Original Article
Economic Impact of Converting from Pen and 10-mL Vial to
3-mL Vial for Insulin Delivery in a Hospital Setting
Elizabeth Eby, MPH*; Lee Smolen, BSEE†; Amber Pitts, BA†; Linda A. Krueger, MHA*; and
Doneen Grimm, PharmD‡
Original Article
Economic Impact of Converting from Pen and 10-mL Vial to
3-mL Vial for Insulin Delivery in a Hospital Setting
Elizabeth Eby, MPH*; Lee Smolen, BSEE†; Amber Pitts, BA†; Linda A. Krueger, MHA*; and
Doneen Grimm, PharmD‡
Original Article
Economic Impact of Converting from Pen and 10-mL Vial to
3-mL Vial for Insulin Delivery in a Hospital Setting
Elizabeth Eby, MPH*; Lee Smolen, BSEE†; Amber Pitts, BA†; Linda A. Krueger, MHA*; and
Doneen Grimm, PharmD‡
Abstract
Purpose: To compare the impact on acquisition cost and purchased volume of rapid‑ and short-acting insulins following conversion from 3-mL disposable pens and 10-mL vials to 3‑mL vials for individual patient supply (IPS) in a hospital setting.
Methods: On February 1, 2010, St. Joseph’s Hospital and Medical Center of Dignity Health in Phoenix, Arizona, converted from pens to 3-mL vials for IPS subcutaneous (SC) injection and from 10-mL short-acting insulin vials to 3-mL vials for intravenous (IV) preparation. Pharmacy purchasing data were analyzed over 6-month periods before and after conversion (March 1 through August 31, 2009, and March 1 through August 31, 2010).
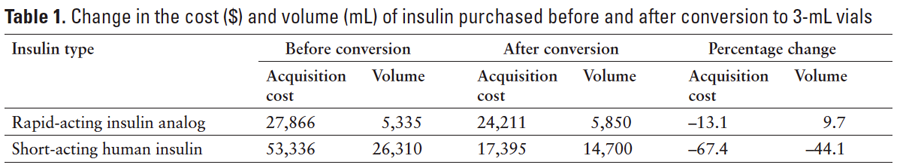
Results: Before conversion, acquisition costs were $27,866 for 5,335 mL of rapid-acting insulins and $53,336 for 26,310 mL of short-acting insulins. After conversion, insulin acquisition costs were $24,211 for 5,850 mL of rapid-acting insulins (13.1% decrease in costs, 9.7% rise in volume), with cost reduction attributable to the lower cost of 3‑mL vials. Acquisition costs were $17,395 for 14,700 mL of short-acting insulins after conversion (67.4% decrease in costs, 44.1% reduction in volume), with cost reduction attributable to lower cost of 3-mL vials versus pens for IPS SC injections and 10-mL vials for IV preparation. The reduction in purchased volumes of short-acting insulins may be partly due to decreased insulin use in IV preparation.
Conclusion: Conversion from pens and 10-mL vials to 3-mL vials for rapid- and short- acting insulins resulted in reduced acquisition costs and decreased use of short-acting insulin in IV preparations.
Key Words—3-mL vial, conversion, cost, insulin, pens
Hosp Pharm—2014;49:1033–1038
Abstract
Purpose: To compare the impact on acquisition cost and purchased volume of rapid‑ and short-acting insulins following conversion from 3-mL disposable pens and 10-mL vials to 3‑mL vials for individual patient supply (IPS) in a hospital setting.
Methods: On February 1, 2010, St. Joseph’s Hospital and Medical Center of Dignity Health in Phoenix, Arizona, converted from pens to 3-mL vials for IPS subcutaneous (SC) injection and from 10-mL short-acting insulin vials to 3-mL vials for intravenous (IV) preparation. Pharmacy purchasing data were analyzed over 6-month periods before and after conversion (March 1 through August 31, 2009, and March 1 through August 31, 2010).
Results: Before conversion, acquisition costs were $27,866 for 5,335 mL of rapid-acting insulins and $53,336 for 26,310 mL of short-acting insulins. After conversion, insulin acquisition costs were $24,211 for 5,850 mL of rapid-acting insulins (13.1% decrease in costs, 9.7% rise in volume), with cost reduction attributable to the lower cost of 3‑mL vials. Acquisition costs were $17,395 for 14,700 mL of short-acting insulins after conversion (67.4% decrease in costs, 44.1% reduction in volume), with cost reduction attributable to lower cost of 3-mL vials versus pens for IPS SC injections and 10-mL vials for IV preparation. The reduction in purchased volumes of short-acting insulins may be partly due to decreased insulin use in IV preparation.
Conclusion: Conversion from pens and 10-mL vials to 3-mL vials for rapid- and short- acting insulins resulted in reduced acquisition costs and decreased use of short-acting insulin in IV preparations.
Key Words—3-mL vial, conversion, cost, insulin, pens
Hosp Pharm—2014;49:1033–1038
Abstract
Purpose: To compare the impact on acquisition cost and purchased volume of rapid‑ and short-acting insulins following conversion from 3-mL disposable pens and 10-mL vials to 3‑mL vials for individual patient supply (IPS) in a hospital setting.
Methods: On February 1, 2010, St. Joseph’s Hospital and Medical Center of Dignity Health in Phoenix, Arizona, converted from pens to 3-mL vials for IPS subcutaneous (SC) injection and from 10-mL short-acting insulin vials to 3-mL vials for intravenous (IV) preparation. Pharmacy purchasing data were analyzed over 6-month periods before and after conversion (March 1 through August 31, 2009, and March 1 through August 31, 2010).
Results: Before conversion, acquisition costs were $27,866 for 5,335 mL of rapid-acting insulins and $53,336 for 26,310 mL of short-acting insulins. After conversion, insulin acquisition costs were $24,211 for 5,850 mL of rapid-acting insulins (13.1% decrease in costs, 9.7% rise in volume), with cost reduction attributable to the lower cost of 3‑mL vials. Acquisition costs were $17,395 for 14,700 mL of short-acting insulins after conversion (67.4% decrease in costs, 44.1% reduction in volume), with cost reduction attributable to lower cost of 3-mL vials versus pens for IPS SC injections and 10-mL vials for IV preparation. The reduction in purchased volumes of short-acting insulins may be partly due to decreased insulin use in IV preparation.
Conclusion: Conversion from pens and 10-mL vials to 3-mL vials for rapid- and short- acting insulins resulted in reduced acquisition costs and decreased use of short-acting insulin in IV preparations.
Key Words—3-mL vial, conversion, cost, insulin, pens
Hosp Pharm—2014;49:1033–1038
Hosp Pharm 2014;49(11):1033–1038
2014 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj4911-1033


In the inpatient setting, a common practice for the management of patients with diabetes is to use insulin from 10‑mL vials of floor stock (FS). The advantage of FS is that it alleviates the burden of tracking each uniquely labeled individual patient supply (IPS) and the varieties of insulins and delivery systems used. However, several organizations recommend the use of IPS. A key disadvantage of IPS use is increased waste. One study reported 25% waste in a hospital setting when 10‑mL vials served as FS and an estimated 90% waste when IPS consisted of 10‑mL vials.1 There have been reports of insulin pens being used on multiple patients, leading to accidental exposure to human immunodeficiency virus2and bloodborne pathogens.3The Institute for Safe Medication Practices (ISMP) is now recommending against the use of insulin pens in the inpatient setting.3
One option for enhancing the economic efficiency of IPS insulin may be the use of 3‑mL sizes, which were introduced for hospital use by Eli Lilly and Company in 2010. A study modeling the costs associated with conversion to 3-mL vials estimated savings across multiple conversion scenarios, including from 10-mL to 3-mL FS and from 10-mL to 3‑mL IPS.1 Ward and Aton also reported results of a study showing that insulin pen use was associated with greater cost savings than with 10‑mL FS vials.4 However, the published literature does not include any real-world studies comparing pens and 3‑mL IPS vials.
Insulin pens were introduced nearly 30 years ago to improve the accuracy and ease of self-administration of insulin, as well as to improve patient compliance.5 Although safety needles are used in hospitals to protect the caregiver when delivering injections, accidental needlestick injuries do occur, both with pens and syringes. Ward and Aton4 reported a reduction in needlestick injuries after conversion from 10-mL vials to pens but noted that the study was conducted shortly after a focused education program was provided to hospital staff, and the subsequent heightened staff attentiveness may have had a favorable impact on study results.
The purpose of this study was to examine the impact on acquisition costs and needlestick injuries of converting from administration of rapid-acting insulin analog (RAIA) and short-acting human insulin (SAHI) from prefilled pens to 3-mL IPS vials and of SAHI from 10-mL to 3‑mL vials for intravenous (IV) preparation in a hospital setting.
METHODS
St. Joseph’s Hospital and Medical Center of Dignity Health is a 607-bed community hospital in Phoenix, Arizona. Beginning February 1, 2010, the hospital converted from prefilled pens to 3-mL IPS vials for IPS subcutaneous (SC) injection of RAIA and SAHI and from 10-mL SAHI vials to 3‑mL vials for IV preparation for treatment of patients. In a study conducted with the approval of the St. Joseph’s Hospital and Medical Center institutional review board, the aggregate costs of insulin acquisition and the volumes purchased were analyzed over a 6‑month period before and after the conversion. To eliminate the potential impact of seasonal variations that might affect patient discharge volumes, costs were compared for matching time periods: March 1, 2009 through August 31, 2009 before the conversion and March 1, 2010 through August 31, 2010 after the conversion. The hospital confirmed that the same insulin delivery protocols impacting the use of RAIAs and SAHIs were in effect during the 2 analyzed 6‑month time periods. The facility’s patient capacities were also the same for the 2 analysis periods.
Pharmacy cost data are not linked to individual patients; thus, pharmacy acquisition data were used to assess the insulin costs and units purchased. The primary outcome of interest was the difference in cost before and after conversion. The insulin acquisition data also included the designation as to whether 340B pricing was in effect for each purchase. The impact of 340B pricing, as well as any other price differences between the insulins across the study period, was incorporated into the analyses.
Monthly insulin purchase data from the two 6-month time periods evaluated were utilized in statistical analysis using the STATA data analysis and statistical software package(Stata/SE Version 12.1 for Windows; StataCorp LP, College Station, TX). The analyses performed were 2-tailed, paired t test analyses, with alpha equal to 0.05 (95% confidence interval).
For the RAIAs, the purpose of the statistical analysis was to validate the assumption that there was no significant difference in the paired monthly insulin volumes purchased between the two 6-month periods from consecutive years. The assumed equivalence in volumes purchased was based on the substitution of IPS 3-mL vials for IPS 3-mL pens. The average monthly purchases for the 2 consecutive years were 889.2 mL and 975.0 mL, respectively. No statistically significant difference was found (P = .55).
For the SAHIs, the purpose of the analysis was to validate the assumption that there was no significant difference in the paired monthly total number of insulin devices (3‑mL pens and 10‑mL vials compared with 3‑mL vials) purchased between the two 6‑month periods. The assumed equivalence in insulin devices purchased was based on the substitution of IPS 3-mL pens (SC use) and 10-mL vials (IV preparation) with 3-mL vials for both SC use (IPS) and IV preparation. The average monthly purchases for the 2 consecutive years were 859.7 devices and 816.7 devices, respectively. No statistically significant difference was found (P = .67).
In addition, an analysis was performed to validate the assumption that there was a significant difference in the paired monthly SAHI volumes purchased between the two 6‑month periods. The assumed decrease in volumes purchased was based on the substitution of 3-mL vials for 10‑mL vials in IV preparation. The average monthly purchases for the 2 consecutive years were 4,385 mL and 2,450 mL, respectively, which were statistically significantly different (P < .01).
Based on the statistical analyses performed, it was determined that overall RAIA and SAHI use was sufficiently similar between the two 6-month time periods to allow for evaluation of the impact of conversion to IPS 3-mL vials on insulin acquisition costs.
In addition, employee incident data were collected to assess needlestick injuries for all medications and for insulins specifically. These events, however, were not reported for the specific medication/insulin brand involved.
RESULTS
Economic Outcomes
Acquisition costs for RAIAs and SAHIs for the 6-month period before and after conversion to 3-mL IPS vials are summarized in Table 1. The volume of RAIA purchased increased from 5,335 mL to 5,850 mL (9.7%) after conversion while the volume of SAHI decreased from 26,310 mL to 14,700 mL (‑44.1%). However, acquisition costs decreased for both the RAIA (‑13.1%) and SAHI (‑67.4%) after the conversion. The hospital did not report any changes to its insulin delivery protocols during the study period.
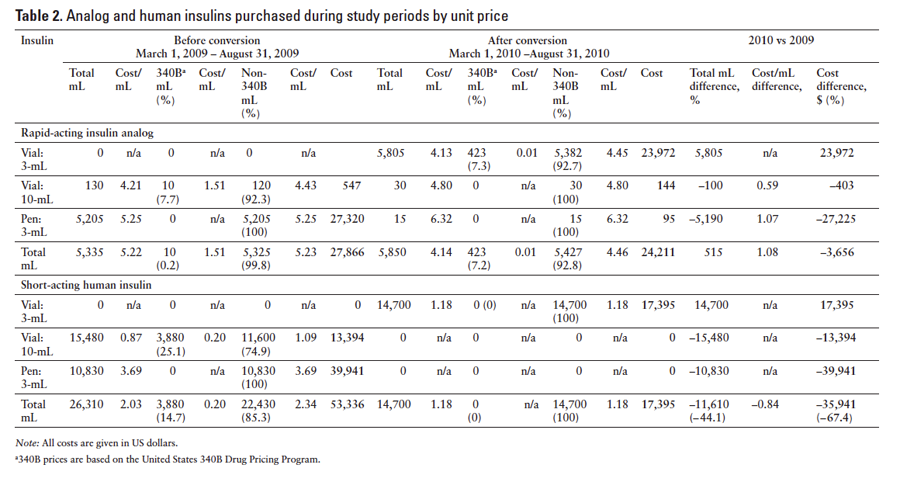
Table 2 summarizes the volume and costs of RAIA and SAHI before and after conversion to the 3-mL vials broken out by cost per milliliter and rate. The majority of insulin purchased was SAHI. In the preconversion period, $53,336 was spent on 26,310 mL of SAHI. Although 58.8% of the SAHI acquired (15,480 mL) was in 10‑mL vials, 74.9% of the expense ($39,941) was for purchase of insulin pens. After conversion to the 3‑mL vials, 14,700 mL of SAHI was purchased for $17,395, which was a decrease in cost of 67.4%, for a savings of $35,941. Notably, 3,880 mL of SAHI (14.7% of the total) acquired in the preconversion period was obtained at the reduced 340B cost. No such 340B cost savings were realized after conversion to the 3‑mL vials.
Although less RAIA than SAHI was acquired during the study periods, RAIA still played a substantial role. In the preconversion period, 5,335 mL of RAIA insulin was acquired at a cost of $27,866. Of this, 97.6% (5,205 mL) was from pens at a cost of $27,320, and 2.4% (130 mL) was in 10‑mL vials at a cost of $547. After conversion to the 3‑mL vials, the amount of RAIA acquired increased to 5,850 mL for a total cost of $24,211, which was a cost decrease of 13.1%, for a savings of $3,656. In the preconversion period, 0.2% (10 mL) of RAIA was obtained at the reduced 340B cost and came from the large vials. In the postconversion period, 7.2% (423 mL) was acquired at 340B costs applied to the 3‑mL vials. No 340B pricing was applied to acquisition costs for the pens either before or after conversion.
Needlestick Injuries
Before conversion to the 3-mL vials, 11 medication-related puncture errors were reported, of which 3 (27.3%) were specific to insulin administration. After conversion, 12 total puncture errors were reported, with 5 (41.7%) related to insulin administration. It is unknown which insulins were involved in the reported needlestick injuries.
DISCUSSION
In all aspects of health care, there is obvious on--going pressure for cost-containment measures. Given the tremendous, worldwide increase in the incidence of diabetes, the economic ramifications of the disease are of acute interest. In the hospital setting, reduction in insulin procurement costs is an important means of curtailing overall diabetes-related costs, assuming similar numbers of patients can be treated using the same delivery protocols, with the same system in place for the purchasing and storage of the insulins.
For RAIAs, the cost difference is primarily due to the conversion from IPS use of 3‑mL pens to less expensive 3-mL vials. Correspondingly, SAHI cost savings are realized from the IPS 3‑mL pen to 3-mL vial conversion. In addition, the conversion from SAHI 10‑mL to 3‑mL vial use in IV preparation results in less purchased insulin volume with corresponding cost savings.
Studies have already shown that conversion from 10‑mL vials to smaller delivery containers can lower costs and reduce waste.1,4 Davis et al6 projected cost reductions for hospitalized patients if pens were used exclusively during their hospital stay. In their budget impact analysis, Lee et al1 reported that not only conversion to smaller 3‑mL vials from larger 10‑mL vials but also conversion from 3‑mL pens to 3‑mL vials resulted in less waste and lower costs.1
The primary purpose of this study was to assess the potential reduction in insulin procurement costs for a hospital that switched from the use of 3‑mL disposable insulin pens to 3‑mL IPS vials. In one hospital, an overall decrease in cost per milliliter of insulin was clearly observed after switching to the smaller 3‑mL IPS vials. For SAHI, conversion to the 3‑mL IPS vials led to a decrease of 67.4%, for a cost savings of $35,941, primarily attributable to the lower acquisition costs of 3‑mL vials compared with the pens. However, the reduction in purchased volumes of SAHI may also partly be due to decreased volume of insulin required for IV insulin preparation because of the use of 3-mL vials.
A secondary endpoint of the study was collection of employee incident data to assess needlestick injuries. The American Society of Health-System Pharmacists expert panel acknowledged that insulin is a high-alert medication in the hospital setting7; in real-world practice, the number of needlestick injuries are presumed to be underreported. In a study of needlestick injuries to nursing staff in nursing homes, the most common injuries involved insulin pens (40.4%), followed by SC injection needles (21.3%), and lancet needles (19.9%).8 Needlestick injuries from insulin pens may result from errors in the user’s technique,9,10 but the variety of pen designs makes it a challenge for health care providers to master all possible devices.9
In this study, the number of overall medication-related punctures increased from 11 to 12, and the number of insulin-related punctures increased from 3 to 5 between the preconversion and postconversion periods. Because the numbers were small and the specific insulins involved in the incidents were unknown, no conclusions can be drawn. It is relevant to note that Ward and Aton4 reported that insulin pen use was associated with fewer needlestick injuries than with 10‑mL vials. However, unlike their study, this study collected data more reflective of natural practice in that the hospital staff had not undergone a focused educational program.
Limitations
Patient-level data were not available for inclusion into this study, so the impact on the number of patients treated, their characteristics, and utilization of insulin during their hospitalizations are unknown. In an effort to overcome some of this bias, data included in the study were from the same seasonal time periods to account for expected seasonal variations. Statistical analyses were performed to justify the assumption of sufficient similarity in overall RAIA and SAHI utilization between the two 6‑month analysis periods. Also, needlestick injuries are voluntary, employee-reported events, and, as such, their incidence is likely underreported. Therefore, the true extent of these events is unknown.
Conclusion
Conversion from pens and 10-mL vials to 3-mL IPS vials for RAIAs and SAHIs resulted in a reduction in acquisition costs for the hospital. Additional research is warranted to examine the insulin administration safety and patient information over the study period.
ACKNOWLEDGMENTS
Financial support/disclosures: Eli Lilly and Company provided financial support for the conduct of this study.
Additional contributions: The authors acknowledge the assistance of Anne S. Packer (INC Research, LLC) and Karla Villines (Eli Lilly and Company) in the preparation of this manuscript.
REFERENCES
- Lee LJ, Smolen LJ, Klein TM, et al. Budget impact analysis of insulin therapies and associated delivery systems. Am J Health Syst Pharm. 2012:69(11):958-965.
- Possible HIV exposure at Buffalo VA hospital. http://www.usatoday.com/story/news/nation/2013/01/13/veterans-hospital-hiv/1831705/. USA Today Web site. Accessed October 2, 2013.
- Ongoing concern about insulin pen reuse shows hospitals need to consider transitioning away from them. Institute for Safe Medication Practices Web site. http://www.ismp.org/Newsletters/acutecare/showarticle.asp?id=41. Accessed October 28, 2013.
- Ward LG, Aton SS. Impact of an interchange program to support use of insulin pens. Am J Health Syst Pharm. 2011;68(14):1349-1352.
- Selam JL. Evolution of diabetes insulin delivery devices.
J Diabetes Sci Technol. 2010;4(3):505-513. - Davis EM, Christensen CM, Nystrom KK, Foral PA, Destache C. Patient satisfaction and costs associated with insulin administered by pen device or syringe during hospitalization. Am J Health Syst Pharm. 2008;65(14):1347-1357.
- Cobaugh DJ, Maynard G, Cooper L, et al. Enhancing insulin-use safety in hospitals: Practical recommendations from an ASHP Foundation expert consensus panel. Am J Health Syst Pharm. 2013;70(16):1404-1413.
- Kiss P, De Meester M, Braeckman L. Needlestick injuries in nursing homes: The prominent role of insulin pens. Infect Control Hosp Epidemiol. 2008;29(12):1192-1194.
- Grissinger M. Avoiding problems with insulin pens in the hospital. PT. 2011:36(10):615-616.
- Insulin pens: Technology is not without imPENding risks. Diabetes in Control Web site. http://www.diabetesincontrol.com/articles/diabetes-news/12891--insulin-pens-technology. Accessed October 28, 2013.

*Eli Lilly and Company, Indianapolis, Indiana; †Medical Decision Modeling Inc., Indianapolis, Indiana; ‡St. Joseph’s Hospital and Medical Center of Dignity Health, Phoenix, Arizona. Corresponding author:Doneen Grimm, PharmD, St. Joseph’s Hospital and Medical Center of Dignity Health, 350 West Thomas Rd., Phoenix, AZ 85013; phone: 602-406-4273; e-mail: Doneen.Grimm@DignityHealth.org