Fertility Preservation Options for Men and Women With Cancer
Malgorzata E. Skaznik-Wikiel, MD,1 Sara Babcock Gilbert, MD, 1 Randall B. Meacham, MD,2 Laxmi A. Kondapalli, MD, MSCE1,3
1Department of Obstetrics and Gynecology, University of Colorado-Denver, Aurora, CO; 2Division of Urology, University of Colorado-Denver, Aurora, CO; 3Colorado Center for Reproductive Medicine, Lone Tree, CO
Approximately 0.2% of Americans aged 20 to 39 years are childhood cancer survivors. Advances in cancer detection and therapy have greatly improved survival rates for young cancer patients; however, treatment of childhood cancers can adversely impact reproductive function. Many cancer patients report a strong desire to be informed of existing options for fertility preservation and future reproduction prior to initiation of gonadotoxic cancer therapies, including surgery, chemotherapy, and radiotherapy. This article discusses, in detail, the effects of cancer treatment on fertility in men and women, and outlines both current and experimental methods of fertility preservation among cancer patients.
[Rev Urol. 2015;17(4):211-219 doi: 10.3909/riu0666]
© 2016 MedReviews®, LLC
Fertility Preservation Options for Men and Women With Cancer
Malgorzata E. Skaznik-Wikiel, MD,1 Sara Babcock Gilbert, MD, 1 Randall B. Meacham, MD,2 Laxmi A. Kondapalli, MD, MSCE1,3
1Department of Obstetrics and Gynecology, University of Colorado-Denver, Aurora, CO; 2Division of Urology, University of Colorado-Denver, Aurora, CO; 3Colorado Center for Reproductive Medicine, Lone Tree, CO
Approximately 0.2% of Americans aged 20 to 39 years are childhood cancer survivors. Advances in cancer detection and therapy have greatly improved survival rates for young cancer patients; however, treatment of childhood cancers can adversely impact reproductive function. Many cancer patients report a strong desire to be informed of existing options for fertility preservation and future reproduction prior to initiation of gonadotoxic cancer therapies, including surgery, chemotherapy, and radiotherapy. This article discusses, in detail, the effects of cancer treatment on fertility in men and women, and outlines both current and experimental methods of fertility preservation among cancer patients.
[Rev Urol. 2015;17(4):211-219 doi: 10.3909/riu0666]
© 2016 MedReviews®, LLC
Fertility Preservation Options for Men and Women With Cancer
Malgorzata E. Skaznik-Wikiel, MD,1 Sara Babcock Gilbert, MD, 1 Randall B. Meacham, MD,2 Laxmi A. Kondapalli, MD, MSCE1,3
1Department of Obstetrics and Gynecology, University of Colorado-Denver, Aurora, CO; 2Division of Urology, University of Colorado-Denver, Aurora, CO; 3Colorado Center for Reproductive Medicine, Lone Tree, CO
Approximately 0.2% of Americans aged 20 to 39 years are childhood cancer survivors. Advances in cancer detection and therapy have greatly improved survival rates for young cancer patients; however, treatment of childhood cancers can adversely impact reproductive function. Many cancer patients report a strong desire to be informed of existing options for fertility preservation and future reproduction prior to initiation of gonadotoxic cancer therapies, including surgery, chemotherapy, and radiotherapy. This article discusses, in detail, the effects of cancer treatment on fertility in men and women, and outlines both current and experimental methods of fertility preservation among cancer patients.
[Rev Urol. 2015;17(4):211-219 doi: 10.3909/riu0666]
© 2016 MedReviews®, LLC
Key words
Fertility preservation • Childhood cancer • Sperm cryopreservation • Testicular tissue cryopreservation • Spermatogonial stem cell cryopreservation • Embryo cryopreservation • Oocyte cryopreservation • Ovarian tissue cryopreservation
Key words
Fertility preservation • Childhood cancer • Sperm cryopreservation • Testicular tissue cryopreservation • Spermatogonial stem cell cryopreservation • Embryo cryopreservation • Oocyte cryopreservation • Ovarian tissue cryopreservation
The optimal time for consideration of fertility preservation is before the initiation of any oncologic therapy that can affect gametogenesis…
Cytotoxic chemotherapy and radiotherapy may produce persistent damage to primordial sperm cells, leading to oligospermia or azoospermia.

Thawed sperm can be used not only for conventional IVF, but also for intrauterine insemination and intracytoplasmic sperm injection.

Spermatogonial stem cells obtained via biopsy of a prepubertal human testis can theoretically be frozen prior to chemotherapy or radiation treatment and reintroduced into the testes after cure of the underlying disease.
Radiation has also been shown to have an effect on uterine volume, elasticity, and vascularity, which impacts pregnancy later in life in the setting of childhood irradiation.

Main Points
• Given that many of the children and young adults diagnosed with cancer will survive treatment, a focus on survivorship and fertility is of the utmost importance. Many cancer patients report a strong desire to be informed of existing options prior to cancer treatment for fertility preservation and future reproduction.
• Obtaining semen for cryopreservation is a common fertility preservation modality used for postpubertal boys and men; sperm can be cryopreserved in liquid nitrogen for several decades. Obtaining semen by alternative methods, such as urine collection after retrograde ejaculation, electroejaculation, and surgical sperm extraction, is an option for postpubertal boys and men who are unable to ejaculate via masturbation.
• Testicular tissue cryopreservation is available for prepubertal boys and postpubertal boys and men; it is considered experimental at this time. A small portion of testicular tissue is cryopreserved using either slow-freezing protocol or vitrification; the tissue can be thawed and subsequently transplanted. However, there may be a risk of reseeding microscopic malignant cells if tissue is reimplanted.
• Unilateral oophorectomy is one method of fertility-sparing surgery performed in women with malignant ovarian germ cell tumors.
• Embryo cryopreservation has reported pregnancy rates after thawing as high as 59%, and live birth rates approximating 50%.
• Due to advancement in vitrification and rapid cooling with a cryoprotectant, oocytes can now be successfully frozen and thawed with rates of success similar to those of embryos.
• Ovarian tissue cryopreservation is an experimental option for pediatric, prepubertal cancer patients. The goal of this method is to preserve eggs within the primordial follicles in the ovarian cortex.
Main Points
• Given that many of the children and young adults diagnosed with cancer will survive treatment, a focus on survivorship and fertility is of the utmost importance. Many cancer patients report a strong desire to be informed of existing options prior to cancer treatment for fertility preservation and future reproduction.
• Obtaining semen for cryopreservation is a common fertility preservation modality used for postpubertal boys and men; sperm can be cryopreserved in liquid nitrogen for several decades. Obtaining semen by alternative methods, such as urine collection after retrograde ejaculation, electroejaculation, and surgical sperm extraction, is an option for postpubertal boys and men who are unable to ejaculate via masturbation.
• Testicular tissue cryopreservation is available for prepubertal boys and postpubertal boys and men; it is considered experimental at this time. A small portion of testicular tissue is cryopreserved using either slow-freezing protocol or vitrification; the tissue can be thawed and subsequently transplanted. However, there may be a risk of reseeding microscopic malignant cells if tissue is reimplanted.
• Unilateral oophorectomy is one method of fertility-sparing surgery performed in women with malignant ovarian germ cell tumors.
• Embryo cryopreservation has reported pregnancy rates after thawing as high as 59%, and live birth rates approximating 50%.
• Due to advancement in vitrification and rapid cooling with a cryoprotectant, oocytes can now be successfully frozen and thawed with rates of success similar to those of embryos.
• Ovarian tissue cryopreservation is an experimental option for pediatric, prepubertal cancer patients. The goal of this method is to preserve eggs within the primordial follicles in the ovarian cortex.
In 2014, an estimated 15,780 new cancer cases were diagnosed among children and adolescents younger than age 20 years, resulting in 1960 deaths. In addition, 1 in 285 children will be diagnosed with cancer before age 20, and approximately 0.2% of Americans aged 20 to 39 years are childhood cancer survivors.1 Advances in cancer detection and therapy have greatly improved survival rates for young cancer patients; however, treatment of childhood cancers can adversely impact reproductive function (eg, men who survive childhood cancer are half as likely as their siblings to father a child).2 Many cancer patients report a strong desire to be informed of existing options for fertility preservation and future reproduction.3 Therefore, the American Society of Clinical Oncology and the American Society for Reproductive Medicine recommend that consideration of fertility preservation be included prior to initiation of gonadotoxic cancer therapies, including surgery, chemotherapy, and radiotherapy.4-6
Infertility as a result of cancer treatment can be psychologically upsetting for many patients,3,7,8 and data suggest that those who pursued fertility preservation usually cope better with their cancer treatment.9 Infertile cancer survivors have an option to become parents through adoption or gamete donation, but most declare a preference for having a biological child.3,10 Schover and colleagues3 found that 51% of newly diagnosed young male cancer patients reported a desire to have children in the future, and this rate increased to 77% for those who did not have children at the time of diagnosis. The desire to become a biological parent persists in male cancer survivors, as 70% reported wanting to father a child after chemotherapy treatment.9 A history of cancer treatment may be perceived by some to pose an increased risk to the health of future offspring; however, several studies have shown that male cancer survivors have not demonstrated an increased risk for having a child with birth defects or cancer.11,12 Recently, a retrospective cohort study conducted in the United States showed no increased risk of malformations or premature birth in the offspring of male cancer survivors.13
The optimal time for consideration of fertility preservation is before the initiation of any oncologic therapy that can affect gametogenesis; thus, it is critical that fertility preservation is discussed with all patients at the time of diagnosis and before treatment starts. Practitioners who provide care for cancer patients should be aware of the relationship between cancer treatment and infertility. Moreover, they need to be able to appropriately refer patients to a reproductive medicine specialist in a timely fashion for further counseling and fertility preservation. Although fertility concerns are paramount to young adults with cancer, many oncologists still do not routinely address these concerns.3,14 In a survey of 200 young male cancer survivors who were primarily treated at a comprehensive cancer center, only 51% recalled being offered sperm cryopreservation prior to their cancer treatment.3 Further, it is important to recognize the psychologic stressors associated with a new cancer diagnosis and associated late effects of cancer treatment, such as infertility or early menopause. Findings from several studies support the importance of counseling patients regarding their risk for fertility issues and educating providers regarding the potential fertility preservation options that are available. For example, Babb and colleagues15 found that, at many institutions, this counseling is already taking place and there is a high rate of discussion with newly diagnosed patients regarding infertility.
Effects of Cancer Treatment on Fertility in Men
The testis is extremely susceptible to the toxic effects of radiation and chemotherapy at all stages of life.16 Testicular damage can affect the somatic cells of the testis (Sertoli and Leydig cells) or the germ cells. Cytotoxic treatments target rapidly dividing cells and as a result, spermatogenesis can be disrupted following treatment. The mechanism of this damage has not been completely defined, but appears to be associated with depletion of the proliferating germ cell pool by the killing of cells at the stage of differentiating spermatogonial, as well as with stem cells themselves.17 Cytotoxic chemotherapy and radiotherapy may produce persistent damage to primordial sperm cells, leading to oligospermia (a sperm density in the ejaculate of less than 20 × 106/mL) or azoospermia (no sperm in the ejaculate).
Germ cells in boys and men are very sensitive to several classes of chemotherapeutic agents, in particular alkylating agents. Chemotherapeutic agents are cytotoxic to cells that have a high mitotic rate. These drugs have the ability to cross the blood-testis barrier and can disrupt the germinal epithelium.18 The impact of combination chemotherapy on the spermatogenic epithelium is dependent on the type and dosage of the drugs used.19-21 Chemotherapy-induced Leydig cell failure resulting in androgen insufficiency that requires testosterone replacement therapy is extremely rare; most boys who survive cancer undergo puberty and have normal adult circulating levels of testosterone.22
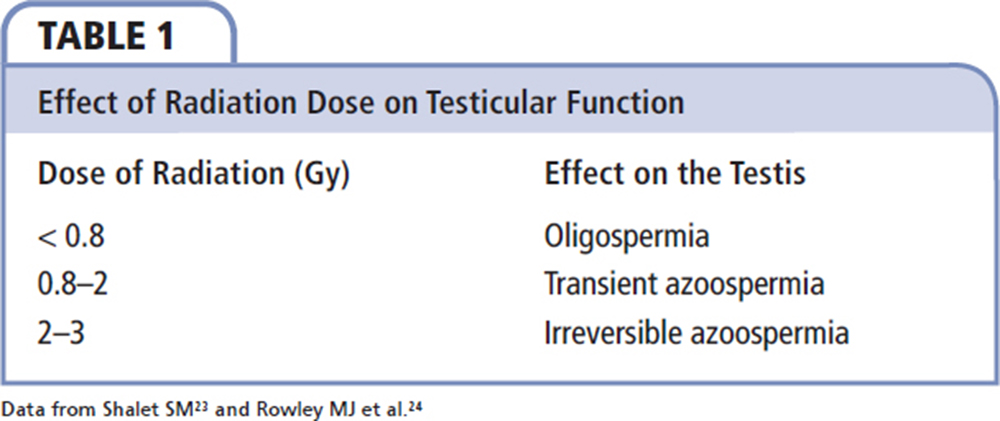
Radiation therapy to the pelvis has been utilized in the treatment of many cancers, including prostate, bladder, penile, and testicular cancer. The testis is one of the most sensitive organs in the body to radiation due to its rapidly dividing germinal epithelium. Radiation-induced testicular dysfunction occurs in a dose-dependent fashion (Table 1).23,24 Further, total body irradiation, which is often incorporated in the conditioning regimen prior to hematologic stem cell transplantation, is associated with 18% germ cell failure.25 Following treatment with 10 or 13 Gy, azoospermia was found in 85% of men, and oligozoospermia was found in the remainder.26 Cranial irradiation is responsible for the development of secondary gonadal failure in some long-term survivors but, overall, overt gonadotropin deficiency is rare and is mainly associated with high doses of radiation.27
Fertility Preservation Strategies
Sperm Cryopreservation
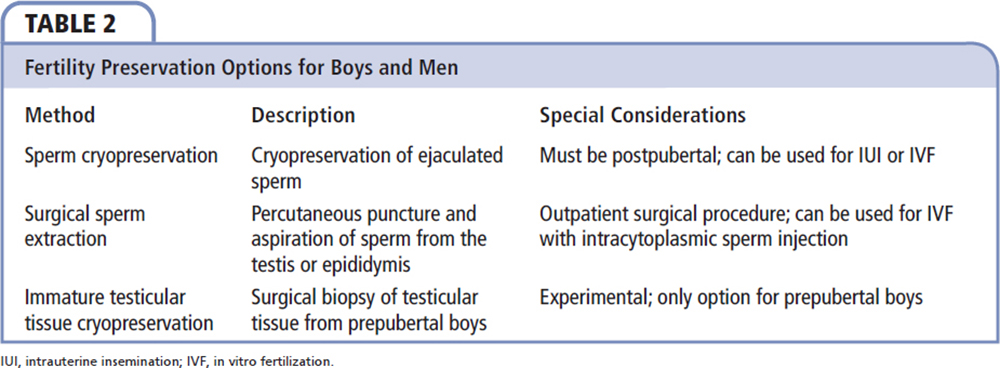
Attempts at fertility preservation (Table 2) should be performed prior to the initiation of cancer therapy due to the vulnerability of the germinal epithelium to gonadotoxic treatment. It should be mentioned that patients diagnosed with cancer often have impaired sperm quality, even before cancer treatment. Pretreatment oligospermia is particularly common in patients with testicular and nontesticular germ cell tumors.28 Sperm analysis has shown that sperm DNA integrity may be affected after just one treatment session. However, if a patient decides to wait until after cancer treatment or needs to initiate cancer therapy immediately, experts recommend waiting at least 12 months after the last treatment session before performing semen analysis.29Obtaining semen for cryopreservation via masturbation and ejaculation is a common fertility preservation modality used for postpubertal boys and men undergoing treatment for cancer. Recent studies demonstrate that postpubertal boys and men can effectively collect and freeze sperm via masturbation prior to starting treatment for cancer.30-32 Sperm can be cryopreserved in liquid nitrogen for several decades; according to one report, sperm frozen for 28 years was used successfully for in vitro fertilization (IVF) that resulted in a live birth.33 Thawed sperm can be used not only for conventional IVF, but also for intrauterine insemination and intracytoplasmic sperm injection. Overall, IVF success rates with cryopreserved sperm from patients with a previous malignancy were comparable with patients who have cryopreserved sperm for other reasons.34
Many male cancer survivors report that having the ability to bank sperm has been comforting to them.35 Survivors who banked sperm prior to treatment have successfully fathered children using cryopreserved semen15,36; however, despite expert consensus recommending sperm banking, as well as the relative ease and high success rates associated with sperm banking, the overall rate for referral and usage of assisted reproductive techniques in patients who cryopreserved sperm remains low.37,38
Sperm Cryopreservation via Alternative Methods
On occasion, patients are unable to ejaculate for a variety of reasons, including sickness, age, pain, psychologic reasons, cultural factors, or religious beliefs.39 Obtaining semen for cryopreservation by alternative methods, such as urine collection after retrograde ejaculation, electroejaculation (EEJ), and surgical sperm extraction, is an option for postpubertal boys and men who are unable to ejaculate via masturbation. For men who have retrograde ejaculation, collection and processing of urine after ejaculation usually allows for isolation of viable sperm for cryopreservation. The process involves medical urine alkalinization and instillation of sperm wash media into the bladder prior to ejaculation.40,41
Additionally, EEJ can be used in patients who are unable to masturbate. EEJ is performed by application of electrical stimulation from a rectal probe to the short postsynaptic fibers in the wall of ejaculatory organs.42 This method has long been used in the treatment of anejaculation due to spinal cord injury and results in sperm retrieval in 90% of patients43; Adank and associates44 have recently reported its feasibility in boys diagnosed with cancer as an alternative to masturbation.
Surgical sperm extraction is an alternative method for patients who cannot ejaculate or have no viable sperm in the ejaculate. Sperm may be obtained via multiple techniques, including testicular sperm extraction, testicular sperm aspiration, and microsurgical epididimal sperm aspiration.45,46 Sperm obtained during these procedures can be used for IVF and intracytoplasmic sperm injection.
Experimental Methods of Fertility Preservation
Testicular Tissue Cryopreservation
Testicular tissue cryopreservation, an emerging fertility preservation method, is available for prepubertal boys and postpubertal boys and men, and is considered experimental at this time. This method involves surgically removing a small portion of testicular tissue, and cryopreserving and storing the specimen.47 Cryopreservation is performed using either slow-freezing protocol or vitrification, which involves the use of increasing concentrations of cryoprotectants and ultrarapid cooling to avoid ice crystal formation.48 In postpubertal boys and men, the tissue can be thawed and subsequently transplanted, either by infusion of a testicular cell suspension into the seminiferous tubules49 or via intratesticular grafting of tissue.48 However, the immature gametes in the testicular tissue from prepubertal boys have yet to be matured for fertility preservation purposes.50 There may be a risk of reseeding microscopic malignant cells if tissue is reimplanted (similar to the risk in women).51 Spermatogonial stem cells (SSCs) obtained via biopsy of a prepubertal human testis can theoretically be frozen prior to chemotherapy or radiation treatment and reintroduced into the testes after cure of the underlying disease.52 However, human testicular tissue has a post-thaw viability of up to 95%, compared with only 66% for post-thaw SSC suspension.50 Cryopreserving testicular tissue allows preservation of supporting Sertoli cells, which may maintain cell-to-cell interactions and account for the improved SSC and germ cell survival.53
Spermatogonial Stem Cell Cryopreservation
SSCs are adult tissue stem cells in the testes that maintain constant sperm production during the postpubertal life of men. Brinster and Zimmermann52 established the technique for SSC transplantation in mice in 1994, demonstrating that donor SSCs could engraft the seminiferous tubules of chemotherapy- treated recipient mice and regenerate spermatogenesis and fertility, leading to the production of viable offspring through normal breeding. Multiple laboratories have reported culturing human SSCs,54-57including from the testes of prepubertal patients.55,56 These preliminary human tissue studies are promising but challenged by the inability to evaluate the full spermatogenic potential of cultured cells by homologous species transplantation into human testes.
Fertility Preservation Strategies for Women
Effects of Cancer Treatment on Fertility in Women
Cancer treatments such as chemotherapy, radiation, and surgery can have gonadotoxic effects that go beyond fertility, such as an impact on growth, puberty, and sexual function.58-60 As women have a finite number of primordial follicles that decline steadily after birth,61 cancer treatments in girls and women can be especially gonadotoxic.60 A number of factors influence the estimated risk of infertility (eg, the risk of gonadotoxicity associated with cancer treatment is increased by the dose of radiation, age at treatment, chemotherapy agent, and dosage of chemotherapy).58
Pelvic radiation therapy for childhood solid or hematologic cancers can have varying effects on the reproductive organs, including the ovaries and the uterus.62 The main effect of radiation to the gonads is reduction of oocyte numbers.63,64 The threshold dose of radiation to the two ovaries and uterus is estimated to be 4 Gy, with possible subfertility with exposures of up to 15 Gy.60,62,65 Radiation has also been shown to have an effect on uterine volume, elasticity, and vascularity, which impacts pregnancy later in life in the setting of childhood irradiation.66 A limited number of studies investigating pregnancy after pelvic radiation have found increased rates of pregnancy complications, including preterm birth, low birth weight offspring, preeclampsia, and stillbirth.67
Chemotherapy with alkylating agents such as chlorambucil, chlormethine, and cyclophosphamide, or cell-cycle-dependent agents such as doxorubicin and paclitaxel and docetaxel, can be quite gonadotoxic to oocytes.68 Likely through apoptosis, germ cells go through elevated rates of cell death during treatment.69,70 Given the finite number of primordial follicles and the biexponential decline in follicle number, younger women have increased reserve compared with women over age 40, making timing of treatment with these agents even more impactful on future fertility rates.71 At age 37, approximately 25,000 follicles are present in the ovary, but because of the biexponential decline, by age 51, only approximately 1000 follicles remain.72
Breast cancer chemotherapy regimens vary depending on the character of the tumor, age of the patient, lymph node status, and menopausal status.73 Although there are many different chemotherapy regimens, the agents most commonly used for adjuvant therapy include cyclophosphamide, methotrexate, paclitaxel, and docetaxel, and 5-fluorouracil or doxorubicin.73 These regimens also often include endocrine-targeted treatments such as tamoxifen—a selective estrogen receptor modulator; anastrozole and letrozole—aromatase inhibitors; and fulvestrant—another estrogen antagonist.74 Women with hormone-sensitive tumors may continue adjuvant therapy with tamoxifen for 5 to 10 years to reduce the risk of recurrence.75 Although tamoxifen is not directly gonadotoxic, fertility is delayed until after this 5- to 10-year treatment duration.76 Animal studies have also raised concern regarding the teratogenic effects of tamoxifen use in pregnancy, leading to a suggested discontinuation of tamoxifen for 12 months prior to conception.77 With potential time delays when tamoxifen is used in women in their 30s and 40s, there is a higher risk of menopause prior to the opportunity to conceive. Given that breast cancer is the leading cancer affecting women, and the therapies used to treat breast cancer have been shown to have a significant impact on fertility later in life, many new strategies are being employed in an attempt to preserve the fertility in these women.
Fertility-preserving Strategies
Surgical Fertility-sparing Methods
There are multiple fertility-preserving approaches for women undergoing cancer treatment (Table 3). Some have been proven through clinical experience and others remain experimental. Moreover, some of these approaches have proven to be more effective than others. One method employed to preserve future fertility is fertility- sparing surgery. An example of this is a unilateral oophorectomy in cases of malignant ovarian germ cell tumors. The outcome and risk of recurrence is similar to that of a bilateral oophorectomy but has the advantage of sparing the contralateral ovary, which can provide a source for hormone production and fertility later in life.78 Even early-stage cervical cancers may be treated with trachelectomy rather than hysterectomy, which allows for fertility maintenance at only a slightly decreased rate from baseline.79
Embryo Cryopreservation
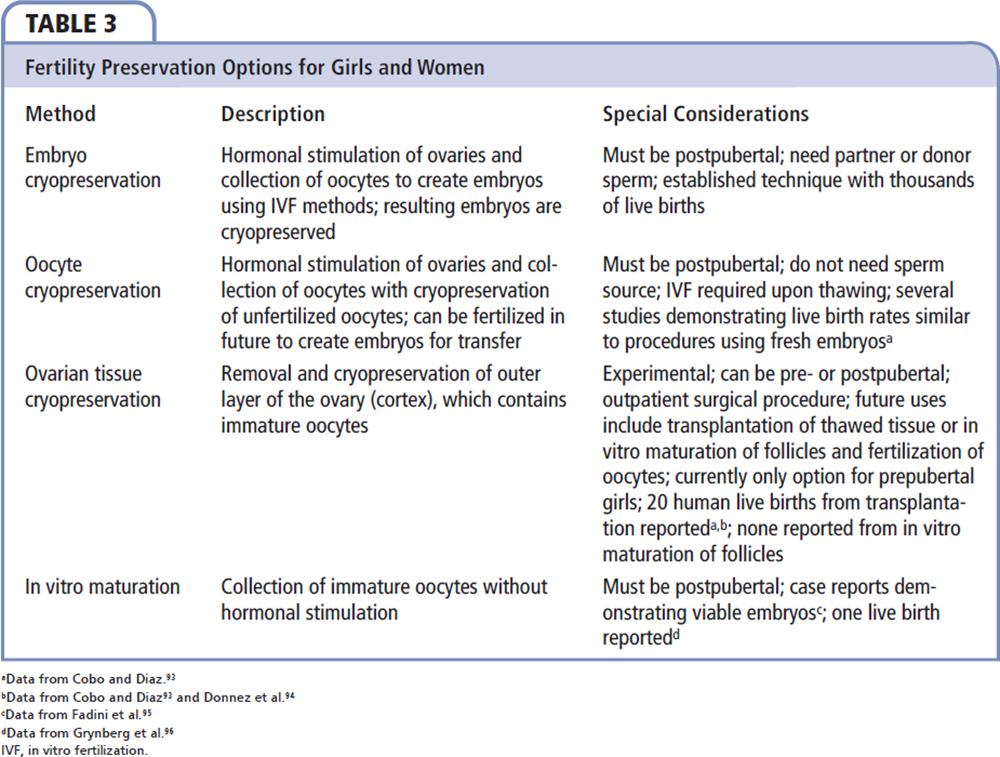
Embryo cryopreservation has been available for almost 30 years, is well studied, and available to most women who desire to conceive. The benefit of this method is that a number of embryos may be frozen, with pregnancy rates after thawing as high as 59%; with live birth rates at approximately 50%.80,81 These rates are similar to those with IVF with fresh embryos. The drawbacks for this procedure include the need for a sexual partner or sperm donor; in addition, the egg donor must be postpubertal.
Oocyte Cryopreservation
Another method gaining popularity is oocyte cryopreservation. Although the first birth from a frozen oocyte used in IVF was reported in 1986, rates of oocyte survival after thawing and live pregnancy rates were previously low secondary to cyroinjury from slow-freeze techniques.82 Due to advancement in vitrification and rapid cooling with a cryoprotectant, oocytes can now be successfully frozen and thawed with similar rates of success to IVF, with intracytoplasmic sperm injection and live birth rates equal to those with fresh oocytes in healthy women under age 35.83,84 This is an attractive option that does not require immediate sperm availability.
Experimental Methods of Fertility Preservation
In Vitro Maturation
In vitro maturation (IVM) is a method in which oocytes at different stages of maturation can be matured in vitro and used for successful IVF procedures or for cryopreservation of oocytes or embryos. This method is only appropriate for postpubertal girls and women, but has an important advantage over standard methods of ovarian stimulation. Women with breast cancer, whether hormone positive or not, are at risk of advancement of their cancer because of high levels of estradiol caused by gonadotropin stimulation during normal ovulation induction.85 By using IVM, the immature oocytes (collected without stimulation) can be matured in vitro.86 Women with breast cancer can undergo this method quickly after diagnosis prior to the start of treatment. They are able to avoid gonadotropin stimulation and high levels of circulating estradiol with the ability to maintain fertility after treatment.
Ovarian Tissue Cryopreservation
Although the above options are available for postpubertal girls and women wishing to preserve fertility, there are currently no established methods for the preservation of fertility among prepubertal girls undergoing cancer treatment. Pediatric cancer patients represent a growing population of patients who may benefit from investigational methods. One experimental option for this age group is ovarian tissue cryopreservation. The goal of this method is to preserve eggs within the primordial follicles in the ovarian cortex, as this contains a large number of eggs. This procedure is usually done laparoscopically prior to cancer treatment with either ovarian cortical biopsy or unilateral oophorectomy. Cortical tissue is then frozen; after cancer treatment is completed, thawed tissue is autotransplanted back into the patient.87 The cortex may contain thousands of primordial follicles that can, after reimplantation, produce antral follicles and result in pregnancy either spontaneously or with assisted reproductive techniques; 20 live births have been reported with this method.88 To date, this approach has been most effective if the thawed tissue is transplanted to the site of a native ovary that still contains the ovarian cortex.88 Reimplantation at this site allows for grafting to the ovarian medulla, which has a vascular network to provide adequate blood supply, and if the fallopian tubes are still patent, can result in spontaneous pregnancy.89 A limitation of this method is that women who have hematologic cancers or ovarian cancers should not undergo autologous reimplantation of ovarian tissue, as it may harbor cancer cells and carry a high risk of cancer recurrence.
Gonadotropin-releasing Hormone Agonists
One additional experimental option available to postpubertal girls and women undergoing cancer treatment is concomitant use of gonadotropin-releasing hormone analogs (GnRHas) for gonadal protection. The concept behind this method is to maintain a prepubertal hormone milieu in an effort to maintain ovarian follicles in a dormant state. The use of GnRHas during adjuvant chemotherapy for breast cancer has been well studied, but with disappointing results. Both nonrandomized90 and randomized studies91 have examined the protective effects of goserelin, leuprolide, or triptorelin on ovarian protection during breast cancer treatment. The benefits of this treatment remain uncertain with conflicting results from the existing studies.92
Conclusions
Given that many of the children and young adults diagnosed with cancer will go on to survive treatment, a focus on survivorship and fertility is of the utmost importance. Treatments need to be discussed early following cancer diagnosis and often need to be individualized based on the disease process and individual patient preference. With continued research and clinical progress in this area, fertility preservation can be a safe, affordable, and successful option for these cancer survivors. ![]()
References
- Ward E, DeSantis C, Robbins A, et al. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83-103.
- Green DM, Kawashima T, Stovall M, et al. Fertility of male survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2010;28:332-339.
- Schover LR, Brey K, Lichtin A, et al. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol. 2002;20:1880-1889.
- Loren AW, Mangu PB, Beck LN, et al; American Society of Clinical Oncology. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500-2510.
- Ethics Committee of American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2013;100:1224-1231.
- Lee SJ, Schover LR, Partridge AH, et al; American Society of Clinical Oncology. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917-2931.
- Rieker PP, Fitzgerald EM, Kalish LA. Adaptive behavioral responses to potential infertility among survivors of testis cancer. J Clin Oncol. 1990;8:347-355.
- Kinahan KE, Didwania A, Nieman CL. Childhood cancer: fertility and psychosocial implications. Cancer Treat Res. 2007;138:191-200.
- Saito K, Suzuki K, Iwasaki A, et al. Sperm cryopreservation before cancer chemotherapy helps in the emotional battle against cancer. Cancer. 2005;104:521-524.
- Nieman CL, Kinahan KE, Yount SE, et al. Fertility preservation and adolescent cancer patients: lessons from adult survivors of childhood cancer and their parents. Cancer Treat Res. 2007;138:201-217.
- Byrne J, Rasmussen SA, Steinhorn SC, et al. Genetic disease in offspring of long-term survivors of childhood and adolescent cancer. Am J Hum Genet. 1998;62: 45-52.
- Green DM, Whitton JA, Stovall M, et al. Pregnancy outcome of partners of male survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21:716-721.
- Chow EJ, Kamineni A, Daling JR, et al. Reproductive outcomes in male childhood cancer survivors: a linked cancer-birth registry analysis. Arch Pediatr Adolesc Med. 2009;163:887-894.
- Anderson RA, Weddell A, Spoudeas HA, et al. Do doctors discuss fertility issues before they treat young patients with cancer? Hum Reprod. 2008;23: 2246-2251.
- Babb A, Farah N, Lyons C, et al. Uptake and outcome of assisted reproductive techniques in long-term survivors of SCT. Bone Marrow Transplant. 2012;47: 568-573.
- Jahnukainen K, Ehmcke J, Hou M, Schlatt S. Testicular function and fertility preservation in male cancer patients. Best Pract Res Clin Endocrinol Metab. 2011;25:287-302.
- Meistrich ML, Finch M, da Cunha MF, et al. Damaging effects of fourteen chemotherapeutic drugs on mouse testis cells. Cancer Res. 1982;42: 122-131.
- Pereira ML, Garcia e Costa F. The blood-testis barrier as a target of some chemotherapeutic agents. Chemotherapy. 2007;53:446-448.
- Aubier F, Flamant F, Brauner R, et al. Male gonadal function after chemotherapy for solid tumors in childhood. J Clin Oncol. 1989;7:304-309.
- Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6: 209-218.
- Rivkees SA, Crawford JD. The relationship of gonadal activity and chemotherapy-induced gonadal damage. JAMA. 1988;259:2123-2125.
- Sklar C. Reproductive physiology and treatment-related loss of sex hormone production. Med Pediatr Oncol. 1999;33:2-8.
- Shalet SM. Effect of irradiation treatment on gonadal function in men treated for germ cell cancer. Eur Urol. 1993;23:148-151.
- Rowley MJ, Leach DR, Warner GA, Heller CG. Effect of graded doses of ionizing radiation on the human testis. Radiat Res. 1974;59:665-678.
- Sarafoglou K, Boulad F, Gillio A, Sklar C. Gonadal function after bone marrow transplantation for acute leukemia during childhood. J Pediatr. 1997;130: 210-216.
- Anserini P, Chiodi S, Spinelli S, et al. Semen analysis following allogeneic bone marrow transplantation. Additional data for evidence-based counselling. Bone Marrow Transplant. 2002;30:447-451.
- Sklar CA. Growth and neuroendocrine dysfunction following therapy for childhood cancer. Pediatr Clin North Am. 1997;44:489-503.
- van Casteren NJ, Boellaard WP, Romijn JC, Dohle GR. Gonadal dysfunction in male cancer patients before cytotoxic treatment. Int J Androl. 2010;33:73-79.
- Fosså SD, Magelssen H. Fertility and reproduction after chemotherapy of adult cancer patients: malignant lymphoma and testicular cancer. Ann Oncol. 2004;15(suppl 4):iv259-iv265.
- Bahadur G, Ling KL, Hart R, et al. Semen quality and cryopreservation in adolescent cancer patients. Hum Reprod. 2002;17:3157-3161.
- Ginsberg JP, Ogle SK, Tuchman LK, et al. Sperm banking for adolescent and young adult cancer patients: sperm quality, patient, and parent perspectives. Pediatr Blood Cancer. 2008;50:594-598.
- Williams DH 4th, Karpman E, Sander JC, et al. Pretreatment semen parameters in men with cancer. J Urol. 2009;181:736-740.
- Feldschuh J, Brassel J, Durso N, Levine A. Successful sperm storage for 28 years. Fertil Steril. 2005;84:1017.
- Hourvitz A, Goldschlag DE, Davis OK, et al. Intracytoplasmic sperm injection (ICSI) using cryopreserved sperm from men with malignant neoplasm yields high pregnancy rates. Fertil Steril. 2008;90:557-563.
- Bonetti TC, Pasqualotto FF, Queiroz P, et al. Sperm banking for male cancer patients: social and semen profiles. Int Braz J Urol. 2009;35:190-197.
- van Casteren NJ, van Santbrink EJ, van Inzen W, et al. Use rate and assisted reproduction technologies outcome of cryopreserved semen from 629 cancer patients. Fertil Steril. 2008;90:2245-2250.
- Chung K, Irani J, Knee G, et al. Sperm cryopreservation for male patients with cancer: an epidemiological analysis at the University of Pennsylvania. Eur J Obstet Gynecol Reprod Biol. 2004;113(suppl 1):S7-S11.
- Ragni G, Somigliana E, Restelli L, et al. Sperm banking and rate of assisted reproduction treatment: insights from a 15-year cryopreservation program for male cancer patients. Cancer. 2003;97:1624-1629.
- Pacey AA. Fertility issues in survivors from adolescent cancers. Cancer Treat Rev. 2007;33:646-655.
- Ohl DA, Quallich SA, Sonksen J, et al. Anejaculation and retrograde ejaculation. Urol Clin North Am. 2008;35:211-220.
- Jefferys A, Siassakos D, Wardle P. The management of retrograde ejaculation: a systematic review and update. Fertil Steril. 2012;97:306-312.
- Chung PH, Yeko TR, Mayer JC, et al. Assisted fertility using electroejaculation in men with spinal cord injury—a review of literature. Fertil Steril. 1995;64:1-9.
- Brackett NL, Ibrahim E, Iremashvili V, et al. Treatment for ejaculatory dysfunction in men with spinal cord injury: an 18-year single center experience. J Urol. 2010;183:2304-2308.
- Adank MC, van Dorp W, Smit M, et al. Electroejaculation as a method of fertility preservation in boys diagnosed with cancer: a single-center experience and review of the literature. Fertil Steril. 2014;102:199. e1-205.e1.
- Berookhim BM, Mulhall JP. Outcomes of operative sperm retrieval strategies for fertility preservation among males scheduled to undergo cancer treatment. Fertil Steril. 2014;101:805-811.
- Ald M, Niederberger CS, Ross LS. Surgical sperm retrieval for assisted reproduction. Minerva Ginecol. 2004;56:217-222.
- Tournaye H, Goossens E, Verheyen G, et al. Preserving the reproductive potential of men and boys with cancer: current concepts and future prospects. Hum Reprod Update. 2004;10:525-532.
- Baert Y, Van Saen D, Haentjens P, et al. What is the best cryopreservation protocol for human testicular tissue banking? Hum Reprod. 2013;28: 1816-1826.
- Ning L, Meng J, Goossens E, et al. In search of an efficient injection technique for future clinical application of spermatogonial stem cell transplantation: infusion of contrast dyes in isolated cadaveric human testes. Fertil Steril. 2012;98:1443-1448.e1.
- Keros V, Hultenby K, Borgström B, et al. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod. 2007;22: 1384-1395.
- Babayev SN, Arslan E, Kogan S, et al. Evaluation of ovarian and testicular tissue cryopreservation in children undergoing gonadotoxic therapies. J Assist Reprod Genet. 2013;30:3-9.
- Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91:11298-11302.
- de Rooij DG. The spermatogonial stem cell niche. Microsc Res Tech. 2009;72:580-585.
- Liu S, Tang Z, Xiong T, Tang W. Isolation and characterization of human spermatogonial stem cells. Reprod Biol Endocrinol. 2011;9:141.
- Sadri-Ardekani H, Mizrak SC, van Daalen SK, et al. Propagation of human spermatogonial stem cells in vitro. JAMA. 2009;302:2127-2134.
- Wu X, Schmidt JA, Avarbock MR, et al. Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc Natl Acad Sci U S A. 2009;106:21672-21677.
- He Z, Kokkinaki M, Jiang J, et al. Isolation, characterization, and culture of human spermatogonia. Biol Reprod. 2010;82:363-372.
- Grigg A. The impact of conventional and high-dose therapy for lymphoma on fertility. Clin Lymphoma. 2004;5:84-88.
- Wallace WH, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte. Hum Reprod. 2003;18:117-121.
- Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738-744.
- Fauser BC. Follicle pool depletion: factors involved and implications. Fertil Steril. 2000;74:629-630.
- Sudour H, Chastagner P, Claude L, et al. Fertility and pregnancy outcome after abdominal irradiation that included or excluded the pelvis in childhood tumor survivors. Int J Radiat Oncol Biol Phys. 2010;76: 867-873.
- Apperley JF, Reddy N. Mechanism and management of treatment-related gonadal failure in recipients of high dose chemoradiotherapy. Blood Rev. 1995;9: 93-116.
- Chiarelli AM, Marrett LD, Darlington G. Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964-1988 in Ontario, Canada. Am J Epidemiol. 1999;150:245-254.
- Brougham MF, Wallace WH. Subfertility in children and young people treated for solid and haematological malignancies. Br J Haematol. 2005;131: 143-155.
- Critchley HO, Wallace WH, Shalet SM, et al. Abdominal irradiation in childhood; the potential for pregnancy. Br J Obstet Gynaecol. 1992;99:392-394.
- Teh WT, Stern C, Chander S, Hickey M. The impact of uterine radiation on subsequent fertility and pregnancy outcomes. Biomed Res Int.2014:482968.
- Familiari G, Caggiati A, Nottola SA, et al. Ultrastructure of human ovarian primordial follicles after combination chemotherapy for Hodgkin’s disease. Hum Reprod. 1993;8:2080-2087.
- Perez GI, Knudson CM, Leykin L, et al. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat Med. 1997;3:1228-1232.
- Tilly JL, Kolesnick RN. Realizing the promise of apoptosis-based therapies: separating the living from the clinically undead. Cell Death Differ. 2003;10: 493-495.
- Fleischer RT, Vollenhoven BJ, Weston GC. The effects of chemotherapy and radiotherapy on fertility in premenopausal women. Obstet Gynecol Surv. 2011; 66:248-254.
- Faddy MJ, Gosden RG. A model conforming the decline in follicle numbers to the age of menopause in women. Hum Reprod. 1996;11:1484-1486.
- Gordon A. The increasing efficacy of breast cancer treatment. Clin Oncol (R Coll Radiol). 1997;9: 338-342.
- Peto R, Davies C, Godwin J, et al; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432-444.
- Strasser-Weippl K, Badovinac-Crnjevic T, Fan L, Goss PE. Extended adjuvant endocrine therapy in hormone-receptor positive breast cancer. Breast. 2013;22(suppl 2):S171-S175.
- Rodriguez-Wallberg KA, Oktay K. Fertility preservation in women with breast cancer. Clin Obstet Gynecol. 2010;53:753-762.
- Halakivi-Clarke L, Cho E, Onojafe I, et al. Maternal exposure to tamoxifen during pregnancy increases carcinogen-induced mammary tumorigenesis among female rat offspring. Clin Cancer Res. 2000;6:305-308.
- Wallberg KA, Keros V, Hovatta O. Clinical aspects of fertility preservation in female patients. Pediatr Blood Cancer. 2009;53:254-260.
- Plante M, Gregoire J, Renaud MC, Roy M. The vaginal radical trachelectomy: an update of a series of 125 cases and 106 pregnancies. Gynecol Oncol. 2011;121:290-297.
- Porcu E, Bazzocchi A, Notarangelo L, et al. Human oocyte cryopreservation in infertility and oncology. Curr Opin Endocrinol Diabetes Obes. 2008;15: 529-535.
- Roy TK, Brandi S, Tappe NM, et al. Embryo vitrification using a novel semi-automated closed system yields in vitro outcomes equivalent to the manual Cryotop method. Hum Reprod. 2014;29:2431-2438.
- Chen C. Pregnancy after human oocyte cryopreservation. Lancet. 1986;1:884-886.
- Cobo A, Meseguer M, Remohí J, Pellicer A. Use of cryo-banked oocytes in an ovum donation programme: a prospective, randomized, controlled, clinical trial. Hum Reprod. 2010;25:2239-2246.
- Practice Committees of American Society for Reproductive Medicine; Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37-43.
- Key T, Appleby P, Barnes I, Reeves G; Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606-616.
- Nogueira D, Sadeu JC, Montagut J. In vitro oocyte maturation: current status. Semin Reprod Med. 2012;30:199-213.
- Bastings L, Liebenthron J, Westphal JR, et al. Efficacy of ovarian tissue cryopreservation in a major European center. J Assist Reprod Genet. 2014;31: 1003-1012.
- Gracia CR, Chang J, Kondapalli L, et al. Ovarian tissue cryopreservation for fertility preservation in cancer patients: successful establishment and feasibility of a multidisciplinary collaboration. J Assist Reprod Genet. 2012;29:495-502.
- Donnez J, Dolmans MM, Pellicer A, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99:1503-1513.
- Recchia F, Saggio G, Amiconi G, et al. Gonadotropin-releasing hormone analogues added to adjuvant chemotherapy protect ovarian function and improve clinical outcomes in young women with early breast carcinoma. Cancer. 2006;106:514-523.
- Elgindy EA, El-Haieg DO, Khorshid OM, et al. Gonadotrophin suppression to prevent chemotherapy-induced ovarian damage: a randomized controlled trial. Obstet Gynecol. 2013;121:78-86.
- Turner NH, Partridge A, Sanna G, et al. Utility of gonadotropin-releasing hormone agonists for fertility preservation in young breast cancer patients: the benefit remains uncertain. Ann Oncol. 2013;24: 2224-2235.
- Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96: 277-285.
- Donnez J, Jadoul P, Pirard C, et al. Live birth after transplantation of frozen-thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril. 2012;98:720-725.
- Fadini R, Dal Canto M, Mignini Renzini M, et al. Embryo transfer following in vitro maturation and cryopreservation of oocytes recovered from antral follicles during conservative surgery for ovarian cancer. J Assist Reprod Genet. 2012;29:779-781.
- Grynberg M, Peltoketo H, Christin-Maître S, et al. First birth achieved after in vitro maturation of oocytes from a woman endowed with multiple antral follicles unresponsive to follicle-stimulating hormone. J Clin Endocrinol Metab. 2013;98:4493-4498.