ISMP Medication Error Report Analysis
Compounded Pain Creams and Adverse Effects
Postanesthesia Care Unit ADC Selection Error
Docetaxel Product Has Unusual Concentration
Tragic Vaccine Diluent Mix-ups
Michael R. Cohen, RPh, MS, ScD,* and Judy L. Smetzer, RN, BSN†
ISMP Medication Error Report Analysis
Compounded Pain Creams and Adverse Effects
Postanesthesia Care Unit ADC Selection Error
Docetaxel Product Has Unusual Concentration
Tragic Vaccine Diluent Mix-ups
Michael R. Cohen, RPh, MS, ScD,* and Judy L. Smetzer, RN, BSN†
ISMP Medication Error Report Analysis
Compounded Pain Creams and Adverse Effects
Postanesthesia Care Unit ADC Selection Error
Docetaxel Product Has Unusual Concentration
Tragic Vaccine Diluent Mix-ups
Michael R. Cohen, RPh, MS, ScD,* and Judy L. Smetzer, RN, BSN†
These medication errors have occurred in health care facilities at least once. They will happen again—perhaps where you work. Through education and alertness of personnel and procedural safeguards, they can be avoided. You should consider publishing accounts of errors in your newsletters and/or presenting them at your inservice training programs.
Your assistance is required to continue this feature. The reports described here were received through the Institute for Safe Medication Practices (ISMP) Medication Errors Reporting Program. Any reports published by ISMP will be anonymous. Comments are also invited; the writers’ names will be published if desired. ISMP may be contacted at the address shown below.
Errors, close calls, or hazardous conditions may be reported directly to ISMP through the ISMP Web site (www.ismp.org), by calling 800-FAIL-SAFE, or via e-mail at ismpinfo@ismp.org. ISMP guarantees the confidentiality and security of the information received and respects reporters’ wishes as to the level of detail included in publications.
These medication errors have occurred in health care facilities at least once. They will happen again—perhaps where you work. Through education and alertness of personnel and procedural safeguards, they can be avoided. You should consider publishing accounts of errors in your newsletters and/or presenting them at your inservice training programs.
Your assistance is required to continue this feature. The reports described here were received through the Institute for Safe Medication Practices (ISMP) Medication Errors Reporting Program. Any reports published by ISMP will be anonymous. Comments are also invited; the writers’ names will be published if desired. ISMP may be contacted at the address shown below.
Errors, close calls, or hazardous conditions may be reported directly to ISMP through the ISMP Web site (www.ismp.org), by calling 800-FAIL-SAFE, or via e-mail at ismpinfo@ismp.org. ISMP guarantees the confidentiality and security of the information received and respects reporters’ wishes as to the level of detail included in publications.
These medication errors have occurred in health care facilities at least once. They will happen again—perhaps where you work. Through education and alertness of personnel and procedural safeguards, they can be avoided. You should consider publishing accounts of errors in your newsletters and/or presenting them at your inservice training programs.
Your assistance is required to continue this feature. The reports described here were received through the Institute for Safe Medication Practices (ISMP) Medication Errors Reporting Program. Any reports published by ISMP will be anonymous. Comments are also invited; the writers’ names will be published if desired. ISMP may be contacted at the address shown below.
Errors, close calls, or hazardous conditions may be reported directly to ISMP through the ISMP Web site (www.ismp.org), by calling 800-FAIL-SAFE, or via e-mail at ismpinfo@ismp.org. ISMP guarantees the confidentiality and security of the information received and respects reporters’ wishes as to the level of detail included in publications.
Hosp Pharm 2015;50(1):009–012
2015 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj5001-009
COMPOUNDED PAIN CREAMS AND ADVERSE EFFECTS
Some compounding pharmacies are preparing compounded pain creams and ointments that contain a combination of multiple potent medications. Many of these include drugs that can cause central nervous system depression or cardiac effects, such as ketamine, baclofen, cyclobenzaprine, lidocaine, tricyclic antidepressants, gabapentin, clonidine, and nifedipine. Most of these drugs have not been US Food and Drug Administration (FDA)–approved for topical use (although off-label use of medications is routine). Some compounding pharmacies are filling prescriptions for these pain creams for specific patients. Their use is growing, prompting some pharmacies to employ a sales force, initiate elaborate marketing campaigns, and provide template prescriptions to make prescribing of these creams easier.
Patients may be unaware of potential dangers with these creams. The products, which are not packaged in containers with a safety closure, also may not be as carefully stored as other medications to prevent accidental child exposures. The Philadelphia Poison Control Center recently received a call about a child who became severely ill after applying his mother’s topical fibromyalgia medication—gabapentin, ketamine, diclofenac, clonidine, nifedipine, bupivacaine, menthol, and cyclobenzaprine—that was compounded at a pharmacy to his body. Pediatric patients may also be at risk of toxicity if the creams are applied to their skin intentionally for a skin condition.
In a case reported in 2013, severe toxicity occurred in an 18-month-old child when his father’s compounded ointment was used to treat a diaper rash (www.ismp.org/sc?id=411). Compounded pain creams need prominent warnings on labels that describe the potential for toxicity. Physicians who prescribe these drugs, as well as pharmacists who compound and dispense them, must ensure that patients are provided with instructions about possible adverse effects, safe storage, and proper use.
Because safety issues can arise with any compounded, unapproved formulations of medications, regulatory or licensing oversight is necessary. With compounded pain creams and ointments, we are specifically concerned about some compounding company statements that may be unproven, such as the products’ safe use with children (www.ismp.org/sc?id=412). However, underfunded state boards of pharmacy are ill-equipped to provide such oversight, and FDA oversight has not been applied to traditional compounding pharmacies. The current Drug Quality and Security Act of 2013 (compounding legislation) does not give the FDA oversight for traditional compounding pharmacies. It is unclear how the Act’s requirement to develop a list of drugs that should never be compounded might affect certain currently compounded products.
POSTANESTHESIA CARE UNIT ADC SELECTION ERROR
An anesthesiologist ordered prochlorperazine 10 mg slow intravenous (IV) push for a postoperative patient in the postanesthesia care unit (PACU). A nurse went to the PACU automated dispensing cabinet (ADC) to obtain the drug but instead removed a vial of phenylephrine 10 mg/mL by mistake and administered it to the patient. The ADC was not profiled and did not allow pharmacy to verify new orders for patients prior to obtaining a medication from the cabinet. Both medications were located in 2 adjacent matrix pockets in the ADC.
To reduce the risk of error, the hospital moved phenylephrine vials to a different ADC cabinet that holds only critical care agents, given that the drug is used primarily as a vasopressor after being diluted in either 5% dextrose injection or 0.9% sodium chloride injection and then administered as a continuous infusion. Open matrix drawers and open cabinet-type configurations are not used in the ADC that stocks critical care drugs, and the phenylephrine is also now stored in its own locked and lidded compartment.
The hospital will be moving to profiled ADC cabinets in the PACU so pharmacy can verify medications in the ADC prior to removal. Barcode scanning technology will also be available with the profiled cabinets to ensure accuracy when stocking ADCs and removing products.
In the Medication Error Report Analysis in October [Phenylephrine injection needs dilution for intravenous bolus use. Hosp Pharm. 2014;49(9):798], we noted that ISMP has received reports of several similar errors during 2014. A diluted form of phenylephrine injection is expected to reach the market in the coming year.
DOCETAXEL PRODUCT HAS UNUSUAL CONCENTRATION
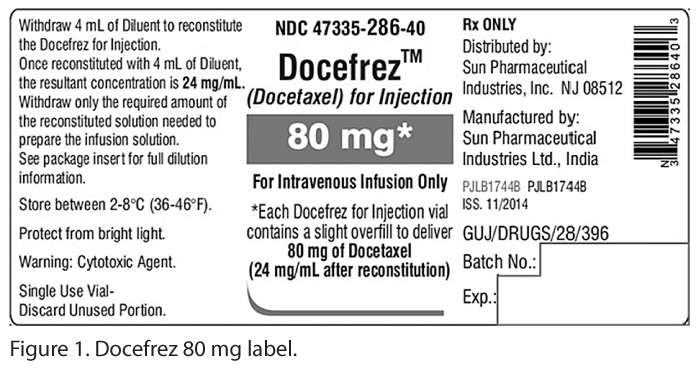
Unlike docetaxel solution products that do not require reconstitution before adding to an infusion container, a Sun Pharmaceutical/Caraco product known as Docefrez is available in 20 mg and 80 mg amounts as a lyophilized powder along with a diluent for reconstitution. While docetaxel solutions are available from various manufacturers in either a 10 mg or 20 mg per mL- concentration, it is important to recognize that the 20 mg Docefrez product results in a 25 mg/mL (20 mg/0.8 mL) concentration after reconstitution using instructions provided with the diluent. The -80 mg product results in a 24 mg/mL (20 mg/0.833 mL)- concentration after reconstitution (see Figure 1). This information is listed on the lyophilized powder container label and the product insert, but it is not prominent and can easily be overlooked because it appears several lines below the total mg amount in each vial.
The unusual final concentration of Docefrez led to a medication error recently when the solution that the hospital normally used was on backorder by the pharmacy’s wholesaler. A pharmacy purchaser saw the 80 mg Docefrez product as an alternative listing. Thinking that it was similar to the product the hospital normally used, he ordered it after checking with a clinical oncology pharmacist specialist. When a dose of 145 mg was later ordered for a patient, a pharmacy technician retrieved the Docefrez product and had a pharmacist check the set-up prior to preparation.
Label instructions were to add 4 mL of diluent to the 80 mg vial, which seemed like it would yield -20 mg/mL, the same as the hospital pharmacy computer system listed for the solution that they normally use. Since the ordered dose was 145 mg, the computer calculated that 7.25 mL was needed, based on a 20 mg/mL final concentration. The final solution of drug was then approved by a second pharmacist and added to an infusion container.
What all parties missed was that reconstituting the 80 mg vial with 4 mL does not yield a 20 mg/mL concentration but rather a 24 mg/mL concentration. The patient received a dose of 174 mg (20% more) instead of 145 mg, which can be a dangerous dose for this drug. Fortunately the patient was monitored and did not experience harm.
To help reduce the risk of an error if Docefrez should be needed due to a manufacturer backorder of the normal docetaxel product the hospital uses, the pharmacy computer system will now list the Docefrez product along with the concentration after reconstitution. There is now also a step during purchasing where the package insert must be reviewed by a pharmacist whenever alternative products are ordered to determine whether there are any inherent risks or potential for errors that need to be communicated to staff.
ISMP has spoken to the manufacturer, Sun Pharma USA, who has agreed to revise the label. There are currently several other docetaxel manufacturers and, as above, various concentrations are listed for purchase in computer systems. Keep in mind when ordering replacement products for any injectable drug on backorder or during a drug shortage that it is the amount of drug by weight that is most important to consider, not the volume. If hospitals or clinics are not able to get the product they normally use, it could be a set-up for a medication error. There must be a process in place to review any new products brought into the hospital to make sure people are aware of the new product and that systems (including strengths and concentrations in the information technology systems) can be adjusted as necessary for safe use.
TRAGIC VACCINE DILUENT MIX-UPS
There were news reports in September 2014 about a terrible tragedy in Syria where 15 children died after being vaccinated against measles. The diluent turned out to be atracurium. Don’t think that something similar couldn’t happen here. It has, many times.
Apparently in the Syrian incident, the manufacturer shipped vials of vaccine as a lyophilized powder along with separate glass ampuls of diluent. Somehow, a mix-up occurred either before shipment from the manufacturer or at the central area where the vaccines and diluents were stored in a refrigerator, prior to distribution to other areas. The diluent ampuls were confused with look-alike ampuls of atracurium, a neuromuscular blocking agent, which is also refrigerated. Of 75 children given vaccine, at least 15 died when the vaccine was accidentally reconstituted with the neuromuscular blocker. The neuromuscular blocker dose is weight based, so the children who died were 18 months old or younger. Older children survived. Below are several similar incidents that have previously been reported by ISMP.
- A US hospital emergency department (ED) nurse administered pancuronium instead of influenza vaccine to several patients. The vials were the same size, and the labels were quite similar. The look-alike vials had been stored next to each other in the refrigerator. The patients experienced dyspnea and respiratory depression but sustained no permanent injuries.
- A nurse mixed up measles vaccine and bacille Calmette-Guerin (BCG) vaccines with pancuronium and administered the drug to healthy infants. One infant died after experiencing seizures and respiratory arrest. The pancuronium vial looked very similar to a vial of sodium chloride injection, the diluent for these vaccines.
- In Taiwan, atracurium was administered subcutaneously instead of hepatitis B vaccine to 7 infants. The infants developed respiratory distress within 30 minutes. Five infants recovered, one sustained permanent injury, and another died. Neuromuscular blocking agents had never been available as floor stock in the nursery. For convenience, an anesthesiologist from a nearby operating room had placed the vial of atracurium in the unit refrigerator near vaccine vials of similar appearance.
- In Mexico, 14 patients presented with hypotonia, cyanosis, and dyspnea 5 minutes following immunization with measles vaccine. One patient died. Succinylcholine and pancuronium vials were found stored with the vaccine and diluent.
- In Kenya, 6 infants immunized with measles vaccine had convulsions and became “floppy.” Pancuronium was found stored with the vaccine.
- In Lesotho, 5 neonates collapsed a few minutes following immunization with BCG and oral polio vaccine. The vaccine was diluted with a neuromuscular blocker. One infant died.
To ensure safety with neuromuscular blocking agents, review the ISMP article, “Paralyzed by mistakes. Preventing errors with neuromuscular blocking agents” (www.ismp.org/sc?id=413). Think through safety changes that might be needed. For example, consider use of prefilled vaccine syringes whenever possible. Eliminate or restrict the storage of paralyzing agents by sequestering the products (eg, in a sealed box with a breakaway lock or rapid sequence intubation kit), and affix “WARNING—PARALYZING AGENT” labels to the vials and storage container. Review refrigerated storage areas regularly to identify the potential for mix-ups, and limit or eliminate the storage of neuromuscular blockers whenever possible.
ISMP is calling upon federal regulators and product vendors to work toward improving vaccine packaging to eliminate tragedies like these, worldwide. Many vaccines that require a diluent could be packaged in a dual chamber container (for the powder and liquid) to ensure that only the proper diluent is always used. ![]()
*President, Institute for Safe Medication Practices, 200 Lakeside Drive, Suite 200, Horsham, PA 19044; phone: 215-947-7797; fax: 215-914-1492; e-mail: mcohen@ismp.org; Web site: www.ismp.org. †Vice President, Institute for Safe Medication Practices, Horsham, Pennsylvania.