Health Economic Impact and Prospective Clinical Utility of Oncotype DX® Genomic Prostate Score
David Albala, MD,1 Michael J. Kemeter, MSPAS,2 Phillip G. Febbo, MD,2 Ruixiao Lu, PhD,2 Vincy John,3 Dylan Stoy,1 Bela Denes, MD,2 Marybeth McCall, MD,3 Alan W. Shindel, MD,2 Frank Dubeck, MD3
1Associated Medical Professionals of NY, PLLC, Syracuse, NY; 2Genomic Health, Inc., Redwood City, CA; 3Excellus BlueCross BlueShield, Rochester, NY
Prostate cancer (CaP) will be diagnosed in approximately 181,000 American men in 2016. Despite the high number of deaths from CaP in the United States, the disease has a protracted natural history and many men diagnosed with CaP will not die of the disease regardless of treatment. Unfortunately, identification of men with truly indolent/nonaggressive CaP is challenging; limitations of conventional diagnostic modalities diminish the ability of physicians to accurately stage every case of CaP based on biopsy results alone. The resulting uncertainty in prognosis may prompt men with low-risk CaP to proceed to morbid and expensive treatments for an unclear survival benefit. Incorporation of the Genomic Prostate Score (GPS) as part of the decision algorithm for patients with National Comprehensive Cancer Network very low-risk and low-risk cancer led to a substantial increase in uptake of active surveillance and substantial cost savings. GPS provides physicians and patients with an additional tool in assessing personalized risk and helps guide individual decision making.
[Rev Urol. 2016;18(3):123-132 doi: 10.3909/riu0725]
© 2016 MedReviews®, LLC
Health Economic Impact and Prospective Clinical Utility of Oncotype DX® Genomic Prostate Score
David Albala, MD,1 Michael J. Kemeter, MSPAS,2 Phillip G. Febbo, MD,2 Ruixiao Lu, PhD,2 Vincy John,3 Dylan Stoy,1 Bela Denes, MD,2 Marybeth McCall, MD,3 Alan W. Shindel, MD,2 Frank Dubeck, MD3
1Associated Medical Professionals of NY, PLLC, Syracuse, NY; 2Genomic Health, Inc., Redwood City, CA; 3Excellus BlueCross BlueShield, Rochester, NY
Prostate cancer (CaP) will be diagnosed in approximately 181,000 American men in 2016. Despite the high number of deaths from CaP in the United States, the disease has a protracted natural history and many men diagnosed with CaP will not die of the disease regardless of treatment. Unfortunately, identification of men with truly indolent/nonaggressive CaP is challenging; limitations of conventional diagnostic modalities diminish the ability of physicians to accurately stage every case of CaP based on biopsy results alone. The resulting uncertainty in prognosis may prompt men with low-risk CaP to proceed to morbid and expensive treatments for an unclear survival benefit. Incorporation of the Genomic Prostate Score (GPS) as part of the decision algorithm for patients with National Comprehensive Cancer Network very low-risk and low-risk cancer led to a substantial increase in uptake of active surveillance and substantial cost savings. GPS provides physicians and patients with an additional tool in assessing personalized risk and helps guide individual decision making.
[Rev Urol. 2016;18(3):123-132 doi: 10.3909/riu0725]
© 2016 MedReviews®, LLC
Health Economic Impact and Prospective Clinical Utility of Oncotype DX® Genomic Prostate Score
David Albala, MD,1 Michael J. Kemeter, MSPAS,2 Phillip G. Febbo, MD,2 Ruixiao Lu, PhD,2 Vincy John,3 Dylan Stoy,1 Bela Denes, MD,2 Marybeth McCall, MD,3 Alan W. Shindel, MD,2 Frank Dubeck, MD3
1Associated Medical Professionals of NY, PLLC, Syracuse, NY; 2Genomic Health, Inc., Redwood City, CA; 3Excellus BlueCross BlueShield, Rochester, NY
Prostate cancer (CaP) will be diagnosed in approximately 181,000 American men in 2016. Despite the high number of deaths from CaP in the United States, the disease has a protracted natural history and many men diagnosed with CaP will not die of the disease regardless of treatment. Unfortunately, identification of men with truly indolent/nonaggressive CaP is challenging; limitations of conventional diagnostic modalities diminish the ability of physicians to accurately stage every case of CaP based on biopsy results alone. The resulting uncertainty in prognosis may prompt men with low-risk CaP to proceed to morbid and expensive treatments for an unclear survival benefit. Incorporation of the Genomic Prostate Score (GPS) as part of the decision algorithm for patients with National Comprehensive Cancer Network very low-risk and low-risk cancer led to a substantial increase in uptake of active surveillance and substantial cost savings. GPS provides physicians and patients with an additional tool in assessing personalized risk and helps guide individual decision making.
[Rev Urol. 2016;18(3):123-132 doi: 10.3909/riu0725]
© 2016 MedReviews®, LLC
Key words
Oncotype DX® GPS • Prostate cancer • Genomic testing • Health economics • Clinical utility
Key words
Oncotype DX® GPS • Prostate cancer • Genomic testing • Health economics • Clinical utility
One particularly compelling approach for improving risk assessment in CaP is genomic testing.
The 17-gene Oncotype DX GPS is an analytically validated, quantitative reverse transcription polymerase chain reaction assay that is performed on prostate carcinoma recovered from formalin-fixed, paraffin-embedded prostate biopsy tissue.
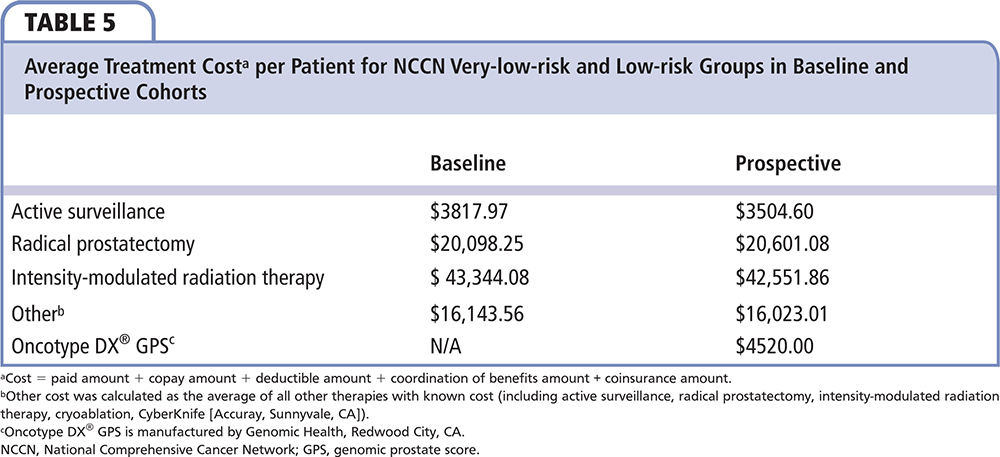
Comparing payer costs in the first 180 days after diagnosis for the baseline and prospective NCCN very-low-risk and low-risk populations, there was an average savings per patient of $2286, including the cost of the GPS of $4520…
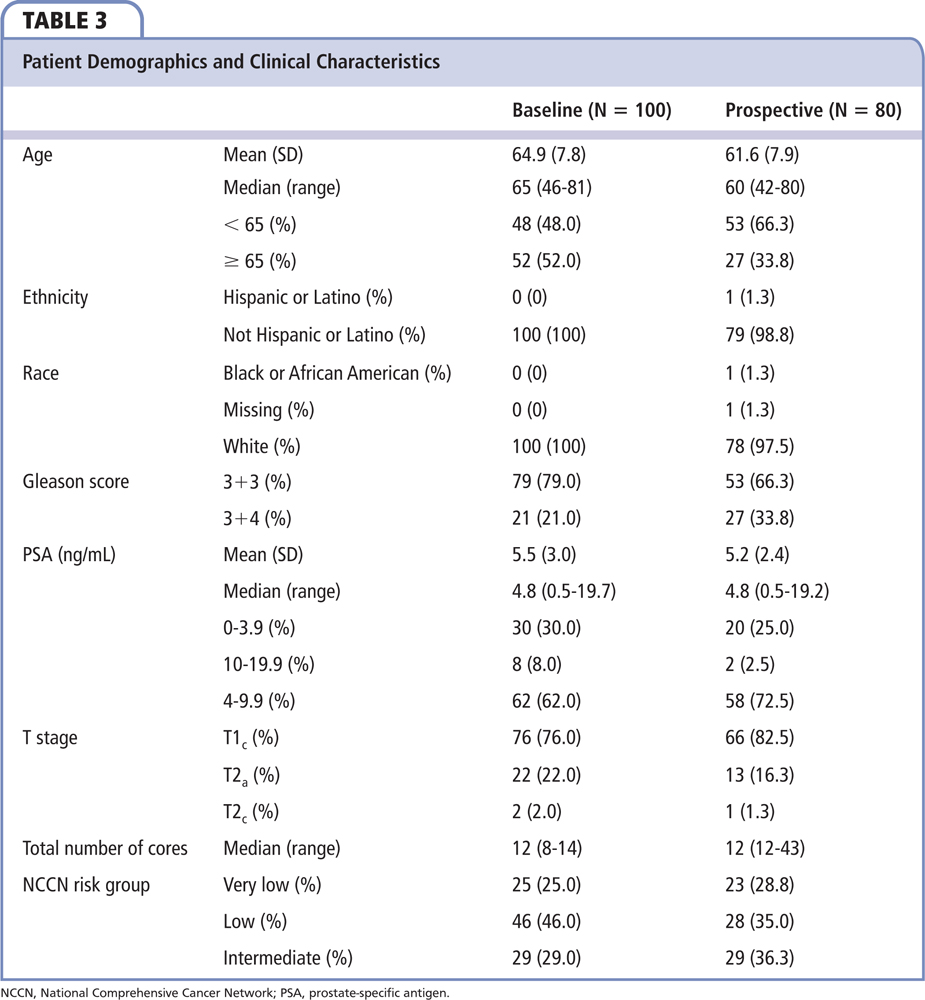
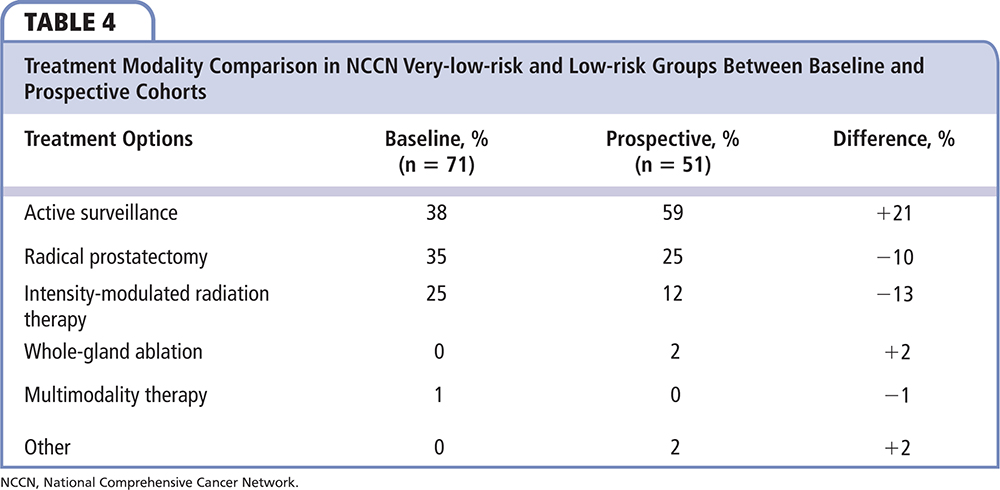
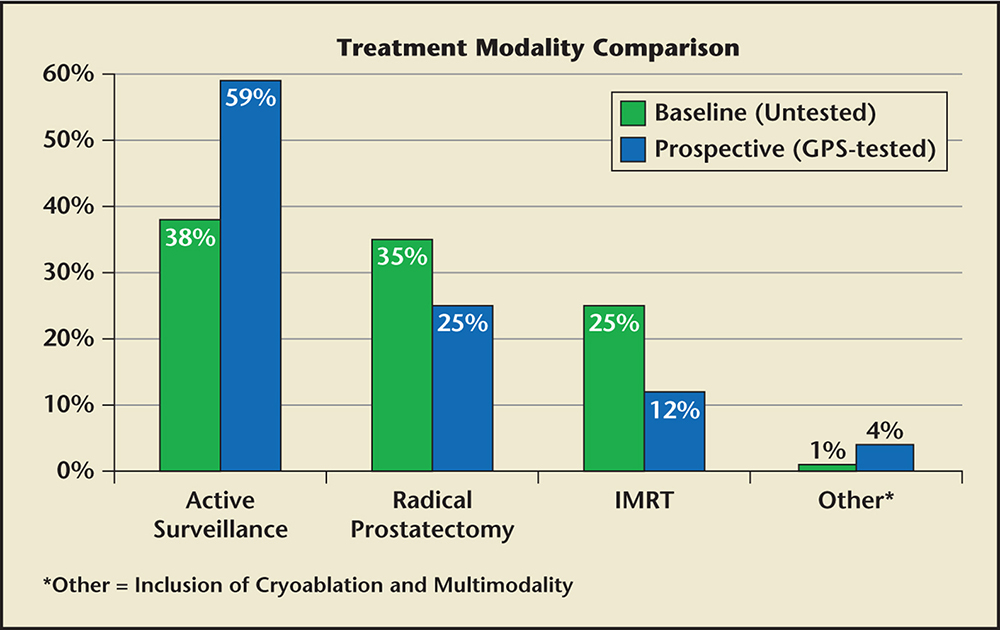
Figure 1. Treatment modality comparison in NCCN very low and low-risk patients between GPS-untested patients (baseline) and GPS-tested patients (prospective). GPS, genomic prostate score; IMRT, intensity-modulated radiation therapy. NCCN, National Comprehensive Cancer Network.

Figure 1. Treatment modality comparison in NCCN very low and low-risk patients between GPS-untested patients (baseline) and GPS-tested patients (prospective). GPS, genomic prostate score; IMRT, intensity-modulated radiation therapy. NCCN, National Comprehensive Cancer Network.
Confidence in management decisions is a key criterion for ensuring recommendation and adherence to AS in men with clinically low-risk CaP.
… a molecular assay such as GPS may be useful in selecting patients for AS and also for confirming which patients are best served with immediate or even adjuvant therapy.
Main Points
• Identification of men with truly indolent/nonaggressive prostate cancer (CaP) is challenging; limitations of conventional diagnostic modalities diminish the ability of physicians to accurately stage every case of CaP based on biopsy results alone. The resulting uncertainty in prognosis may prompt men with low-risk CaP to proceed to morbid and expensive treatments for an unclear survival benefit.
• The goal of the management of CaP is to screen, diagnose, and treat men based on the best available evidence for their individual risk and likelihood of benefit from treatment. With greater certainty regarding prognosis, men with CaP and their health care providers are able to make more confident decisions about the appropriateness of conservative management with active surveillance (AS) versus the advisability of immediate treatment.
• Development of new techniques for identification of patients with truly indolent CaP is a public health priority. To be considered clinically useful, any new technique or intervention must demonstrate that it provides unique information not available with standard clinical parameters alone. One compelling approach for improving risk assessment in CaP is genomic testing. Genomic assays may be performed on serum, urine, or tissue samples to provide information about gene expression in various disease states.
• Incorporation of the Genomic Prostate Score (GPS) as part of the decision algorithm for patients with very low-risk and low-risk cancer led to substantial increase in uptake of AS and substantial cost savings. GPS provides physicians and patients with an additional tool in assessing personalized risk and helps guide individual decision making.
Main Points
• Identification of men with truly indolent/nonaggressive prostate cancer (CaP) is challenging; limitations of conventional diagnostic modalities diminish the ability of physicians to accurately stage every case of CaP based on biopsy results alone. The resulting uncertainty in prognosis may prompt men with low-risk CaP to proceed to morbid and expensive treatments for an unclear survival benefit.
• The goal of the management of CaP is to screen, diagnose, and treat men based on the best available evidence for their individual risk and likelihood of benefit from treatment. With greater certainty regarding prognosis, men with CaP and their health care providers are able to make more confident decisions about the appropriateness of conservative management with active surveillance (AS) versus the advisability of immediate treatment.
• Development of new techniques for identification of patients with truly indolent CaP is a public health priority. To be considered clinically useful, any new technique or intervention must demonstrate that it provides unique information not available with standard clinical parameters alone. One compelling approach for improving risk assessment in CaP is genomic testing. Genomic assays may be performed on serum, urine, or tissue samples to provide information about gene expression in various disease states.
• Incorporation of the Genomic Prostate Score (GPS) as part of the decision algorithm for patients with very low-risk and low-risk cancer led to substantial increase in uptake of AS and substantial cost savings. GPS provides physicians and patients with an additional tool in assessing personalized risk and helps guide individual decision making.
Prostate cancer (CaP) will be diagnosed in approximately 181,000 American men in 2016, making it the most common noncutaneous solid tumor in the United States.1 CaP is also the second leading cause of cancer-related death in American men; 26,000 men are expected to die of CaP in the United States in 2016.1 Despite the high number of deaths from CaP in the United States, the disease has a protracted natural history and many men diagnosed with CaP will not die of the disease regardless of treatment.2 Unfortunately, identification of men with truly indolent/nonaggressive CaP is challenging.3 Limitations of conventional diagnostic modalities diminish the ability of physicians to accurately stage every case of CaP based on biopsy results alone.4 The resulting uncertainty in prognosis may prompt men with low-risk CaP to proceed to morbid and expensive treatments for an unclear survival benefit.2,5
The United States Preventive Services Task Force (USPSTF) and other agencies have recommended against screening for CaP so as to minimize the dual risks of overdiagnosis and overtreatment.5,6 Although this strategy may reduce the number of indolent cancers treated unnecessarily, it deprives men with potentially life-threatening cancers the opportunity for early detection and cure. Bhindi and colleagues7 reported that the post-USPSTF decline in incident CaP was most pronounced in the intermediate- and high-risk categories (median 17.5 cases per month declining to 10 per month) as compared with the low-risk category (median 8.5 cases per month declining to 5.5 cases per month).7 The implication is that even aggressive CaP may often go undetected under the new practice paradigm. Furthermore, given heterogeneity and multifocality of CaP, some men who appear low risk after biopsy results are subsequently found to harbor aggressive lesions with the potential for metastasis.3
The ideal goal for the management of CaP is to screen, diagnose, and treat men based on the best available evidence for their individual risk and likelihood of benefit from treatment. Development of new techniques for identification of patients with truly indolent CaP is a public health priority.8 With greater certainty regarding prognosis, men with CaP and their health care providers are able to make more confident decisions about the appropriateness of conservative management with active surveillance (AS) versus the advisability of immediate treatment.
To be considered clinically useful, any new technique or intervention must demonstrate that it provides unique information not available with standard clinical parameters alone.9 The technique must have clinical utility to influence treatment decisions.9 One particularly compelling approach for improving risk assessment in CaP is genomic testing.10 Genomic assays may be performed on serum, urine, or tissue samples10 to provide information about gene expression in various disease states. In the setting of CaP, molecular diagnostics have been shown to provide prognostic information that is independent from clinical parameters.11,12 Furthermore, molecular diagnostics have demonstrated proven clinical utility in CaP decision making.13,14
Although there is increasing enthusiasm for genomic testing in CaP, the health economic impact remains in question. Economic models are often utilized to estimate or predict the economic impact an intervention (such as molecular diagnostics for CaP) would have on the health care system. Unfortunately, these models are, by necessity, based on a number of assumptions that may not be accurate and may have an adverse impact on both the internal and external validity of health economic studies. For example, a health economics study of genomic testing for decision making in CaP assumed that testing would lead to a greater than fourfold increase in AS utilization among low-risk patients (15% pregenomic test increased to 69% postgenomic test) and a greater than fivefold increase in AS utilization among intermediate-risk patients (5% pregenomic test increased to 27% postgenomic test).15 Such dramatic increases in AS utilization are not consistent with real-world data16 and tend to overstate the economic benefit of genomic tests in CaP.
In this prospective study, we evaluated in a real-world setting the clinical utility and economic impact of the Oncotype DX® Genomic Prostate Score (GPS; Genomic Health, Inc., Redwood City, CA), a 17-gene molecular assay designed to improve risk stratification for men diagnosed with clinically low-risk CaP to inform decisions for initial management. Rather than modelling cost assumptions, the study was conducted at a single large urology group practice (Associated Medical Professionals [AMP], Syracuse, NY) and enrolled patients with a single insurance carrier (Excellus BlueCross BlueShield [BCBS], Rochester, NY). Excellus BCBS calculated cost data from the first 180 days after diagnosis (including the cost of the diagnostic biopsy) and provided the average treatment cost per patient from their analysis, obviating the need for modelling. This analysis compares management patterns and costs from a baseline, untested population to a similar prospective, GPS-tested population. This unique study design allowed us to assess both the clinical utility and economic impact of using GPS in this real-world setting.
Methods
Study Design
Baseline Cohort. A retrospective cohort of 100 clinically low-risk patients (Table 1)—who did not have genomic testing—were identified to provide baseline management patterns at AMP and associated costs. These patients were identified through a review of the electronic medical records at AMP, starting with patients seen on December 31, 2013, and working backward in time until 100 evaluable patients with clinically low-risk CaP were identified. This method of baseline capture helped remove selection bias because the first consecutive 100 cases that met criteria were selected for evaluation. Because molecular markers were commercially available during this 2013 assessment, patients in whom other genomic markers had been used were excluded from the baseline cohort.
Prospective Cohort. The prospective, noninterventional, decision impact and cost study was conducted at AMP. AMP is an integrated urology practice of urologists, pathologists, and radiation oncologists with onsite laboratory and intensity-modulated radiation therapy (IMRT) facilities. To allow collection of cost data, all patients were required to be insured by Excellus BCBS, which insures approximately 1.5 million members across upstate New York.
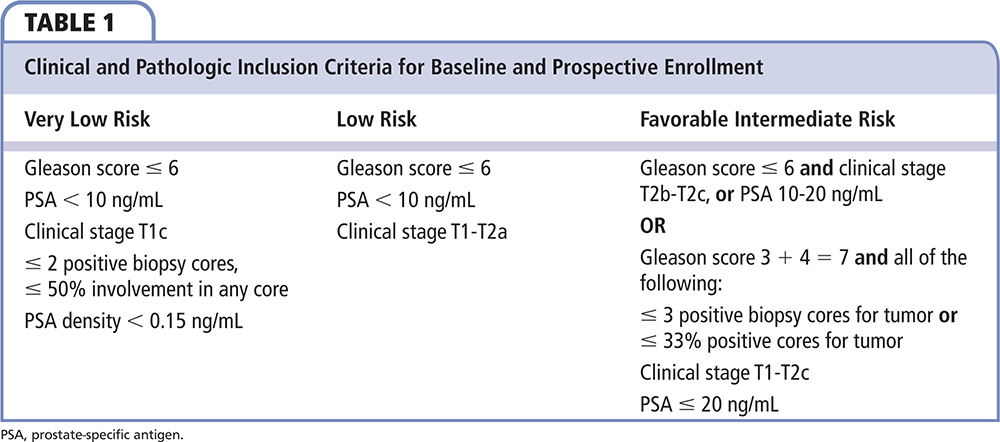
Patients were eligible if they had National Comprehensive Cancer Network (NCCN) very-low-risk, low-risk, or favorable intermediate-risk CaP. For this study, favorable intermediate risk was defined as a prostate-specific antigen (PSA) level of 10.1 to 20 ng/mL, clinical stage T2b-c, and/or biopsy Gleason score of 3+4 with 3 cores and/or 33% positive cores for tumor. Prespecified enrollment targets were a minimum of 15% and a maximum of 30% enrollment for the NCCN very-low-risk and favorable intermediate-risk groups, with the remainder having NCCN low-risk cancer. This prespecified design was done before baseline capture and ensured a more accurate comparison of clinical risk between the baseline and prospective groups. The study actively enrolled patients from July 2, 2014 through March 11, 2016.
All participating physicians were provided education on the development and validation of the Oncotype DX GPS prior to study initiation. Patients who were eligible for the study (new diagnosis of clinically low-risk CaP, seen at AMP for care, and insured by Excellus BCBS) were offered the option of participation by a study coordinator at the research site. Biopsy tissues from patients who agreed to participate were submitted to Genomic Health, Inc. for Oncotype DX GPS testing according to standard procedures detailed below. GPS results were reviewed by participating AMP physicians and incorporated into decision making with their patients. Following this GPS discussion, the investigator and patient made an initial management decision, which was recorded via questionnaire.
Institutional review board (IRB) approval was obtained from Asentral, Inc. IRB (Newburyport, MA). All prospective participants provided written informed consent. Participants were not compensated for participation in the study. All the clinical data collected for both the retrospective and the prospective cohort patients were captured using the electronic data capture system eClinicalOS® (Merge, Morrisville, NC), which is compliant with the Title 21 Code of Federal Regulation Part 11 and other standard guidelines and regulations.
Assay Methods
The 17-gene Oncotype DX GPS is an analytically validated, quantitative reverse transcription polymerase chain reaction assay that is performed on prostate carcinoma recovered from formalin-fixed, paraffin-embedded prostate biopsy tissue. The expression of 12 cancer-related genes from four molecular pathways is normalized to expression of five reference genes (scaled from 0 to 100) and is calculated using a proprietary algorithm. A GPS result is indexed to NCCN risk category to produce an estimated likelihood for low-grade disease (defined as surgical pathologic Gleason score 3+4 or less), organ-confined disease (pT2), and composite favorable pathology (both pathologic Gleason score 3+4 or less AND pT2 disease). The GPS has been clinically validated as a predictor of adverse pathology11,12 and biochemical recurrence after radical prostatectomy (RP).11
All specimens for this study were reviewed by a Genomic Health pathologist and appropriate tissues microdissected for testing. All analytic methods were performed as previously described.12,17
Cost Data
Participant Excellus BCBS subscriber identification was collected by the AMP study team and transmitted to Excellus for cost capture. All claims data for 180 days after and including the diagnostic biopsy were extracted. Claims with diagnoses not related to the treatment of CaP or its complications were excluded (eg, traumatic injuries, motor vehicle accidents). A list of diagnoses was used on both the baseline and prospective cohorts. Table 2 provides an example of some of the codes utilized for cost data. Those patients’ claims data were extracted, filtered by the diagnoses, and analyzed for 180 days after the date of their prostate biopsy. Claims were analyzed for type of treatment (AS, RP, or radiation) and their actual paid claim costs. Cost was defined by Excellus BCBS as the total amount paid by all parties involved (ie, paid amount + copay amount + deductible amount + other insurer’s payments and copays [coordination of benefits]). These data were anonymized and transmitted to the study team for integration into the patient database and subsequently to Genomic Health Inc. for statistical analysis.
Statistical Analysis
The primary endpoint of the study was the net percentage difference in prospective treatment decisions with use of Oncotype DX GPS as compared with the baseline treatment patterns without use of GPS. For each treatment modality reported for the baseline patients based on electronic medical records, the proportion of patients who received this modality was computed. Similarly, for the prospective patients, the proportion of the patients whose shared decision was to pursue each treatment modality was computed based on the post-GPS questionnaire. The change in utilization for each treatment modality was computed as the difference between the two in the percent of patients.
Physician confidence in shared decisions post-GPS was summarized using the physician-reported confidence questionnaire, which was captured on a seven-point Likert scale. The number and percentage of responses in each of the seven categories was computed, as well as the total number and proportion of cases where physicians responded as somewhat agree, agree, or strongly agree that their confidence in their management decision was greater after using the assay.
The total cost in each treatment modality was calculated by multiplying the average cost received from Excellus and number of patients per modality. The total cost for a given NCCN risk group was the summation of the cost across all modalities in the group. Because IMRT therapies are provided over a longer course, average cost for IMRT was calculated from those patients who completed a full episode of care for IMRT (ie, obtained all treatment sessions). The cost differences between the baseline and prospective cohort were first calculated on a per-patient basis, and total cost difference was computed assuming both groups had the same number of patients. The cost in the prospective cohort also included the price of GPS testing. All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc, Cary, NC).
Results
The prospective portion of the study enrolled 80 evaluable patients during the period of July 2, 2014 through March 11, 2016. Among these 80 patients, 23 (29%) were NCCN very low risk, 28 (35%) were NCCN low risk, and 29 (36%) were NCCN favorable intermediate risk. Median age was 60 years (range, 42-80 y) and 27 (34%) were aged 65 years or older; 53 (66%) had Gleason score 3+3. Median PSA level was 4.8 ng/mL (range, 0.5-19.2 ng/mL). The majority of patients had stage T1c disease (83%).
The untested baseline portion of the study extracted data on 100 evaluable patients with diagnosis of CaP from February through December 2013. Twenty-five (25%) patients were NCCN very low risk, 46 (46%) were NCCN low risk, and 29 (29%) were NCCN favorable intermediate risk. Median age was 65 years (range, 46-81 y) and 52% were aged 65 years or older; 79 (79%) had a Gleason score of 6. Median PSA level was 4.8 ng/mL (range, 0.5-19.7 ng/mL); 76 (76%) baseline patients had stage T1c disease. The two cohorts had similar racial and ethnic distribution; clinical characteristics were also similar (Table 3).
Among the 80 GPS-tested prospective patients, median GPS was 34 (range, 9-73). The median GPS within NCCN very-low-risk, NCCN low-risk, and NCCN favorable intermediate-risk groups was 32 (range, 9-59), 28 (range, 9-70), and 40 (range, 18-73), respectively. Among the 23 NCCN very-low-risk patients, 7 patients (30.4%) and 1 patient (4.3%) had risk more consistent with NCCN low-risk and favorable intermediate-risk categories after GPS, respectively. Among the 28 NCCN low-risk patients, 7 patients (25%) had a lower risk consistent with NCCN very low-risk, and 10 patients (35.7%) had a higher risk consistent with NCCN intermediate-risk categories, respectively. Although a wide range of GPS distribution was seen in the NCCN intermediate-risk group, none of the patients were restratified into a lower-risk group.
Of the 71 men in the baseline group who were NCCN very low risk and low risk, 27 (38%) were managed with AS, 25 (35%) had RP, 18 (25%) were managed with IMRT and 1 (1%) had whole-gland cryoablation. In the 51 GPS-tested NCCN very-low-risk and low-risk patients, 30 (59%) were managed with AS, 13 (25%) had an RP, 6 (12%) were managed with IMRT, 1 (2%) was managed with multimodal therapy (IMRT and brachytherapy), and 1 (2%) chose focal cryoablation. AS utilization was 21% higher in the prospective GPS-tested cohort of very-low-risk and low-risk men compared with the baseline cohort of risk-group–matched men. The rate of RP was 10% lower and the rate of IMRT was 14% lower in the prospective cohort of very-low-risk and low-risk men when GPS was incorporated into treatment decisions compared with the baseline cohort of risk group-matched men (Table 4 and Figure 1).
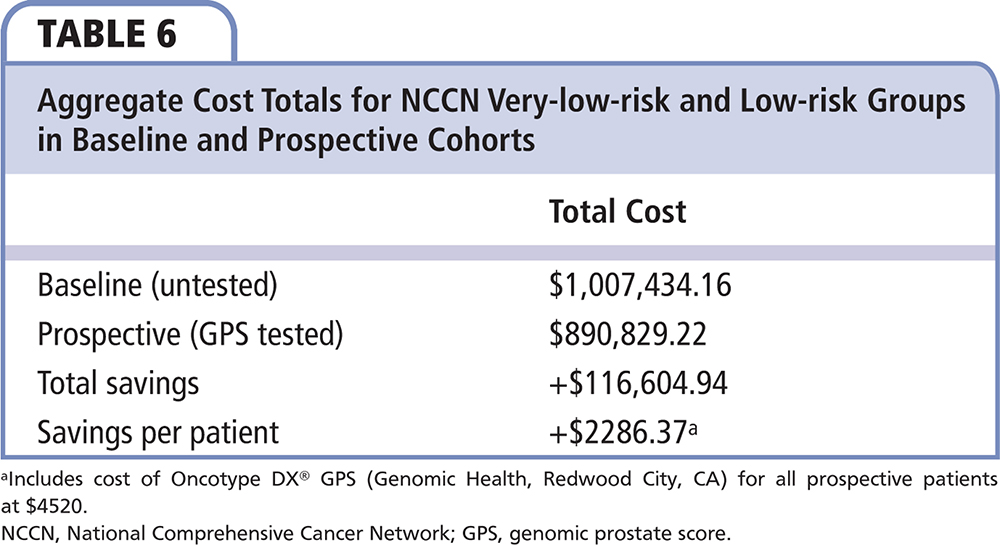
Comparing payer costs in the first 180 days after diagnosis for the baseline and prospective NCCN very-low-risk and low-risk populations, there was an average savings per patient of $2286, including the cost of the GPS of $4520 (total net savings of $116,605 for the entire GPS-tested very-low-risk and low-risk groups [n = 51]) (Table 5 and Table 6). In this assessment of direct cost, the savings is noted from the increase in AS and decrease of intervention, mainly the significant decrease in IMRT. In the 29 GPS-untested NCCN intermediate-risk patients, 5 (17.2%) were managed by AS, 12 (41.4%) by RP, 11 (37.9%) by IMRT, and 1 (3.4%) by CyberKnife (Accuray, Sunnyvale, CA) radio surgery. In the 29 GPS-tested NCCN favorable intermediate-risk patients, no patient chose AS, 14 (48%) chose RP, 11 (38%) chose RT, 1 (3%) chose brachytherapy, and 3 (10%) chose multimodal treatment. AS utilization decreased and RP slightly increased in NCCN favorable intermediate-risk group patients after using GPS. IMRT usage remained unchanged between the baseline and prospective groups. Comparing payer costs in the first 180 days after diagnosis for the entire NCCN risk population (n = 80), there was an average cost addition of $1023 per patient, including the cost of the GPS at $4520 (total net addition of $81,855 for the entire GPS-tested population).
Using GPS also increased the physician’s confidence in treatment decision making. In 91% of cases, physicians indicated increased confidence in decision making after GPS use. Similarly, in 90% of cases, physicians found the GPS useful in clinical practice.
Discussion
In this study, we demonstrated increased utilization of AS for men with NCCN very-low-risk and low-risk CaP who received GPS as part of their CaP risk assessment compared with men who did not. Importantly, the rate of AS in the baseline cohort was consistent with published national norms for utilization of AS in low-risk patients18; this demonstrates that the GPS is effective at increasing AS in practices that currently utilize AS according to national norms at baseline. In addition to increasing uptake of AS in NCCN very-low-risk and low-risk patients, GPS was considered useful and informative in decision making in 90% of cases. Confidence in management decisions is a key criterion for ensuring recommendation and adherence to AS in men with clinically low-risk CaP.
Furthermore, we demonstrated that utilization of the GPS led to decreased aggregate health care costs (average $2286 per patient) for men with NCCN very-low-risk and low-risk CaP in the first 180 days. This decrease is driven primarily by reduced utilization of RP and IMRT. These data are uniquely informative in that they are calculated based on real-world costs for a single payer during the first 180 days after diagnosis and treatment. Cost-effectiveness studies are not new in the cancer molecular diagnostics landscape; however, most studies to date are based on estimations of cost.19 To our knowledge, this is the first study of a molecular marker in CaP to utilize real-world cost data in the analyses.
Diagnosis and treatment of CaP represents a substantial expense in the United States. A 2011 analysis estimated that the direct costs of CaP care in 2020 will exceed $16 billion (indexed to 2010 dollars); the estimated increase in CaP care costs between 2010 and 2020 was the greatest relative increase (42%) of all cancer types studied in this analysis.20 The costs of CaP care include the expense of managing the common side effects of treatment, including but not limited to erectile dysfunction and urinary incontinence. These symptoms may become chronic after treatment; the Prostate Cancer Intervention versus Observation Trial (PIVOT) reported that, at 2 years post surgery, urinary incontinence was present in 17% of men treated with RP versus 6% of those observed; similarly, erectile dysfunction was present in 81% of men treated with RP compared with 44% who were not.5 Indirect expenses from CaP treatment include loss of work productivity for men treated, and friends and family who may take time off from work to provide care; the estimated annual aggregate loss in terms of productivity for men and their partners and families is $5.4 billion and $3 billion dollars, respectively.21
The expense of CaP management is substantial, particularly in light of the fact that many treated CaPs are found to be low-risk, indolent lesions that are unlikely to pose a significant risk over a given man’s lifespan. PIVOT reported that there was no significant difference in overall survival between men randomized to surgery or observation as management.5 The rate of AS in men with low-risk CaP has increased markedly in the past decade but remains relatively low, at approximately 40% in a large US registry study.18 A recent review of numerous AS studies reported < 1% risk of metastasis/CaP-specific death in low-risk patients at a median of 6 years after diagnosis.22 Given the very low rate of early adverse events in this population, AS is clearly underutilized as a management strategy.23 The confidence provided by GPS may help drive acceptance and utilization of AS in clinical practice. Finally, and perhaps most importantly, there is the mental and emotional toll of treatment, which is difficult to quantify in financial terms.24 A test such as GPS that enhances uptake of AS and improves confidence in appropriate patients may not only drive cost savings but also improve patient quality of life.
Particular care must be taken in interpretation of the favorable intermediate-risk patient population. In this practice that has opted to utilize AS for intermediate-risk patients, the additional biologic information provided by GPS led to a decline in AS rate and an attendant increase in costs for this particular group of patients. The optimal utility of genomic assays is to personalize management. In some cases, GPS will identify patients at low risk for progression who can be safely managed with AS. In other cases, GPS will identify patients who are at risk for harboring aggressive disease and who are best served by immediate intervention. Selection of the appropriate management for each individual man is likely to be most cost-effective option over the long term, and (more importantly) provides the optimal care for CaP patients. One must also consider that the initial rate of AS recommendation for intermediate-risk patients was high, at 17%; a recent publication reported the uptake of AS in intermediate-risk CaP was < 10% in the years 2010 to 2013.18 Low utilization of AS in intermediate-risk patients is to be expected, particularly as the NCCN had not included consideration for AS in intermediate-risk patients during the time course of this study.25 It must also be considered that some men with intermediate-risk CaP do benefit from treatment; a molecular assay such as GPS may be useful in selecting patients for AS and also for confirming which patients are best served with immediate or even adjuvant therapy.
As data on favorable intermediate-risk patients on AS mature, we expect that there will be greater acceptance of AS for select intermediate-risk patients; molecular testing will aid in stratification of risk and optimization of patient selection. Genomic information will likely be useful to tailor AS protocols but prospective studies will be required to inform this sort of decision making.
Some specific limitations of this trial must be noted. The sample size of this study was small and did not meet its accrual target of 100 patients in the prospective cohort. All patients were seen in a single group practice and a single insurance payer was involved; costs may differ among plans and practices. There was a higher proportion of favorable intermediate-risk patients in the prospective tested group versus the retrospective group. This imbalance resulted in an overall higher-risk cohort, likely biasing results toward higher treatment intensity and contributing to the increased cost associated with the full prospective cohort. Furthermore, the temporal lag between the baseline and prospective patients occurred in the context of increasing acceptance for AS nationwide; shifting practice patterns may account for some of the change in management, although the degree of change over a relatively short interval argues against time being the only driving factor. Identifying a cost cutoff of 180 days does provide a limitation in those instances in which IMRT was selected for treatment; however, treatment visits were driven past the 180-day cost capture. In this instance, some IMRT therapies may not have captured all costs for the full IMRT procedure; however, the number of cases with incomplete IMRT data was similar in both groups.
Conclusions
Incorporation of GPS as part of the decision algorithm for patients with NCCN very-low-risk and low-risk cancer led to substantial increase in uptake of AS and substantial cost savings (average, $2286 per patient) for insurance carriers. Using the GPS list price of $4520, the $2286 savings represents a return on investment of over 50% ($2286/$4520) over 6 months. Further assessment of GPS in a larger pool of intermediate risk patients is needed to assess the potential impact on treatment planning. GPS provides physicians and patients with an additional tool in assessing personalized risk and helps guide individual decision making. ![]()
References
- SEER Stat Fact Sheets: Prostate Cancer. National Cancer Institute website. http://seer.cancer.gov/statfacts/html/prost.html. Accessed August 20, 2016.
- Xia J, Trock BJ, Cooperberg MR, et al. Prostate cancer mortality following active surveillance versus immediate radical prostatectomy. Clin Cancer Res. 2012;18:5471-5478.
- Yamamoto T, Musunuru B, Vesprini D, et al. Metastatic prostate cancer in men initially treated with active surveillance. J Urol. 2016;195:1409-1414.
- Epstein JI, Feng Z, Trock BJ, Pierorazio PM. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol. 2012;61:1019-1024.
- Wilt TJ, Brawer MK, Jones KM, et al; Prostate Cancer Intervention versus Observation Trial (PIVOT) Study Group. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203-213.
- Livingston CJ, Freeman RJ, Mohammad A, et al; Choosing Wisely® Task Force. Choosing Wisely® in Preventive Medicine: the American College of Preventive Medicine’s Top 5 List of Recommendations. Am J Prev Med. 2016;51:141-149.
- Bhindi B, Mamdani M, Kulkarni GS, et al. Impact of the U.S. Preventive Services Task Force recommendations against prostate specific antigen screening on prostate biopsy and cancer detection rates. J Urol. 2015;193:1519-1524.
- Martin NE, Mucci LA, Loda M, Depinho RA. Prognostic determinants in prostate cancer. Cancer J. 2011;17:429-437.
- Teutsch SM, Bradley LA, Palomaki GE, et al; EGAPP Working Group. The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Initiative: methods of the EGAPP Working Group. Genet Med. 2009;11:3-14.
- Bostrom PJ, Bjartell AS, Catto JW, et al. Genomic predictors of outcome in prostate cancer. Eur Urol. 2015;68:1033-1044.
- Cullen J, Rosner IL, Brand TC, et al. A biopsy-based 17-gene genomic prostate score predicts recurrence after radical prostatectomy and adverse surgical pathology in a racially diverse population of men with clinically low- and intermediate-risk prostate cancer. Eur Urol. 2015;68:123-131.
- Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol. 2014;66:550-560.
- Dall’Era MA, Maddala T, Polychronopoulos L, et al. Utility of the Oncotype DX prostate cancer assay in clinical practice for treatment selection in men newly diagnosed with prostate cancer: a retrospective chart review analysis. Urology Practice. 2015;2:343-348.
- Badani KK, Kemeter MJ, Febbo PG, et al. The impact of a biopsy based 17-gene genomic prostate score on treatment recommendations in men with newly diagnosed clinically prostate cancer who are candidates for active surveillance. Urology Practice. 2015;2:181-189.
- Crawford DE, Gustavsen G, Brawer MB, et al. Evaluation of the economic impact of the CCP assay in localized prostate cancer. Presented at: Society of Urologic Oncology Annual Meeting; December 3-5, 2014; Bethesda, MD.
- Shore ND, Kella N, Moran B, et al. Impact of the cell cycle progression test on physician and patient treatment selection for localized prostate cancer. J Urol. 2016;195:612-618.
- Knezevic D, Goddard AD, Natraj N, et al. Analytical validation of the Oncotype DX prostate cancer assay - a clinical RT-PCR assay optimized for prostate needle biopsies. BMC Genomics. 2013;14:690.
- Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990-2013. JAMA. 2015;314:80-82.
- Vanderlaan BF, Broder MS, Chang EY, et al. Cost-effectiveness of 21-gene assay in node-positive, early-stage breast cancer. Am J Manag Care. 2011;17:455-464.
- Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117-128.
- Rizzo JA, Zyczynski TM, Chen J, et al. Lost labor productivity costs of prostate cancer to patients and their spouses: evidence from US National Survey Data. J Occup Environ Med. 2016;58:351-358.
- Tosoian JJ, Loeb S, Epstein JI, et al. Active surveillance of prostate cancer: use, outcomes, imaging, and diagnostic tools. Am Soc Clin Oncol Educ Book. 2016;35:e235-e245.
- Hayes JH, Ollendorf DA, Pearson SD, et al. Active surveillance compared with initial treatment for men with low-risk prostate cancer: a decision analysis. JAMA. 2010;304:2373-2380.
- Johansson E, Steineck G, Holmberg L, et al; SPCG-4 Investigators. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Prostate Cancer Group-4 randomised trial. Lancet Oncol. 2011;12:891-899.
- Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate Cancer, Version 1.2016. J Natl Compr Canc Netw. 2016;14:19-30.