Original Article
Improved Pharmacy Department Workflow with New Method
of Order Entry for Single-Agent, High-Dose Methotrexate
Polly E. Kintzel, PharmD, BCPS, BCOP*; Thomas H. VanDyke, RPh*; Paul W. Athmann, PharmD*; Lisa B. Mills,
PharmD†; Michael P. Bonter, PharmD‡; Matthew W. Bremer, PharmD§; Mary L. Dougherty, DP, RN, AOCNS*;
Ryan W. Foster, PharmD*; Sandra K. Knight, RN*; Martha G. Slot, PharmD*; and Laura L. Steinmetz-Malato, PharmD¶
Original Article
Improved Pharmacy Department Workflow with New Method
of Order Entry for Single-Agent, High-Dose Methotrexate
Polly E. Kintzel, PharmD, BCPS, BCOP*; Thomas H. VanDyke, RPh*; Paul W. Athmann, PharmD*; Lisa B. Mills,
PharmD†; Michael P. Bonter, PharmD‡; Matthew W. Bremer, PharmD§; Mary L. Dougherty, DP, RN, AOCNS*;
Ryan W. Foster, PharmD*; Sandra K. Knight, RN*; Martha G. Slot, PharmD*; and Laura L. Steinmetz-Malato, PharmD¶
Original Article
Improved Pharmacy Department Workflow with New Method
of Order Entry for Single-Agent, High-Dose Methotrexate
Polly E. Kintzel, PharmD, BCPS, BCOP*; Thomas H. VanDyke, RPh*; Paul W. Athmann, PharmD*; Lisa B. Mills,
PharmD†; Michael P. Bonter, PharmD‡; Matthew W. Bremer, PharmD§; Mary L. Dougherty, DP, RN, AOCNS*;
Ryan W. Foster, PharmD*; Sandra K. Knight, RN*; Martha G. Slot, PharmD*; and Laura L. Steinmetz-Malato, PharmD¶
Abstract
Purpose: To determine whether a process change impacted the proportion of orders for single-agent, high-dose methotrexate entered by chemotherapy pharmacists instead of general pharmacy staff. Coordination of antiemetic premedication and leucovorin rescue with the new method of order entry was evaluated.
Methods: Adults treated with single-agent, high-dose methotrexate were identified retrospectively. Order entry of methotrexate and ancillary medications was examined to determine whether the old or new method was used and whether it was performed by a chemotherapy pharmacist. The fundamental difference between the old and new methods for order entry is use of the “unscheduled” frequency of medication administration to replace the administration frequency of “once” with a specified date and time. Timing of antiemetic premedication and leucovorin rescue relative to methotrexate administration were tallied for the new method. Chi-square analysis was performed for the primary objective. Observational statistics were performed otherwise.
Results: The number of evaluable encounters identified was 158. A chemotherapy pharmacist entered a greater proportion of orders when the new method was utilized (P < .0001). The proportion of orders entered by a chemotherapy pharmacist increased during the hours of 0700 and 2259 with the new method. Appropriate coordination of antiemetic and leucovorin administration was documented for 96% and 100% of cases with the new method of order entry.
Conclusion: The proportion of orders for single-agent, high-dose methotrexate entered by a chemotherapy pharmacist was significantly greater with the use of the new method. Administration of antiemetic premedication and leucovorin rescue were appropriately coordinated with the use of the new method for order entry of single-agent, high-dose methotrexate.
Key Words—antineoplastic agents; intravenous administration; medication systems, hospital; methotrexate; patient safety; workflow
Hosp Pharm—2014;49:1039–1043
Abstract
Purpose: To determine whether a process change impacted the proportion of orders for single-agent, high-dose methotrexate entered by chemotherapy pharmacists instead of general pharmacy staff. Coordination of antiemetic premedication and leucovorin rescue with the new method of order entry was evaluated.
Methods: Adults treated with single-agent, high-dose methotrexate were identified retrospectively. Order entry of methotrexate and ancillary medications was examined to determine whether the old or new method was used and whether it was performed by a chemotherapy pharmacist. The fundamental difference between the old and new methods for order entry is use of the “unscheduled” frequency of medication administration to replace the administration frequency of “once” with a specified date and time. Timing of antiemetic premedication and leucovorin rescue relative to methotrexate administration were tallied for the new method. Chi-square analysis was performed for the primary objective. Observational statistics were performed otherwise.
Results: The number of evaluable encounters identified was 158. A chemotherapy pharmacist entered a greater proportion of orders when the new method was utilized (P < .0001). The proportion of orders entered by a chemotherapy pharmacist increased during the hours of 0700 and 2259 with the new method. Appropriate coordination of antiemetic and leucovorin administration was documented for 96% and 100% of cases with the new method of order entry.
Conclusion: The proportion of orders for single-agent, high-dose methotrexate entered by a chemotherapy pharmacist was significantly greater with the use of the new method. Administration of antiemetic premedication and leucovorin rescue were appropriately coordinated with the use of the new method for order entry of single-agent, high-dose methotrexate.
Key Words—antineoplastic agents; intravenous administration; medication systems, hospital; methotrexate; patient safety; workflow
Hosp Pharm—2014;49:1039–1043
Abstract
Purpose: To determine whether a process change impacted the proportion of orders for single-agent, high-dose methotrexate entered by chemotherapy pharmacists instead of general pharmacy staff. Coordination of antiemetic premedication and leucovorin rescue with the new method of order entry was evaluated.
Methods: Adults treated with single-agent, high-dose methotrexate were identified retrospectively. Order entry of methotrexate and ancillary medications was examined to determine whether the old or new method was used and whether it was performed by a chemotherapy pharmacist. The fundamental difference between the old and new methods for order entry is use of the “unscheduled” frequency of medication administration to replace the administration frequency of “once” with a specified date and time. Timing of antiemetic premedication and leucovorin rescue relative to methotrexate administration were tallied for the new method. Chi-square analysis was performed for the primary objective. Observational statistics were performed otherwise.
Results: The number of evaluable encounters identified was 158. A chemotherapy pharmacist entered a greater proportion of orders when the new method was utilized (P < .0001). The proportion of orders entered by a chemotherapy pharmacist increased during the hours of 0700 and 2259 with the new method. Appropriate coordination of antiemetic and leucovorin administration was documented for 96% and 100% of cases with the new method of order entry.
Conclusion: The proportion of orders for single-agent, high-dose methotrexate entered by a chemotherapy pharmacist was significantly greater with the use of the new method. Administration of antiemetic premedication and leucovorin rescue were appropriately coordinated with the use of the new method for order entry of single-agent, high-dose methotrexate.
Key Words—antineoplastic agents; intravenous administration; medication systems, hospital; methotrexate; patient safety; workflow
Hosp Pharm—2014;49:1039–1043
Hosp Pharm 2014;49(11):1039–1043
2014 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj4911-1039
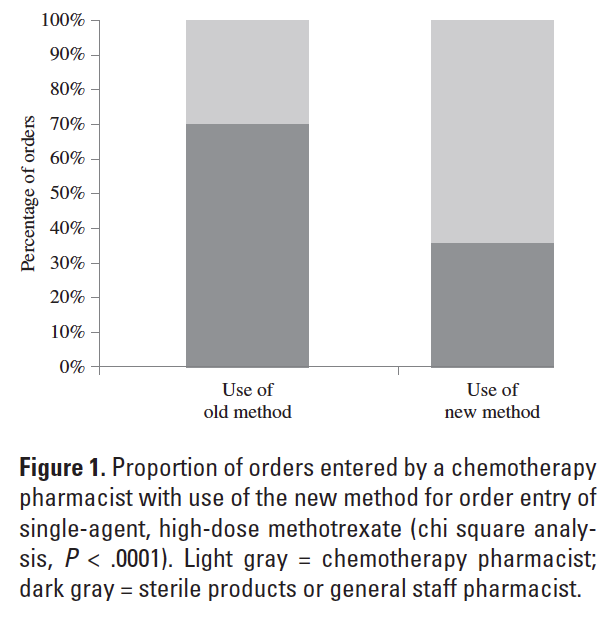


Interdisciplinary and departmental workflow impact the safety of chemotherapy order management. Chemotherapy orders for anticancer treatments are generally complex and entail preparation of high-alert medications. Chemotherapy orders should be processed in the pharmacy department when trained personnel are available to check the orders and manage the order entry process.1-3 Coordination of chemotherapy order review and entry can be challenging when the administration time is a moving target. One such example is the administration time for high-dose methotrexate, which is contingent on urinary alkalinization and robust urine output to reduce treatment-related nephrotoxicity.4 The start time for administration of single-agent, high-dose methotrexate is dependent on achieving a sustained target urine output (>100 mL/h) and pH (>7) in addition to the logistics of the patient admission process and care delivery.5 The safe and timely administration of single-agent, high-dose methotrexate is also conditional on the coordinated timing of ancillary treatments, such as antiemetics and leucovorin.4-7 Alkaline hydration can be initiated upon the patient’s arrival to the nursing unit so that target values for urine output and pH are achieved in a timely manner. At Spectrum Health Hospitals, Grand Rapids, Michigan, the time from start of alkaline hydration to urine pH greater than 7 in this population ranges from 5.4 to 11.8 hours.8
Cerner Millenium is the software program used by Spectrum Health Hospitals for electronic medication management. Complex chemotherapy regimens used for anticancer treatments are handled using paper order sets; computerized prescriber order entry (CPOE) is utilized for most other medication orderables. A chemotherapy-dedicated pharmacist is scheduled daily from 0630 to 1700 to direct order review, order entry, and product preparation of anticancer treatments. This schedule was implemented June 30, 2010, with the opening of a new infusion center pharmacy. Prior to that date, sterile product and general staff pharmacists provided coverage of an oncology infusion center pharmacy on business days; chemotherapy orders were handled in the sterile products area at other times. The sterile products pharmacist handles chemotherapy orders when the chemotherapy pharmacist is unavailable. A general staff pharmacist handles chemotherapy orders when the sterile products pharmacist is unavailable. The pharmacy department handles chemotherapy order review for hospitalized adult oncology patients as delineated by the American Society of Health-System Pharmacists guidelines on preventing medication errors with chemotherapy products. Pharmacist double check is accomplished by collaboration between the chemotherapy pharmacist or sterile products pharmacist and another staff pharmacist.
Historically at Spectrum Health Hospitals, order entry of single-agent, high-dose methotrexate used for the treatment of hospitalized adult oncology patients was performed with designation of a specified date and time for chemotherapy administration following documentation of the target urine output and pH. Due to the variable time frame during which pretreatment parameters were met, use of this method resulted in 12% of orders being entered between the hours of 2300 and 06599; there was a perception that many chemotherapy orders were being handled by general staff instead of pharmacists dedicated to chemotherapy preparation. To address these concerns, the pharmacy department changed the method for order entry of single-agent, high-dose methotrexate to improve the dynamics of workflow and support safe medication practice. Interdisciplinary collaboration and education supported the use of the new method for order entry of single-agent, high-dose methotrexate to treat hospitalized adult patients beginning February 1, 2010.
Historically, pharmacist review and order entry of single-agent, high-dose methotrexate would happen once the patient’s urine output and pH were at the designated target values and otherwise the patient was ready for drug administration. The methotrexate and ancillary medications were entered in Cerner Millenium Pharmacy Medication Manager for administration to hospitalized adult oncology patients on designated dates and times. Following pharmacist order entry, the methotrexate and ancillary medications are viewable in the electronic medication administration record (MAR), which allows nursing to double check the active orders relative to the original chemotherapy order submitted by the medical oncologist. Due to the parameter-dependent timeframe for methotrexate administration, this process generated a cumbersome amount of high-alert medication order processing at variable and possibly inopportune times. To improve the dynamics of workflow and support safe medication practice, the pharmacy department modified the method of order entry for hospitalized adult oncology patients receiving single-agent, high-dose methotrexate, allowing pharmacist order entry to occur at a time much earlier than product preparation and administration without having a deleterious effect on the interdisciplinary chain of safe medication practice. The fundamental difference between the old and new methods for order entry is use of the “unscheduled” frequency of medication administration to replace the administration frequency of “once” with a specified date and time. Use of the unscheduled frequency for order entry provides an active medication in the MAR that remains in the current view until it is charted at the time of administration by nursing; at that point, the MAR entry moves to the section for discontinued medications with the date and time stamp charted by nursing for administration. Antiemetics administered as chemotherapy premedication are also entered using the unscheduled frequency. Leucovorin rescue is initially entered with a scheduled date and time to begin on the day following anticipated methotrexate administration. Immediately after the leucovorin is entered, it is put into a “suspended” state by the pharmacist to prevent inadvertent premature initiation of therapy. Following methotrexate administration, the leucovorin entry is modified by the chemotherapy pharmacist such that the first infusion is timed for 24 hours after the charted methotrexate infusion or as otherwise specified by the prescriber. Pro re nata medications ordered as part of the chemotherapy regimen are entered at the time of pharmacist order review. Alkaline hydration is ordered by the prescriber using CPOE, which facilitates prompt initiation of this therapy. Routine home medications are entered by the prescriber using CPOE.
The primary objective of this analysis was to determine whether our process change impacted the proportion of orders for single-agent, high-dose methotrexate entered by the chemotherapy pharmacists versus sterile products or general staff pharmacists. A secondary objective was to analyze the time of day that orders for single-agent, high-dose methotrexate were entered. Our project also evaluated whether antiemetic premedication and leucovorin rescue were administered in an appropriately timed manner with our new method of order entry.
METHODS
Hospitalized adult patients treated with single-agent, high-dose methotrexate during the time frame of January 1, 2008 to December 31, 2012 were identified retrospectively from pharmacy records by an electronic medication use search. Order entry of methotrexate and ancillary medications was examined for use of the old method or the new method. Order entry was examined to determine whether or not it was performed by a chemotherapy pharmacist. During the period following June 30, 2010, work performed by a chemotherapy pharmacist was determined by the name of the person attached to order entry. During the period of January 1, 2008
to January 31, 2010, when sterile products and general staff pharmacists rotated through the infusion center pharmacy, the time frame of 0700 to 1459 was used as a surrogate marker to determine order entry by a chemotherapy pharmacist. Encounters between the dates of February 1, 2010 and June 29, 2010 were censored from analysis because data from this period represented the new method of order entry prior to designation of specific personnel for chemotherapy order entry.
The date and time of methotrexate order entry were collected; these data were categorized by occurrence during the time intervals of 0700-1459, 1500-2259, or 2300-0659. The coordination of antiemetic premedication and leucovorin rescue relative to methotrexate administration were assessed for cases with order entry using the new method. Antiemetic premedication administration occurring within 60 minutes prior to methotrexate administration was tallied. Initiation of leucovorin rescue 23 to 25 hours following the start time of methotrexate, or as otherwise ordered by the prescriber, was tallied.
Chi-square analysis was performed for the primary objective. Observational statistics were performed for secondary endpoints. The project was approved with waiver of consent/HIPAA authorization by the institutional review board.
RESULTS
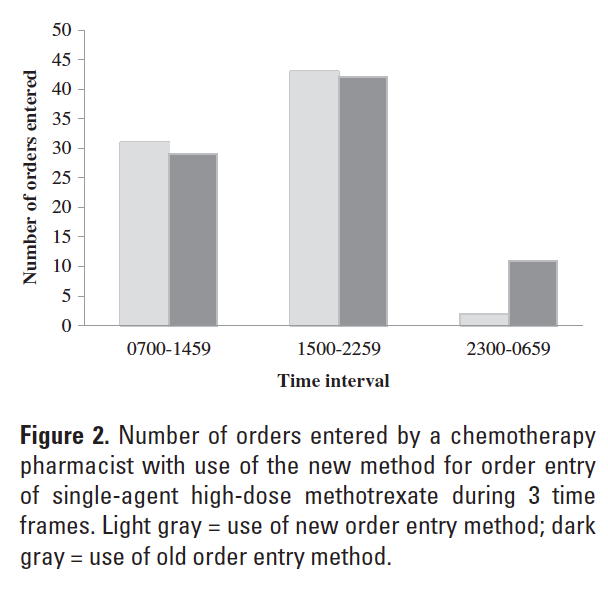
A total of 182 patient encounters for administration of single-agent, high-dose methotrexate were identified retrospectively. Twenty-four cases were censored. Twenty-three cases were censored because they occurred during the time frame of February 1, 2010 to June 29, 2010. One case was censored due to incomplete data. The new method of order entry was used for 76 of 158 evaluable encounters (48%). A significantly greater proportion of orders entered using the new method was handled by a chemotherapy pharmacist (P < .0001) (Figure 1). The proportion of orders entered by a chemotherapy pharmacist increased during the hours of 0700-2259 and decreased during the hours of 2300-0659 with use of the new method for order entry of single-agent, high-dose methotrexate (Figure 2). Examination of order entry times demonstrated that during the period beginning June 30, 2010, all orders for single-agent, high-dose methotrexate entered during the time frame of 1500-2259 (41 orders) were done so using the new method of order entry. Of these, 24 orders were entered during the time frame of 1500-1700 (21 orders) or shortly after 1700 by a chemotherapy--dedicated pharmacist (3 orders). The remaining 17 orders, which represented 41% of this subset, were entered during the evening work shift by pharmacists not routinely dedicated to chemotherapy order management. Two orders for single-agent, high-dose methotrexate that were entered after the date of June 30, 2010 were done so during the time frame of 2300-0659. The exact time of methotrexate order entry was 2311 and 2340 for these occurrences, which is thought to represent extended work on the part of a pharmacist scheduled during the evening shift versus shifted work to the formal 2300-0659 work interval.
Administration of antiemetic premedication within 60 minutes preceding the charted start time for methotrexate infusion was documented for 73 of 76 evaluable encounters (96%) processed using the new method of order entry. One case fell outside of the predetermined 60-minute interval because administration of antiemetic premedication was charted 62 minutes before the start of methotrexate infusion. Two cases were designated as outliers due to ambiguous charting that confounded data collection. Both of the cases with ambiguous antiemetic premedication charting were examined for evidence of chemotherapy-induced nausea and vomiting. For both cases, there were no reports of nausea or vomiting within 24 hours following methotrexate administration documented by the physician, advanced practice provider, or staff nurse and no pro re nata antiemetic were administered. Administration of the first dose of leucovorin rescue was documented within 23 to 25 hours following the start of the methotrexate infusion, or as ordered by the physician, for 76 of 76 (100%) of evaluable encounters using the new method of order entry.
DISCUSSION
Use of the new method for order entry of single-agent, high-dose methotrexate supports safe medication practice. One essential aspect of the new method is that it maintains the interdisciplinary continuum of chemotherapy order verification. In fact, with the use of the new method, the chemotherapy order can be evaluated by pharmacist and nurse checkpoints in a less time-pressured manner than occurred with the old method. Another key aspect is that use of the new method directs workflow such that complex chemotherapy orders are reviewed and managed by pharmacy personnel dedicated to this process instead of defaulting to an arbitrary assignment based on the timing of auxiliary events.
Use of the new method for order entry of single-agent, high-dose methotrexate facilitates well-timed order verification with respect to the overall itinerary of the patient’s hospital stay. The duration of hospitalization for patients admitted for treatment with single-agent, high-dose methotrexate is influenced by the time required to coordinate delivery of their treatments in addition to the time required for appropriate urinary alkalinization and hydration.6 The new method of order entry facilitates the multifaceted process of pharmacist and nursing chemotherapy order verification while the patient is receiving initial alkaline hydration.
Different elements may have impacted the proportion of single-agent, high-dose methotrexate orders entered by a chemotherapy-dedicated pharmacist. The primary one was the adoption of the new method for order entry of single-agent, high-dose methotrexate. The main advantage conferred by the use of the new method for order entry was flexibility to the timing of interdisciplinary chemotherapy order review and verification without weakening the stringency of these checkpoints. The second was expansion of the time frame for utilization of a chemotherapy-dedicated pharmacist from an 8-hour (0700-1459; surrogate marker) to a 10-hour (0630-1700 daily) work shift. Implementing a 10-hour work shift was complementary to supporting patient care in the infusion center and beneficial to the organization of tasks and workload. With the new method of order entry, 59% of orders entered during the time interval of 1500-2259 were done so by a chemotherapy pharmacist; however, retrospective analysis cannot definitively delineate the effect of extending the chemotherapy pharmacist shift by 2 hours versus increased work flexibility with the new method for order entry. One caveat to this report is that the author did not verify dates that the front-line chemotherapy pharmacists were concurrently not working. This is an infrequent event because 2 pharmacists share an alternating 7-day-on/7-day-off schedule to provide primary coverage for the daily 10-hour shift that dedicates a pharmacist to chemotherapy order processing. However, it does introduce the possibility of spuriously increasing that tally of single-agent, high-dose methotrexate orders that were not entered by a chemotherapy pharmacist for the chronologic period beginning June 30, 2010.
Ideally, all orders for high-risk and complex chemotherapy treatments should be entered when a pharmacist dedicated to checking and processing these orders is available. However, the variable timing of high-dose methotrexate administration relative to hydration and urinary alkalinization makes this challenging. One option is to delay chemotherapy
preparation until the following day for orders received after a designated time. However, a major drawback to this is the potential for prolonging the duration of hospitalization, which increases the cost of health care delivery and can inadvertently delay provision of care to other patients requiring admission to a busy medical center. Additional factors that can impact the timing of single-agent, high-dose methotrexate administration include tight bed availability, findings on the initial clinical evaluation, intravenous access issues, staffing levels, and availability of radiographic or laboratory results. All of which add to the variability of administration time for single-agent, high-dose methotrexate. It is important to identify and evaluate process-related events that prolong hospitalization, increase the cost of providing care, and can inadvertently delay the care of other patients.
CONCLUSION
The proportion of orders for single-agent, high-dose methotrexate entered by a chemotherapy-dedicated pharmacist versus sterile products or staff pharmacist was significantly greater with use of the unscheduled frequency in Cerner Millenium Pharmacy Medication Manager. Administration of antiemetic premedication and leucovorin rescue were appropriately coordinated with use of the new system for order entry of single-agent, high-dose methotrexate.
REFERENCES
- American Society of Health-System Pharmacists. ASHP guidelines on preventing medication errors with antineoplastic agents. Am J Health Syst Pharm. 2002;59:1648-1668.
- Jacobson JO, Polovich M, Gilmore TR, et al. Revisions to the 2009 American Society of Clinical Oncology/Oncology Nursing Society Chemotherapy Administration Safety Standards: Expanding the Scope to Include Inpatient Settings. J Oncol Prac. 2011;8:2-6.
- Jacobson JO, Polovich M, McNiff KK,et al. American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards. J Clin Oncol. 2009;27:5469-5475.
- Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694-703.
- Methotrexate [package insert]. Lakefield, IL; Hospira, Inc; Oct 2011.
- Camitta BM, Holcenberg JS. Safety of delayed leucovorin “rescue” following high-dose methotrexate in children. Med Pediatr Oncol. 1978;5(1):55-59.
- Sirotnak FM, Moccio DM, Dorick DM. Optimization of high dose methotrexate with leucovorin rescue therapy in the L1210 Leukemia and Sarcoma 180 murine tumor models. Cancer Res. 1978;38:345-353.
- Kintzel PE, Campbell AD, Yost KJ, et al. Reduced time for urinary alkalinization before high dose methotrexate with preadmission oral bicarbonate. J Oncol Pharm Prac. 2012;18:239-244.
- Kintzel PE, VanDyke TH, Athmann PW, Bonter MP, Mills LB. Improved pharmacy department workflow with order entry project for single agent high dose methotrexate. Presented at: 2011 American Society of Hospital Pharmacy Summer Meeting; June 13, 2011; Denver, CO.

*Department of Pharmacy, Spectrum Health Hospitals, Grand Rapids, Michigan; †Department of Pharmacy, Coram Healthcare, Oklahoma City, Oklahoma; ‡Dell Services, Caledonia, Michigan; §Department of Pharmacy, Trihealth, Cincinnati, Ohio; ¶Department of Pharmacy, Swedish Health Services, Seattle, Washington. Corresponding author:Polly Kintzel, PharmD, Department of Pharmacy, Spectrum Health Hospitals, 100 Michigan Street, NE, MC 001, Grand Rapids, MI 49503; phone: 616-391-3159; e-mail: polly.kintzel@spectrum-health.com