/sofosbuvir when used for the treatment of HCV genotype 1 infections following therapy for 8, 12, or 24 weeks. These 2 drugs have also been used in combination with ribavirin for the treatment of chronic HCV infection with good results; however, the incidence of adverse effects is increased because of the ribavirin portion of the triple-drug regimen. This new all-oral, dual-drug regimen appears to be a promising option for the treatment of HCV genotype 1 infections, but data are lacking regarding its long-term efficacy (eg, SVR beyond 12 weeks) and its effects on the long-term complications associated with HCV infections (eg, cirrhosis, cancer).
REFERENCES
- Harvoni (ledipasvir/sofosbuvir) [prescribing information]. Foster City, CA: Gilead Sciences Inc; October 2014.
- Cox E. FDA approval letter: Harvoni (ledipasvir/sofosbuvir NSA 205834). US Food and Drug Administration Web site. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/205834Orig1s000ltr.pdf. Published October 10, 2014. Accessed October 24, 2014.
- Afdhal N, Reddy KR, Nelson DR, et al; ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–1493.
- Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–1898.
- Kowdley KV, Gordon SC, Reddy KR, et al; ION-3 Investigators. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879–1888.
- Lawitz E, Poordad FF, Pang PS, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in the treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): An open-label, randomised, phase 2 trial. Lancet. 2014;383(9916):515–523.
- Ledipasvir and sofosbuvir. ClinicalTrials.gov Web site. http://www.clinicaltrials.gov/ct2/results?term=ledipasvir+AND+sofosbuvir. Accessed October 24, 2014.
- Wyles DL, Rodriguez-Torres M, Lawitz E, et al. All-oral combination of ledipasvir, vedroprevir, tegobuvir, and ribavirin in treatment-naïve patients with genotype 1 HCV infection. Hepatology. 2014;60(1):56–64.
- Thompson A, Han S, Shiffman ML, et al. GS-5885 + GS-9451 + peginterferon and ribavirin (PR) for six or twelve weeks achieves high SVR12 rates in treatment-naïve genotype 1 IL28B CC patients [abstract]. J Hepatol. 2013;59:Abstract s29.
- Everson GT, Di Bisceglie AM, Vierling JM, et al. Combination of the NS5A inhibitor, GS-5885, the NS3 protease inhibitor, GS-9451, and pegylated interferon plus ribavirin in treatment-experienced patients with genotype 1 hepatitis C infection [abstract]. J Hepatol. 2013;58:Abstract s6.
- Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368(20):1859–1861.
- Klevens RM, Hu DJ, Jiles R, Holmberg SD. Evolving epidemiology of hepatitis C virus in the United States. Clin Infect Dis. 2012;55(suppl 1):S3–S9.
- Victrelis (boceprevir) [prescribing information]. Whitehouse Station, NJ: Merck & Co Inc; 2014.
- Olysio (simeprevir) [prescribing information]. Titusville, NJ: Janssen Products LP; 2014.
- Sovaldi (sofosbuvir) [prescribing information]. Foster City, CA: Gilead Sciences; December 2013.
- Link JO, Taylor JG, Xu L, et al. Discovery of ledipasvir (GS-5885): A potent, once-daily oral NS5A inhibitor for the treatment of hepatitis C virus infection. J Med Chem. 2014;57(5):2033–2046.
- Lawitz EJ, Gruener D, Hill JM, et al. A phase 1, randomised, placebo-controlled, 3-day, dose-ranging study of GS-5885, an NS5A inhibitor, in patients with genotype 1 hepatitis C. J Hepatol. 2012;57(1):24–31.
- Wong KA, Worth A, Martin R, et al. Characterization of hepatitis C virus resistance from a multiple-dose clinical trial of the novel NS5A inhibitor GS-5885. Antimicrob Agents Chemother. 2013;57(12):6333–6340.
- Hernandez D, Zhou N, Ueland J, Monikowski A, McPhee F. Natural prevalence of NS5A polymorphisms in subjects infected with hepatitis C virus genotype 3 and their effects on the antiviral activity of NS5A inhibitors. J Clin Virol. 2013;57(1):13–18.
- Rodriguez-Torres M, Lawitz E, Kowdley KV, et al. Sofosbuvir (GS-7977) plus peg-interferon/ribavirin in treatment-naïve patients with HCV genotype 1: A randomized, 28-day, dose-ranging trial. J Hepatol. 2013;58(4):663-668.
- Hebner C, Lee YJ, Han B, et al. In vitro pan-genotypic and combination activity of sofosbuvir (SG-7977) in stable replican cell lines [abstract]. Hepatology. 2012;56(4: suppl):1066A.
- Han B, Ma H, Wong KA. In vitro analyses of HCV NS5B S282T mutants in multiple HCV genotypes show low levels of reduced susceptibility to sofosbuvir (GS-7977), no cross resistance to other classes of direct-acting antivirals, and hypersensitivity to ribavirin [abstract]. Hepatology. 2012;56(4 suppl):711A.
- Svarovskaia ES, Dvory-Sobol H, Gunicharova V, et al. Comprehensive resistance testing in patients who relapsed after treatment with sofosbuvir (GS-7977)- containing regimens in phase 2 studies [abstract]. Hepatology. 2012;56(4 suppl):551A.
- Kirby B, Li H, Keamey BP, Mathias A. Population pharmacokinetic analysis of ledipasvir (GS-5885) in healthy and hepatitis C virus infected subjects. Presented at: 15th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy; May 19-21, 2014; Washington DC. Abstract P_33.
- German P, Moorehead L, Pang P, Vimal M, Mathias A. Lack of a clinically important pharmacokinetic interaction between sofosbuvir or ledipasvir and hormonal oral contraceptives norgestimate/ethinyl estradiol in HCV-uninfected female subjects. J Clin Pharmacol. 2014;54(11):1290-1298.
- German P, Yang J, West S, Han L, Sajwani K, Mathias A. Effect of food and acid reducing agents on the relative bioavailability and pharmacokinetics of ledipasvir and sofosbuvir fixed dose combination tablet. Presented at: 15th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy; May 19-21, 2014; Washington DC. Abstract P_15.
- Guidelines: Recommendations for testing, managing, and treating hepatitis C. American Association for the Study of Liver Disease & Infectious Diseases Society of America. http://www.hcvguidelines.org/fullreport. Published August 11, 2014. Accessed September 9, 2014.
- Chronic hepatitis C virus (HCV) infection: Treatment considerations from the Department of Veterans Affairs National Hepatitis C Resource Center Program and the Office of Public Health. http://www.hepatitis.va.gov/pdf/2014hcv.pdf. Revised May 13, 2014. Accessed September 9, 2014.
- Gane EJ, Stedman CA, Hyland RH, et al. Efficacy of nucleotide polymerase inhibitor sofosbuvir plus the NS5A inhibitor ledipasvir or the NS5B non-nucleoside inhibitor GS-9669 against HCV genotype 1 infection. Gastroenterology. 2014;146(3):736–743.
- German P, Pang P, Wu X, Buacharem M, Mathias A. Evaluation of the effect of ledipasvir on the QT/QTc interval in healthy subjects. Presented at: 15th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy; May 19-21, 2014. Washington DC. Abstract P_47.
- German P, Pang P, West S, Han L, Sajwani K, Mathias A. Drug interactions between direct acting anti-HCV antivirals sofosbuvir and ledipasvir and HIV antiretrovirals. Presented at: 15th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy; May 19-21, 2014. Washington DC. Abstract O_06.

*Founder and Contributing Editor, The Formulary; †Director, Drug Information Center, and Professor of Pharmacy Practice, College of Pharmacy, Washington State University Spokane, PO Box 1495, Spokane, Washington 99210-1495; ‡Drug Information Resident, College of Pharmacy, Washington State University. The authors indicate no relationships that could be perceived as a conflict of interest.