Sorafenib-induced Scrotal Eczema
Selahattin Çalişkan, MD
Department of Urology, Hitit University Çorum Training and Research Hospital, Çorum, Turkey
Sorafenib is an orally active, small-molecule multikinase inhibitor that blocks tumor cell proliferation and angiogenesis. Studies have shown that it is a highly potent, selective inhibitor of vascular endothelial growth factor receptors 2 and 3, platelet-derived growth factor-β, RAF, FLT-3, and c-Kit. This drug was recently approved for the treatment of metastatic renal cell carcinoma and hepatocellular carcinoma. We report a case of a patient treated with sorafenib for metastatic renal cell carcinoma who developed scrotal eczema.
[Rev Urol. 2015;17(4):250-251 doi: 10.3909/riu0648]
© 2016 MedReviews®, LLC
Sorafenib-induced Scrotal Eczema
Selahattin Çalişkan, MD
Department of Urology, Hitit University Çorum Training and Research Hospital, Çorum, Turkey
Sorafenib is an orally active, small-molecule multikinase inhibitor that blocks tumor cell proliferation and angiogenesis. Studies have shown that it is a highly potent, selective inhibitor of vascular endothelial growth factor receptors 2 and 3, platelet-derived growth factor-β, RAF, FLT-3, and c-Kit. This drug was recently approved for the treatment of metastatic renal cell carcinoma and hepatocellular carcinoma. We report a case of a patient treated with sorafenib for metastatic renal cell carcinoma who developed scrotal eczema.
[Rev Urol. 2015;17(4):250-251 doi: 10.3909/riu0648]
© 2016 MedReviews®, LLC
Sorafenib-induced Scrotal Eczema
Selahattin Çalişkan, MD
Department of Urology, Hitit University Çorum Training and Research Hospital, Çorum, Turkey
Sorafenib is an orally active, small-molecule multikinase inhibitor that blocks tumor cell proliferation and angiogenesis. Studies have shown that it is a highly potent, selective inhibitor of vascular endothelial growth factor receptors 2 and 3, platelet-derived growth factor-β, RAF, FLT-3, and c-Kit. This drug was recently approved for the treatment of metastatic renal cell carcinoma and hepatocellular carcinoma. We report a case of a patient treated with sorafenib for metastatic renal cell carcinoma who developed scrotal eczema.
[Rev Urol. 2015;17(4):250-251 doi: 10.3909/riu0648]
© 2016 MedReviews®, LLC
Key words
Sorafenib • Scrotal eczema • Hand-foot skin reaction
Key words
Sorafenib • Scrotal eczema • Hand-foot skin reaction
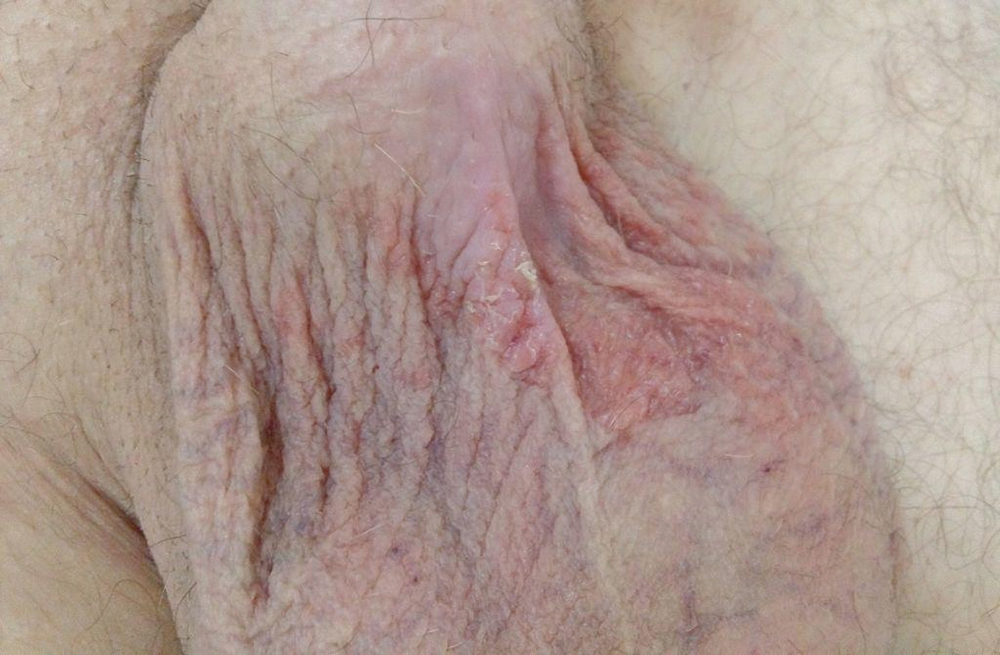
Figure 1. Scrotal eczema caused by sorafenib treatment.

Figure 1. Scrotal eczema caused by sorafenib treatment.
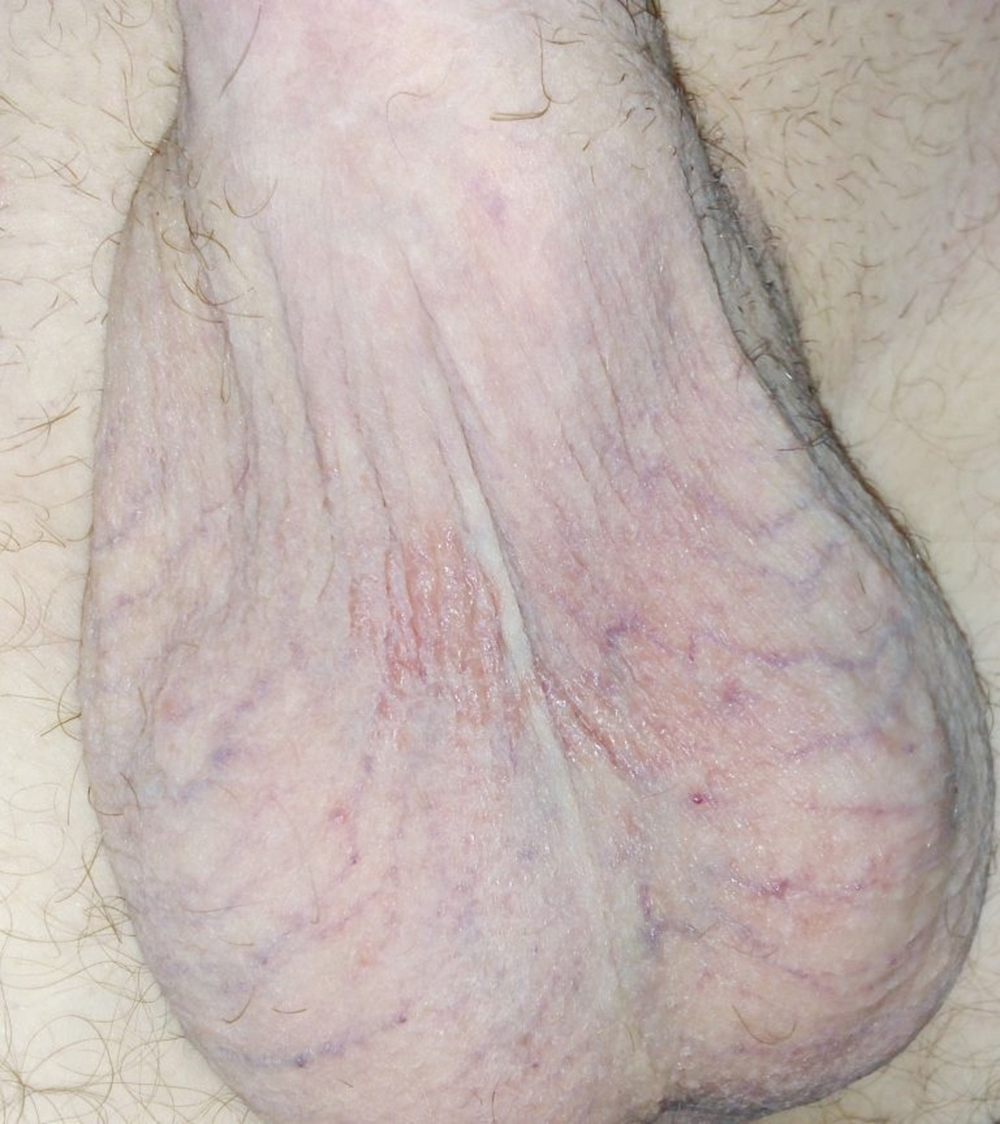
Figure 2. Sorafenib-induced scrotal eczema after topical steroid treatment.

Figure 2. Sorafenib-induced scrotal eczema after topical steroid treatment.
Main Points
• Sorafenib is an orally active, small-molecule multikinase inhibitor that blocks tumor cell proliferation and angiogenesis.
• Up to 90% of patients receiving sorafenib have reported dermatologic symptoms, including hand-foot skin reaction, facial erythema, subungual hemorrhage, alopecia, pruritus, and xerosis.
• It has been suggested that sorafenib has a toxic effect on the skin, and such adverse effects can resolve with topical treatments.
Main Points
• Sorafenib is an orally active, small-molecule multikinase inhibitor that blocks tumor cell proliferation and angiogenesis.
• Up to 90% of patients receiving sorafenib have reported dermatologic symptoms, including hand-foot skin reaction, facial erythema, subungual hemorrhage, alopecia, pruritus, and xerosis.
• It has been suggested that sorafenib has a toxic effect on the skin, and such adverse effects can resolve with topical treatments.
Sorafenib is approved by the US Food and Drug Administration for use in metastatic renal cell carcinoma.1 It is a multikinase inhibitor that blocks both tumor cell proliferation and angiogenesis.2 Up to 90% of patients receiving sorafenib have reported dermatologic symptoms, including hand-foot skin reaction, facial erythema, subungual hemorrhage, alopecia, pruritus, and xerosis.3 Here we report on a patient on sorafenib who presents with the uncommon adverse effect of scrotal eczema.
Case Report
A 56-year-old man presented with a scrotal lesion after 15 days of treatment with sorafenib, 400 mg twice daily (Figure 1). The patient underwent a right radical nephrectomy 4 years prior to this presentation. Abdominal computed tomography revealed a 36 × 66 mm lesion in the liver that was suggestive of metastasis. The patient consulted with the oncology department and sorafenib treatment was initiated. The scrotal eczema resolved with topical steroid treatment and sorafenib was continued (Figure 2).
Discussion
Several novel agents have been developed for anticancer therapy in the past decade.2 These agents are generally well tolerated when compared with standard chemotherapies. However, they can cause several distinct and unique skin reactions. Although sorafenib is an oral multikinase inhibitor and has an excellent safety profile, more than 90% of patients develop skin reactions after treatment. The hand-foot skin reaction is one of the most frequent side effects, occurring in more than 60% of patients.3 The National Cancer Institute has classified the lesions into three grades of severity (grade I, II, and III).4 Most patients reported grade I and II lesions. The exact mechanism of action of this reaction is unclear; it has been suggested that sorafenib has a toxic effect on the skin.4
This case of scrotal eczema is an uncommon skin reaction that was induced by sorafenib. This is the first report of sorafenib-induced scrotal eczema in the literature. This adverse effect can resolve with topical treatments. ![]()
References
- Haraldsdottir S, Li Q, Villalona-Calero MA, et al. Case of sorafenib-induced thyroid storm. J Clin Oncol. 2013;31:e262-e264.
- Yang CH, Lin WC, Chang CK, et al. Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol. 2008;158:592-596.
- Cuesta L, Betlloch I, Toledo F, et al. Severe sorafenibinduced hand-foot skin reaction. Dermatol Online J. 2011;17:14.
- Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/ MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66: 11851-11858.