Case Report
Ventricular Tachycardia Precipitated by the Use of the Diet Supplement Hydroxycut Gummies
Drayton A. Hammond, PharmD, MBA, BCPS*; Estela Thano, MD†; Kristin A. Bohnenberger, PharmD‡; Matthew W. McAllister, PharmD§; Thomas Wannenburg, MD¶; Steve Hsu, MD¶; Bill J. Gurley, PhD**; and Robert Kim, MD¶
Case Report
Ventricular Tachycardia Precipitated by the Use of the Diet Supplement Hydroxycut Gummies
Drayton A. Hammond, PharmD, MBA, BCPS*; Estela Thano, MD†; Kristin A. Bohnenberger, PharmD‡; Matthew W. McAllister, PharmD§; Thomas Wannenburg, MD¶; Steve Hsu, MD¶; Bill J. Gurley, PhD**; and Robert Kim, MD¶
Case Report
Ventricular Tachycardia Precipitated by the Use of the Diet Supplement Hydroxycut Gummies
Drayton A. Hammond, PharmD, MBA, BCPS*; Estela Thano, MD†; Kristin A. Bohnenberger, PharmD‡; Matthew W. McAllister, PharmD§; Thomas Wannenburg, MD¶; Steve Hsu, MD¶; Bill J. Gurley, PhD**; and Robert Kim, MD¶
Abstract
Background: Dietary supplements have a long history of causing adverse effects. Ventricular arrhythmias have not been described with Hydroxycut Gummies.
Objective: To report a case of ventricular arrhythmia after prolonged use of a popular dietary supplement, Hydroxycut Gummies.
Case Report: An 18-year-old female with no significant past medical history presented with life-threatening ventricular arrhythmia following about 10 days of use of Hydroxycut Gummies, a legal dietary supplement previously unreported to cause this complication. The patient received external cardioversion due to progressive decline in mental status and persistent hypotension and was initiated on intravenous procainamide at an outside hospital. Left ventricular ejection fraction was 45% to 50%, and cardiac MRI showed no definite finding of infarct, myocarditis, or fibrosis. Beta-blocker therapy was initiated, and there was a progressive reduction in ventricular arrhythmia burden with an improvement of symptoms over the next few days. Two and a half months after the initial hospitalization, follow-up Holter monitor revealed occasional accelerated idioventricular rhythm events and a significant reduction in, but still occasional, long monomorphic ventricular tachycardia events. None of the ingredients listed in this product have been associated with cardiac dysrhythmias in the literature. One phytochemical potentially in the product is alpha-quinidine, which could be the cause of the adverse event. However, there was no other identifiable etiology for the ventricular tachycardia, which resolved after the discontinuation of supplement and the addition of beta-blocker therapy.
Conclusion: Hydroxycut Gummies should be considered a probable cause of this patient’s arrhythmia given the lack of another etiology and a Naranjo Scale score of 6.
Key Words—dietary supplement, Hydroxycut Gummies, ventricular tachycardia
Hosp Pharm—2015;50:615–618
Abstract
Background: Dietary supplements have a long history of causing adverse effects. Ventricular arrhythmias have not been described with Hydroxycut Gummies.
Objective: To report a case of ventricular arrhythmia after prolonged use of a popular dietary supplement, Hydroxycut Gummies.
Case Report: An 18-year-old female with no significant past medical history presented with life-threatening ventricular arrhythmia following about 10 days of use of Hydroxycut Gummies, a legal dietary supplement previously unreported to cause this complication. The patient received external cardioversion due to progressive decline in mental status and persistent hypotension and was initiated on intravenous procainamide at an outside hospital. Left ventricular ejection fraction was 45% to 50%, and cardiac MRI showed no definite finding of infarct, myocarditis, or fibrosis. Beta-blocker therapy was initiated, and there was a progressive reduction in ventricular arrhythmia burden with an improvement of symptoms over the next few days. Two and a half months after the initial hospitalization, follow-up Holter monitor revealed occasional accelerated idioventricular rhythm events and a significant reduction in, but still occasional, long monomorphic ventricular tachycardia events. None of the ingredients listed in this product have been associated with cardiac dysrhythmias in the literature. One phytochemical potentially in the product is alpha-quinidine, which could be the cause of the adverse event. However, there was no other identifiable etiology for the ventricular tachycardia, which resolved after the discontinuation of supplement and the addition of beta-blocker therapy.
Conclusion: Hydroxycut Gummies should be considered a probable cause of this patient’s arrhythmia given the lack of another etiology and a Naranjo Scale score of 6.
Key Words—dietary supplement, Hydroxycut Gummies, ventricular tachycardia
Hosp Pharm—2015;50:615–618
Abstract
Background: Dietary supplements have a long history of causing adverse effects. Ventricular arrhythmias have not been described with Hydroxycut Gummies.
Objective: To report a case of ventricular arrhythmia after prolonged use of a popular dietary supplement, Hydroxycut Gummies.
Case Report: An 18-year-old female with no significant past medical history presented with life-threatening ventricular arrhythmia following about 10 days of use of Hydroxycut Gummies, a legal dietary supplement previously unreported to cause this complication. The patient received external cardioversion due to progressive decline in mental status and persistent hypotension and was initiated on intravenous procainamide at an outside hospital. Left ventricular ejection fraction was 45% to 50%, and cardiac MRI showed no definite finding of infarct, myocarditis, or fibrosis. Beta-blocker therapy was initiated, and there was a progressive reduction in ventricular arrhythmia burden with an improvement of symptoms over the next few days. Two and a half months after the initial hospitalization, follow-up Holter monitor revealed occasional accelerated idioventricular rhythm events and a significant reduction in, but still occasional, long monomorphic ventricular tachycardia events. None of the ingredients listed in this product have been associated with cardiac dysrhythmias in the literature. One phytochemical potentially in the product is alpha-quinidine, which could be the cause of the adverse event. However, there was no other identifiable etiology for the ventricular tachycardia, which resolved after the discontinuation of supplement and the addition of beta-blocker therapy.
Conclusion: Hydroxycut Gummies should be considered a probable cause of this patient’s arrhythmia given the lack of another etiology and a Naranjo Scale score of 6.
Key Words—dietary supplement, Hydroxycut Gummies, ventricular tachycardia
Hosp Pharm—2015;50:615–618
Hosp Pharm 2015;50(7):615–618
2015 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj5007-615
The prevalence of obesity in the United States is increasing, affecting 35.7% of US adults in 2010.1 The obesity epidemic has led to the increased use of widely available over-the-counter diet aids. As most herbal remedies are classified as dietary supplements, and thus not regulated as strictly by the US Food and Drug Administration (FDA), evidence of safety, efficacy, and quality is not required prior to marketing. Furthermore, these remedies often lack the rigorous quality and production standards that prescription medications have to adhere to. In part because of the lesser degree of regulation, herbal supplements are responsible for over 13,000 adverse events annually. Liver failure, colitis, and rhabdomyolysis are the most well-documented adverse events.2,3
Since the withdrawal of ephedra-containing weight loss supplements from the market, newer formulations of supplements that are devoid of sympathomimetic amines have been developed. One of the primary components of Hydroxycut is epigallocatechin, the pharmacologically active component of green tea extract previously reported to potentially induce atrial fibrillation by inhibiting cardiac ion channels.4 Newer formulations of Hydroxycut claim not to contain epigallocatechin or caffeine as active ingredients.5 It is nevertheless possible that a sympathomimetic component of some type, potentially as an adulterant or a previously unreported effect of the listed ingredients, could lead to the development of ventricular arrhythmias.6 We report a case of ventricular tachycardia related to Hydroxycut Gummies (Muscle Tech).
CASE PRESENTATION
An 18-year-old White female Navy recruit with no prior medical history of cardiac or obstructive sleep disorders presented to the emergency department with a 1-week history of sustained palpitation, dyspnea, and fatigue. Prior to this event, she consistently exercised 3 to 5 times each week, running and performing weight-lifting exercises. She was not exercising when her symptoms developed. She weighed 75.7 kg, and had a body mass index of 28.63 kg/m2. Upon presentation to an outside hospital, a 12-lead electrocardiogram (ECG) demonstrated irregular, rapid, wide-complex tachycardia, which initially was interpreted as atrial fibrillation with aberrant conduction. Several hours later, the patient required an external cardioversion due to progressive decline in mental status and persistent hypotension. She subsequently was initiated on intravenous procainamide and transferred to University of Florida Health Jacksonville for further care.
Upon arrival, the patient was noted again to be in irregular wide-complex tachycardia albeit at a slower rate. Closer examination of the ECGs revealed that the rhythm was actually ventricular in origin (evidence of atrioventricular dissociation and fusion beats). The patient provided a history of taking 2 HydroxycutGummies by mouth 3 times daily in accordance with labeled instructions for weight loss for about 10 days prior to the onset of symptoms but not taking prescription medications or other herbal or dietary supplements. She denied drinking coffee, soft drinks, green tea, or energy drinks. She denied illicit drug use, and a standard urine drug screen that included amphetamines, cocaine, and phencyclidine was negative. Serum electrolytes were within normal limits. There was no family history of arrhythmia or sudden cardiac death. Transthoracic echocardiogram demonstrated mild global systolic dysfunction with a left ventricular ejection fraction of 45% to 50%. Cardiac MRI that was performed several days later showed no definite finding of infarct, myocarditis, or fibrosis. Oral beta-blocker therapy was instituted. Over the course of the next several days, there was a progressive reduction in ventricular arrhythmia burden with an improvement of symptoms. The patient was seen at an outside hospital 1 month after discharge, and a repeat echocardiogram found a left ventricular ejection fraction of 60% to 65%. The patient returned to our clinic 2.5 months after discharge. The follow-up Holter monitor revealed occasional accelerated idioventricular rhythm events and a significant reduction in long, monomorphic ventricular tachycardia events with a heart rate in the range of 180-190 beats per minute. The patient reported that she was asymptomatic during these episodes and continued to exercise 3 to 5 times per week. She refused a formal electrophysiology study due to her current asymptomatic nature.
CASE DISCUSSION
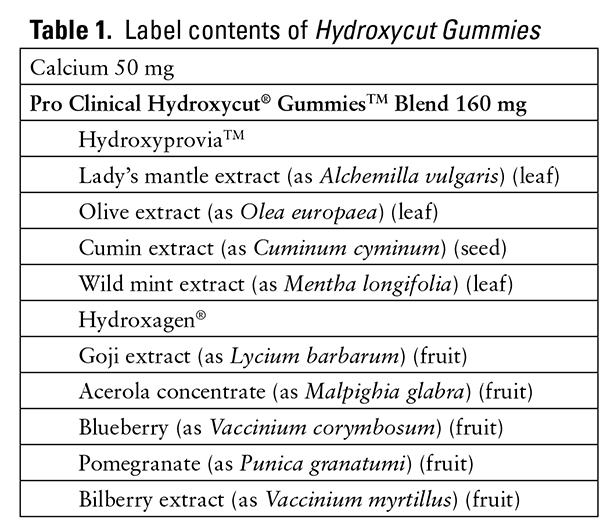
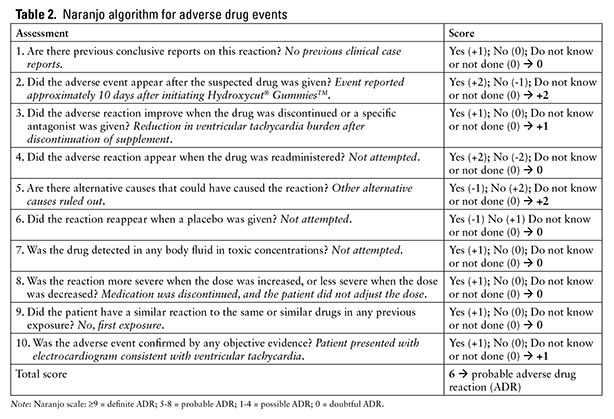
The patient may have experienced chronic, asymptomatic ventricular tachycardia episodes; however, she did not endorse any cardiac or obstructive sleep disorder history. None of the product ingredients explicitly listed on the product packaging have been associated with cardiac dysrhythmias in the literature7,8 (Table 1). However, one phytochemical found in the leaves of Olea europaea is cinchonidine, which is also known as alpha-quinidine. Class I antiarrhythmics have been known to demonstrate proarrhythmic properties.9 The manufacturer does not describe the extract procedure or phytochemical composition of the extract, which means the amount of cinchonidine, if any, in the products is unknown.5,10 However, there was no other identifiable etiology for her ventricular tachycardia, which was significantly reduced after the discontinuation of the supplement and the addition of beta-blocker therapy. Given the lack of another etiology and a Naranjo Scale score of 6 (Table 2), Hydroxycut Gummies is a probable cause of this patient’s arrhythmia.11
CONCLUSION
This is the first case report that establishes a probable association between the onset of ventricular tachycardia and use of Hydroxycut Gummies. Pharmacists should continue to scrutinize dietary supplements critically as potential causes of adverse events and utilize every resource at their disposal to provide their most educated assessments of these events when they occur.
ACKNOWLEDGMENTS
The authors declare no conflicts of interest.
REFERENCES
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief. 2012;82:1-7.
- Morrow JD, Edeki TI, El Mouelhi M, et al. American Society for Clinical Pharmacology and Therapeutics position statement on dietary supplement safety and regulation. Clin Pharmacol Ther. 2002;77(3):113-122.
- Morrow JD. Why the United States still needs improved dietary supplement regulation and oversight. Clin Pharmacol Ther. 2008;83(3):391-393.
- Kang J,Cheng H, Ji J, Incardona J, Rampe D. In vitro electrocardiographic and cardiac ion channel effects of (-)-epigallocatechin-3-gallate, the main catechin of green tea. J Pharmacol Exp Ther. 2010;334:619-626.
- Hydroxycut Gummies [product information]. http://www.hydroxycut.com/products/gummies/. Accessed January 18, 2014.
- Whitsitt V, Beehner C, Welch C. The role of good manufacturing practices for preventing dietary supplement adulteration. Anal Bioanal Chem. 2013;405(13):4353-4358.
- POISINDEX System [Internet database]. Greenwood Village, CO: Thomson Micromedex. Updated periodically. Accessed January 18, 2014.
- Natural Medicines. Updated periodically. naturalmedicines.therapeuticresearch.com. Accessed January 18, 2014.
- Farkas A, Leprán I, Papp JG. Proarrhythmic effects of intravenous quinidine, amiodarone, D-sotalol, and almokalant in the anesthetized rabbit model of torsade de pointes. J Cardiovasc Pharmacol. 2002;39(2):287-297.
- Gurley BJ. Emerging technologies for improving phytochemical bioavailability: Benefits and risks. Clin Pharmacol Ther. 2011;89(6):915-919.
- Busto U, Naranjo CA, Sellers EM. Comparison of two recently published algorithms for assessing the probability of adverse drug reactions. Br J Clin Pharmacol. 1982;13(2):223-227.

*Clinical Assistant Professor, Department of Pharmacy Practice, University of Arkansas for Medical Sciences, Little Rock, Arkansas; †Cardiovascular Medicine Fellow, Department of Medicine, University of Florida Health Jacksonville, Florida; ‡Clinical Toxicology/Emergency Medicine Fellow, Florida/USVI Poison Information Center – Jacksonville at UF Health Jacksonville, Florida; §Clinical Pharmacy Specialist – Emergency Medicine, Department of Pharmacy, Columbus Regional Health Midtown Medical Center, Columbus, Georgia; ¶Assistant Professor of Medicine, Section of Cardiac Electrophysiology, University of Florida Health Jacksonville, Florida; **Professor, Department of Pharmaceutical Sciences, University of Arkansas for Medical Sciences, Little Rock, Arkansas. Corresponding author: Drayton A. Hammond, PharmD, MBA, BCPS, Clinical Assistant Professor, Department of Pharmacy Practice, University of Arkansas for Medical Sciences, 4301 West Markham Street, Little Rock, AR 72205; phone: 501-686-6683; e-mail: dahammond@uams.edu