Metabolic Syndrome and Nephrolithiasis Risk: Should the Medical Management of Nephrolithiasis Include the Treatment of Metabolic Syndrome?
John Michael DiBianco, MD, T.W. Jarrett, MD, Patrick Mufarrij, MD
Department of Urology, George Washington University Medical School, Washington, DC
This article reviews the relationship between metabolic syndrome (MetS) and nephrolithiasis, as well as the clinical implications for patients with this dual diagnosis. MetS, estimated to affect 25% of adults in the United States, is associated with a fivefold increase in the risk of developing diabetes, a doubling of the risk of acquiring cardiovascular disease, and an increase in overall mortality. Defined as a syndrome, MetS is recognized clinically by numerous constitutive traits, including abdominal obesity, hypertension, dyslipidemia (elevated triglycerides, low high-density lipoprotein cholesterol), and hyperglycemia. Urologic complications of MetS include a 30% higher risk of nephrolithiasis, with an increased percentage of uric acid nephrolithiasis in the setting of hyperuricemia, hyperuricosuria, low urine pH, and low urinary volume. Current American Urological Association and European Association of Urology guidelines suggest investigating the etiology of nephrolithiasis in affected individuals; however, there is no specific goal of treating MetS as part of the medical management. Weight loss and exercise, the main lifestyle treatments of MetS, counter abdominal obesity and insulin resistance and reduce the incidence of cardiovascular events and the development of diabetes. These recommendations may offer a beneficial adjunctive treatment option for nephrolithiasis complicated by MetS. Although definitive therapeutic recommendations must await further studies, it seems both reasonable and justifiable for the urologist, as part of a multidisciplinary team, to recommend these important lifestyle changes to patients with both conditions. These recommendations should accompany the currently accepted management of nephrolithiasis.
[Rev Urol. 2015;17(3):117-128 doi: 10.3909/riu0650]
© 2015 MedReviews®, LLC
Metabolic Syndrome and Nephrolithiasis Risk: Should the Medical Management of Nephrolithiasis Include the Treatment of Metabolic Syndrome?
John Michael DiBianco, MD, T.W. Jarrett, MD, Patrick Mufarrij, MD
Department of Urology, George Washington University Medical School, Washington, DC
This article reviews the relationship between metabolic syndrome (MetS) and nephrolithiasis, as well as the clinical implications for patients with this dual diagnosis. MetS, estimated to affect 25% of adults in the United States, is associated with a fivefold increase in the risk of developing diabetes, a doubling of the risk of acquiring cardiovascular disease, and an increase in overall mortality. Defined as a syndrome, MetS is recognized clinically by numerous constitutive traits, including abdominal obesity, hypertension, dyslipidemia (elevated triglycerides, low high-density lipoprotein cholesterol), and hyperglycemia. Urologic complications of MetS include a 30% higher risk of nephrolithiasis, with an increased percentage of uric acid nephrolithiasis in the setting of hyperuricemia, hyperuricosuria, low urine pH, and low urinary volume. Current American Urological Association and European Association of Urology guidelines suggest investigating the etiology of nephrolithiasis in affected individuals; however, there is no specific goal of treating MetS as part of the medical management. Weight loss and exercise, the main lifestyle treatments of MetS, counter abdominal obesity and insulin resistance and reduce the incidence of cardiovascular events and the development of diabetes. These recommendations may offer a beneficial adjunctive treatment option for nephrolithiasis complicated by MetS. Although definitive therapeutic recommendations must await further studies, it seems both reasonable and justifiable for the urologist, as part of a multidisciplinary team, to recommend these important lifestyle changes to patients with both conditions. These recommendations should accompany the currently accepted management of nephrolithiasis.
[Rev Urol. 2015;17(3):117-128 doi: 10.3909/riu0650]
© 2015 MedReviews®, LLC
Metabolic Syndrome and Nephrolithiasis Risk: Should the Medical Management of Nephrolithiasis Include the Treatment of Metabolic Syndrome?
John Michael DiBianco, MD, T.W. Jarrett, MD, Patrick Mufarrij, MD
Department of Urology, George Washington University Medical School, Washington, DC
This article reviews the relationship between metabolic syndrome (MetS) and nephrolithiasis, as well as the clinical implications for patients with this dual diagnosis. MetS, estimated to affect 25% of adults in the United States, is associated with a fivefold increase in the risk of developing diabetes, a doubling of the risk of acquiring cardiovascular disease, and an increase in overall mortality. Defined as a syndrome, MetS is recognized clinically by numerous constitutive traits, including abdominal obesity, hypertension, dyslipidemia (elevated triglycerides, low high-density lipoprotein cholesterol), and hyperglycemia. Urologic complications of MetS include a 30% higher risk of nephrolithiasis, with an increased percentage of uric acid nephrolithiasis in the setting of hyperuricemia, hyperuricosuria, low urine pH, and low urinary volume. Current American Urological Association and European Association of Urology guidelines suggest investigating the etiology of nephrolithiasis in affected individuals; however, there is no specific goal of treating MetS as part of the medical management. Weight loss and exercise, the main lifestyle treatments of MetS, counter abdominal obesity and insulin resistance and reduce the incidence of cardiovascular events and the development of diabetes. These recommendations may offer a beneficial adjunctive treatment option for nephrolithiasis complicated by MetS. Although definitive therapeutic recommendations must await further studies, it seems both reasonable and justifiable for the urologist, as part of a multidisciplinary team, to recommend these important lifestyle changes to patients with both conditions. These recommendations should accompany the currently accepted management of nephrolithiasis.
[Rev Urol. 2015;17(3):117-128 doi: 10.3909/riu0650]
© 2015 MedReviews®, LLC
Key words
Nephrolithiasis • Metabolic syndrome • Uric acid nephrolithiasis
Key words
Nephrolithiasis • Metabolic syndrome • Uric acid nephrolithiasis
A history of kidney stones is approximately twice as common in individuals with three criteria for MetS and three times as common in those with five criteria for MetS…
… patients with a history of nephrolithiasis are significantly more likely to have multiple risk factors for cardiovascular disease, premature atherosclerosis, and cardiovascular events.
… obesity is associated with an increased risk of nephrolithiasis …
The presence of type 2 diabetes was identified as the strongest risk factor for the development of uric acid stones.
… persistent urine acidity was the most important contributing factor to uric acid nephrolithiasis.
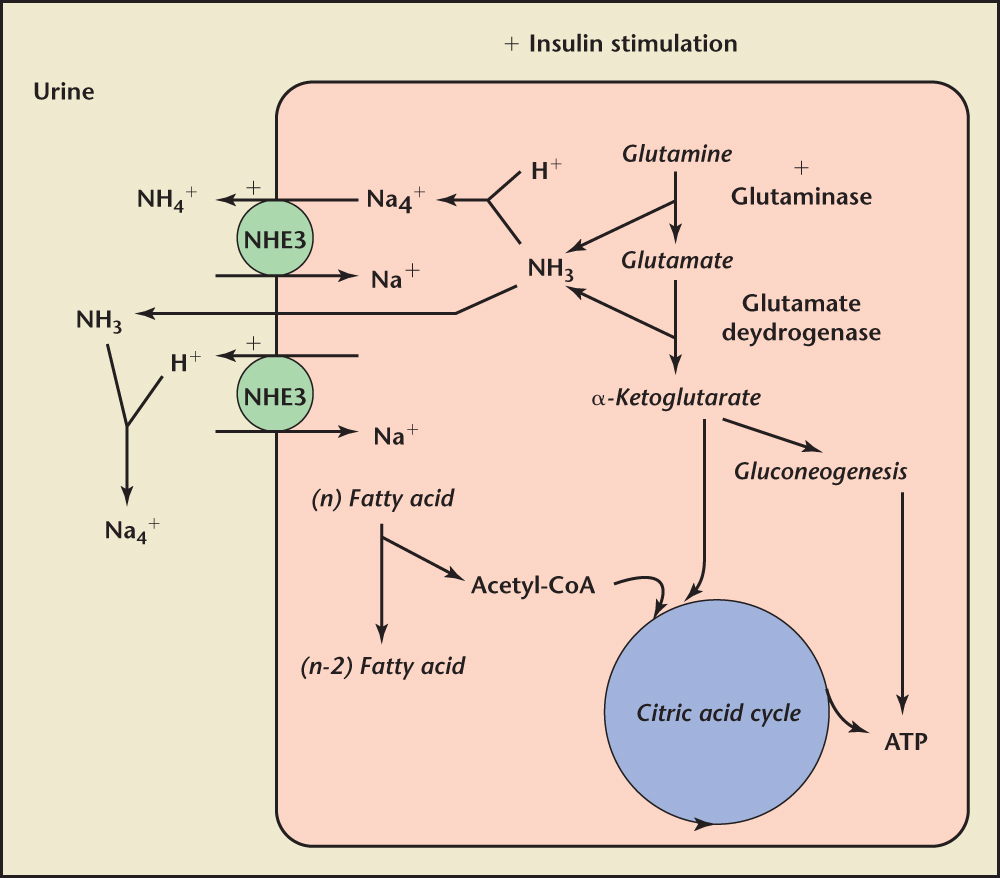
Figure 1. Potential mechanisms of ammonium generation and secretion in the proximal tubule in the setting of insulin resistance. ATP, adenosine triphosphate; CoA, coenzyme A; NHE3, sodium-hydrogen exchanger 3. Reprinted with permission from Pearle and Lotan.53

Figure 2. Possible pathophysiologic pathways interrelating the metabolic syndrome and nephrolithiasis. Reprinted with permission from Pearle and Lotan.53



Main Points
• Metabolic syndrome (MetS), defined by the presence of at least three of the following criteria: central obesity, and low high-density lipoprotein cholesterol, hypertriglyceridemia, hypertension, and elevated fasting glucose, affects more than 22% of US adults. It is associated with an almost fivefold increase in the risk of developing diabetes and a doubling of the risk of acquiring cardiovascular disease. Beyond cardiometabolic risks, MetS has a wide range of long-term complications, including nonalcoholic fatty liver disease, polycystic ovarian syndrome, obstructive sleep apnea, hypogonadism, lipodystrophy, microvascular disease, and chronic renal disease. An important urologic complication of MetS, not routinely cited, is nephrolithiasis.
• Nephrolithiasis continues to be a major cause of morbidity and healthcare spending. A history of kidney stones is approximately twice as common in individuals with three criteria for MetS and three times as common in those with five criteria for MetS, as compared with those with none. Patients with a history of nephrolithiasis are significantly more likely to have multiple risk factors for cardiovascular disease, premature atherosclerosis, and cardiovascular events. It is unknown whether this is primarily a reflection of factors associated with nephrolithiasis, such as obesity, hypertension, or glucose intolerance/diabetes, or due to components of MetS, such as insulin resistance.
• Poor diabetes control was associated with a higher prevalence of urolithiasis, and type 2 diabetes was associated with a higher prevalence of uric acid stones. The presence of type 2 diabetes was identified as the strongest risk factor for the development of uric acid stones. The association of MetS and diabetes with uric acid urolithiasis has been suggested to involve insulin resistance, which is the fundamental metabolic disorder in both MetS and diabetes, and is known to create defective ammoniagenesis, resulting in low urine pH, thus promoting uric acid stone formation.
• Diet and lifestyle changes are recognized as effective tools for reducing the formation of nephrocalculi, although the magnitude of benefit has not been directly compared with pharmacologic therapy. In view of this, recommendations remain inclusive of both diet therapy and pharmacotherapy in most clinical settings. Recommendations to improve lifestyle, through weight loss and exercise, will likely offer a beneficial adjunctive treatment option for nephrolithiasis complicated by MetS.
Main Points
• Metabolic syndrome (MetS), defined by the presence of at least three of the following criteria: central obesity, and low high-density lipoprotein cholesterol, hypertriglyceridemia, hypertension, and elevated fasting glucose, affects more than 22% of US adults. It is associated with an almost fivefold increase in the risk of developing diabetes and a doubling of the risk of acquiring cardiovascular disease. Beyond cardiometabolic risks, MetS has a wide range of long-term complications, including nonalcoholic fatty liver disease, polycystic ovarian syndrome, obstructive sleep apnea, hypogonadism, lipodystrophy, microvascular disease, and chronic renal disease. An important urologic complication of MetS, not routinely cited, is nephrolithiasis.
• Nephrolithiasis continues to be a major cause of morbidity and healthcare spending. A history of kidney stones is approximately twice as common in individuals with three criteria for MetS and three times as common in those with five criteria for MetS, as compared with those with none. Patients with a history of nephrolithiasis are significantly more likely to have multiple risk factors for cardiovascular disease, premature atherosclerosis, and cardiovascular events. It is unknown whether this is primarily a reflection of factors associated with nephrolithiasis, such as obesity, hypertension, or glucose intolerance/diabetes, or due to components of MetS, such as insulin resistance.
• Poor diabetes control was associated with a higher prevalence of urolithiasis, and type 2 diabetes was associated with a higher prevalence of uric acid stones. The presence of type 2 diabetes was identified as the strongest risk factor for the development of uric acid stones. The association of MetS and diabetes with uric acid urolithiasis has been suggested to involve insulin resistance, which is the fundamental metabolic disorder in both MetS and diabetes, and is known to create defective ammoniagenesis, resulting in low urine pH, thus promoting uric acid stone formation.
• Diet and lifestyle changes are recognized as effective tools for reducing the formation of nephrocalculi, although the magnitude of benefit has not been directly compared with pharmacologic therapy. In view of this, recommendations remain inclusive of both diet therapy and pharmacotherapy in most clinical settings. Recommendations to improve lifestyle, through weight loss and exercise, will likely offer a beneficial adjunctive treatment option for nephrolithiasis complicated by MetS.
Metabolic syndrome (MetS), as defined by the National Cholesterol Education Program and the Adult Treatment Panel III in 2001 (and updated in 2005), represents a growing medical problem affecting more than 22% of US adults.1-4 It is associated with an almost fivefold increase in the risk of developing diabetes and a doubling of the risk of acquiring cardiovascular disease.5 MetS is a clinical disorder defined by the presence of at least three of the following criteria: central obesity (abdominal girth > 102 cm [40 in] men and > 88 cm [35 in] women), low high-density lipoprotein (HDL) cholesterol (< 40 mg/dL in men and < 50 mg/dL in women), hypertriglyceridemia (> 150 mg/dL), hypertension (blood pressure > 130/85 mm Hg), and elevated fasting glucose (> 100 mg/dL).2,4 The development of MetS appears to result from a complex interaction of genetics, phenotypic visceral fat accumulation (central obesity), insulin resistance, and sedentary behavior.5,6 Beyond cardiometabolic risks, MetS has a wide range of long-term complications, including nonalcoholic fatty liver disease, polycystic ovarian syndrome, obstructive sleep apnea, hypogonadism, lipodystrophy, microvascular disease, and chronic renal disease.6 An important urologic complication of MetS, not routinely cited, is nephrolithiasis.6-8
Nephrolithiasis continues to be a major cause of morbidity and healthcare spending.9 A history of kidney stones is approximately twice as common in individuals with three criteria for MetS and three times as common in those with five criteria for MetS, as compared with those with none.10 These trends were confirmed in a large-scale, nationwide study of 30,448 Japanese patients with urolithiasis, who demonstrated that MetS was associated with a significantly increased risk of hypercalciuria, hyperuricosuria, hyperoxaluria, and hypocitraturia, independent of age and sex.11 Additionally, patients with a history of nephrolithiasis are significantly more likely to have multiple risk factors for cardiovascular disease, premature atherosclerosis, and cardiovascular events.10,12,13 It is unknown whether this is primarily a reflection of factors associated with nephrolithiasis, such as obesity, hypertension, or glucose intolerance/diabetes, or due to components of MetS, such as insulin resistance.14 The current American Urological Association (AUA) guidelines on the medical management of kidney stones suggests a need for future research on advising patients to exercise and lose weight, but does not make definitive recommendations on these lifestyle changes.15
Risk of Nephrolithiasis
Obesity
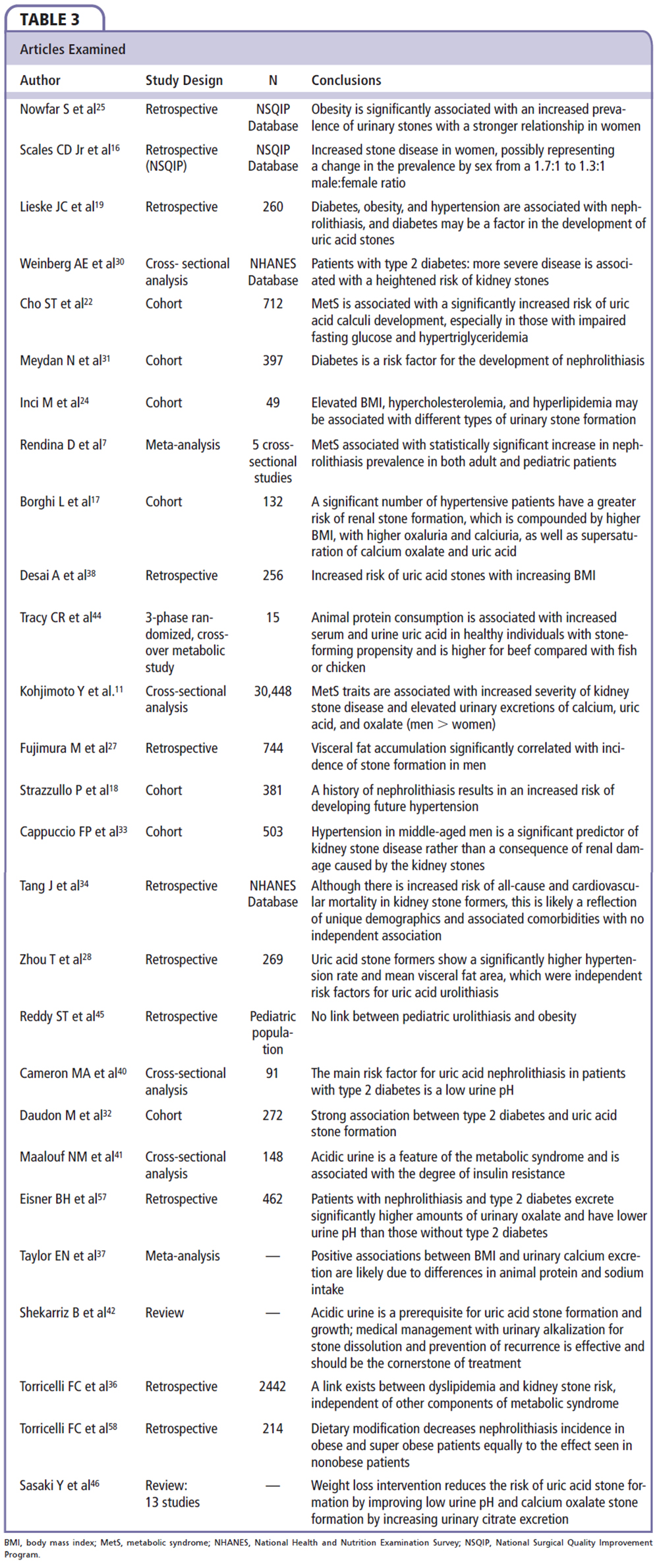
The prevalence of nephrolithiasis in the United States is 8.8%, affecting 1 of 11 individuals, with a significantly growing incidence.16 Diabetes, obesity, hypertension, and MetS are interrelated clinical problems that have all individually been associated with an increased risk of nephrolithiasis.9,17-22 Obesity has been considered a major contributor to the increased frequency of nephrolithiasis seen in recent years.9,23,24 Obesity may also explain the apparent change in the predominance of nephrolithiasis in men, as there is an increasing frequency of kidney stones in obese women.9,23,24 Supporting the association of obesity with nephrolithiasis is the finding of a parallel increase of both disorders observed in a national cohort study of 181,092,957 inpatients, reported by Nowfar and colleagues.25 These authors analyzed the International Classification of Diseases, 9th Revision, diagnostic codes for renal or ureteral calculus and obesity, derived from discharges between 1998 and 2003. They demonstrated that both the incidence of obesity and nephrolithiasis increased significantly over this 5-year period. Other studies concur with the findings of Nowfar and colleagues,25 demonstrating that obesity has increased from 30.5% to 35.7% between 2000 and 2010.26 Nowfar and colleagues25 concluded that obesity is associated with an increased risk of nephrolithiasis (all ages, both sexes), and further commented that the prevalence was disproportionately higher in obese women as compared with obese men. Although representing a large, nationwide experience and documenting a clinical correlation, the data do not demonstrate that obesity contributes directly to nephrolithiasis. Moreover, relying on diagnostic coding contributes to the study’s lack of specificity regarding the absolute percentage of obese subjects, with and without MetS, and the frequency and magnitude of the individual clinical characteristics comprising the diagnostic criteria for MetS.20
Visceral fat accumulation (abdominal obesity), defined as a waist circumference in men ≥ 102 cm (40 in) and ≥ 88 cm (35 in) in women, is a common and frequent component of the diagnostic criteria for MetS.4,6,20 Fujimura and associates,27 using computed tomography-derived measure of visceral fat in 372 patients with known nephrolithiasis and 372 age-matched control subjects, found that visceral fat accumulation was a significant covariant associated with nephrolithiasis (P < .0001). The strength of this relationship is enhanced by the demonstration of a significant correlation in all age groups studied (< 40 y, 41-60 y, and > 60 y), although the inherent difficulties of eliminating confounders is recognized. Reviewing the association of visceral fat area, a recognized better correlate of MetS than body mass index (BMI), and uric acid nephrolithiasis, Zhou and associates28 retrospectively reviewed data from 269 patients who underwent percutaneous nephrolithotomy. Using logistic regression analysis, they demonstrated a significantly higher mean visceral fat area in those patients with uric acid stones compared with those who did not form uric acid stones (odds ratio [OR] 3.64; 95% confidence interval [CI], 1.22-10.85; P = .02).23 They also noted that hypertension was an independent risk factor for uric acid nephrolithiasis (OR 2.16; 95% CI, 1.05-4.45; P = .04).28 An incidental finding was an almost threefold increase in coronary artery disease in the stone formers compared with those who did not form stones (14.3% vs 4.6%; P = .011).23 Although the data from this study cannot inform us as to the specific relationship of visceral fat accumulation and hypertension to uric acid lithogenicity, the results raise consideration for studies to investigate these very points.
Diabetes
Diabetes and urolithiasis have a bidirectional relationship; that is, type 2 diabetes increases the risk of urolithiasis, and is increased in prevalence in patients with nephrolithiasis, as reported by Lieske and associates19 (unadjusted OR for diabetes 1.29; 95% CI, 1.09-1.53).29-31 Daudon and colleagues,32 in their analysis of 2464 renal calculi from 272 patients with type 2 diabetes and 2192 patients without type 2 diabetes, confirmed an increased incidence of nephrolithiasis in diabetes independent of age and BMI.32 Additionally, poor diabetes control was associated with a higher prevalence of urolithiasis, and type 2 diabetes patients had a higher prevalence of uric acid stones (35.7% vs 11.3% in patients without type 2 diabetes; P < .0001).19,22,24,30,32 The presence of type 2 diabetes was identified as the strongest risk factor for the development of uric acid stones.30 The association of MetS and diabetes with uric acid urolithiasis has been suggested to involve insulin resistance, which is the fundamental metabolic disorder in both MetS and diabetes, and is known to create defective ammoniagenesis, resulting in low urine pH, thus promoting uric acid stone formation.16
Hypertension
Observed in epidemiologic studies, hypertension is associated with an increased frequency of nephrolithiasis, independent of age, body mass, or renal function.17,18,33,34 Kohjimoto and coworkers11 confirmed a correlation of hypertension with recurrent or multiple stones in a nationwide survey, independent of age, sex, and other MetS traits (OR 1.14; 95% CI, 1.03-1.26). Although the exact understanding of this relationship remains obscure, the association of hypertension and nephrolithiasis appears to be bidirectional33,35; patients with nephrolithiasis have an increased incidence of hypertension (and higher mean arterial pressures than the general population), and patients with hypertension have higher rates of kidney stone formation.33,35
Dyslipidemia
In a large nationwide survey of nephrolithiasis in Japan, Kohjimoto and coworkers11 found dyslipidemia to be associated with an increased frequency of recurrent or multiple stones, independent of age, sex, or the presence of other MetS traits (OR 1.36, 95% CI, 1.22-1.52). Although they used a slightly more inclusive definition of dyslipidemia (low-density lipoprotein cholesterol ≥ 140 mg/dL, HDL < 40 mg/dL, or triglyceride level ≥ 150 mg/dL), the authors also confirmed that of all of the metabolic traits reviewed, only dyslipidemia was associated, independently, with urinary lithogenic changes, including hypercalciuria, hyperuricosuria, hyperoxaluria, and hypocitraturia.11 These correlations of dyslipidemia raise the possible participation of a common mechanism such as insulin resistance, inflammation, and oxidative stress that may contribute to nephrolithiasis in MetS.11
Torricelli and coauthors36 conducted a review of 2442 patients with nephrolithiasis who also underwent 24-hour urine studies and lipid profiles. They found that the serum concentrations of lipid components correlated with urinary changes. High total cholesterol predicted a significantly higher urinary potassium and calcium, whereas low HDL or high triglyceride levels correlated with an elevated urine sodium, oxalate, and uric acid, and a lower urine pH.36 Furthermore, a high total cholesterol or triglyceride level was associated with a significantly higher risk of uric acid stone formation.36 Cho and colleagues,22 studying 712 patients with nephrolithiasis in South Korea, found significantly higher serum uric acid, lower urine pH, and a markedly increased percentage of uric acid stones in patients with MetS. In this population, triglyceride elevation and reduced HDL were associated with an increase in uric acid nephrolithiasis; however, after correction for age and sex, only triglyceride elevation maintained a statistically significant correlation.22 Although the evidence is limited, there appears to be a definite association of dyslipidemia with urinary constituent changes that favor uric acid nephrolithiasis.
Urine Composition and pH Studies
Whether urine compositional changes predict nephrolithiasis in patients with the interrelated problems of obesity, diabetes, or MetS is unknown. Studying the relationship between BMI and urinary excretion of lithogenic factors in a cohort of 2176 stone-forming adults and 1097 nonstone-forming adults from the Health Professionals Follow-Up Study and the Nurses Health Studies I and II, Taylor and colleagues9 and Taylor and Curhan37 found that patients with elevated BMIs excreted more urinary oxalate (P < .04), uric acid (P < .001), sodium (P < .001), and phosphate (P < .001) than those with lower BMIs. They also found an inverse relationship between BMI and urine pH (P < .02).9,37 The authors concluded that their findings of positive associations between BMI and urinary calcium excretion were due to differences in animal protein and sodium consumption.9,37 They believe that the increased incidence of nephrolithiasis in the obese subjects is most likely due to the increased incidence of uric acid nephrolithiasis.37 Similar observations have been made in patients with nephrolithiasis by Desai and associates38 in a retrospective study of 256 patients (145 men and 111 women) and in a nationwide survey in Japan by Kohjimoto and colleagues,11 as previously noted.
Desai and associates38 found that an increased BMI raises the risk of calcium oxalate monohydrate and dihydrate, calcium phosphate, and uric acid stones with positive correlations between BMI and urine sodium and calcium oxalate. Studies of the relationship between nephrolithiasis and urine composition in patients with type 2 diabetes parallel the aforementioned findings in obese subjects. Eisner and coworkers,39 in a retrospective analysis of 462 patients with a history of nephrolithiasis, found that patients with type 2 diabetes had a significantly higher amount of oxaluria and a significantly lower urine pH than those without type 2 diabetes. In a smaller study of patients with type 2 diabetes, Cameron and associates40 confirmed an inverse relationship between urine pH and body weight. They concluded that the main risk contributor for nephrolithiasis in patients with type 2 diabetes is a low urine pH.40 Using a cross-sectional study of MetS to investigate these observations further, Maalouf and colleagues41 examined 148 adults who were known stone formers, 44 of whom were identified as having MetS. The authors found that those with MetS had a significantly lower urine pH than those without MetS, and that persistent urine acidity was the most important contributing factor to uric acid nephrolithiasis.41,42 They concluded that an abnormally low urine pH is a feature of MetS and is associated with the degree of insulin resistance.41 The authors further uncovered a strong inverse relationship between 24-hour urine pH and the various components of MetS, which also correlate to the degree of insulin resistance.21,41 This finding was independent of known factors to influence urinary pH (age, sex, creatinine clearance, and urine sulfate).21,41
Proposed Mechanism of Action
The mechanisms by which MetS may decrease urine pH include an increase in titratable acid and a decrease in base in the form of ammonium, and citrate, yielding higher hydrogen ion concentrations and a more acidic urine (Figure 1).15,43 Several studies support the finding of low urinary pH in patients with increased body weight.41 These authors proposed that a low urine pH results from resistance to insulin’s renal actions, leading to excessive urinary acidification (Figure 1). They also found that urinary pH is inversely related to body weight among patients with nephrolithiasis, and that an increase in the incidence of uric acid stones is associated with low urine pH.41 These authors, and others, have suggested that uric acid nephrolithiasis be considered a sequela of insulin resistance and prompt investigation for type 2 diabetes and/or the components for MetS.22,32
Hyperuricemia can result from increased production or decreased excretion of uric acid, or from a combination of the two processes, and may be classified as primary or secondary depending on whether the cause is innate or acquired (Figure 2).20 Increased animal protein intake may also contribute to urine acidification, as higher ingestion and excretion of sulfur-containing acidic amino acids decrease urine pH.44,45 Attendant increases in the ingestion and excretion of purines within animal proteins facilitates development of hyperuricosuria.44 Uric acid, a weak acid, is more soluble in neutral or basic solutions than in acidic solutions; hence, as urine pH is lowered, the solubility of uric acid in the urine decreases and there is a greater likelihood of crystallization, especially in supersaturated solutions.15 Coe and colleagues43 reported that, at 37°C, pH 5.35, only one-third of the usual total urate concentration of healthy individuals is soluble, predisposing to precipitation of uric acid crystals and calculi. They concur with others that the low urine pH, as a consequence of reduced ammonia excretion, is a concomitant of insulin resistance, resulting from diabetes and MetS, among other causes.43
Oxalate, the compound most commonly combining with calcium in calcium-containing stones, is another lithogenic compound, making hyperoxaluria a common risk factor for nephrolithiasis.9 Patients with obesity, MetS, and type 2 diabetes are predisposed to hyperoxaluria and low urine pH.37,39-42 These factors, especially if exacerbated by dehydration, may stimulate nephrolithiasis.42
Intervention
It remains unknown whether specific interventions exist that can reduce the increased risk of nephrolithiasis, and uric acid nephrolithiasis specifically, in patients with hypertension, obesity, diabetes, and MetS—all insulin-resistant states.28 Data from a basic research model suggest that it can. A study utilizing a rat model was performed by Sasaki and associates46 in order to examine the effect of dietary calorie restriction and/or exercise on urinary stone formation. Weight loss intervention, in this study, reduced the risk of both uric acid and calcium oxalate nephrolithiasis, by improving low urine pH and increasing urinary citrate excretion.46 This study provides the first theoretical evidence, albeit in a rat model, suggesting that weight loss and/or an exercise intervention program may reduce nephrolithiasis. Although this result cannot be extrapolated to humans, it is an intriguing finding that deserves serious consideration and future study.24,25
Concurrently with the increases of obesity, there has been an increase in the number of bariatric procedures performed.47 These procedures cause a significant decrease in weight loss; however, there is a well-known causal relationship of nonanatomic procedures (jejunoileal bypass) and nephrolithiasis due to the malabsorptive state created.47,48 Recent trends have demonstrated an increase in the utilization of anatomic or restrictive procedures that obtain similar weight loss and health outcomes, without creating as significant malabsorptive states.47,48 One study in particular retrospectively analyzed the rate of nephrolithiasis after 332 restrictive bariatric procedures (gastric banding and sleeve gastrectomy), and found that those patients after restrictive procedures have a low incidence of nephrolithiasis.49 Unfortunately, Chen and coworkers49 were not able to compare these rates before and after surgery. Understanding of patients’ nephrolithiasis risks prior to, during, and after weight loss intervention is paramount to prevention of stone recurrence.
Discussion
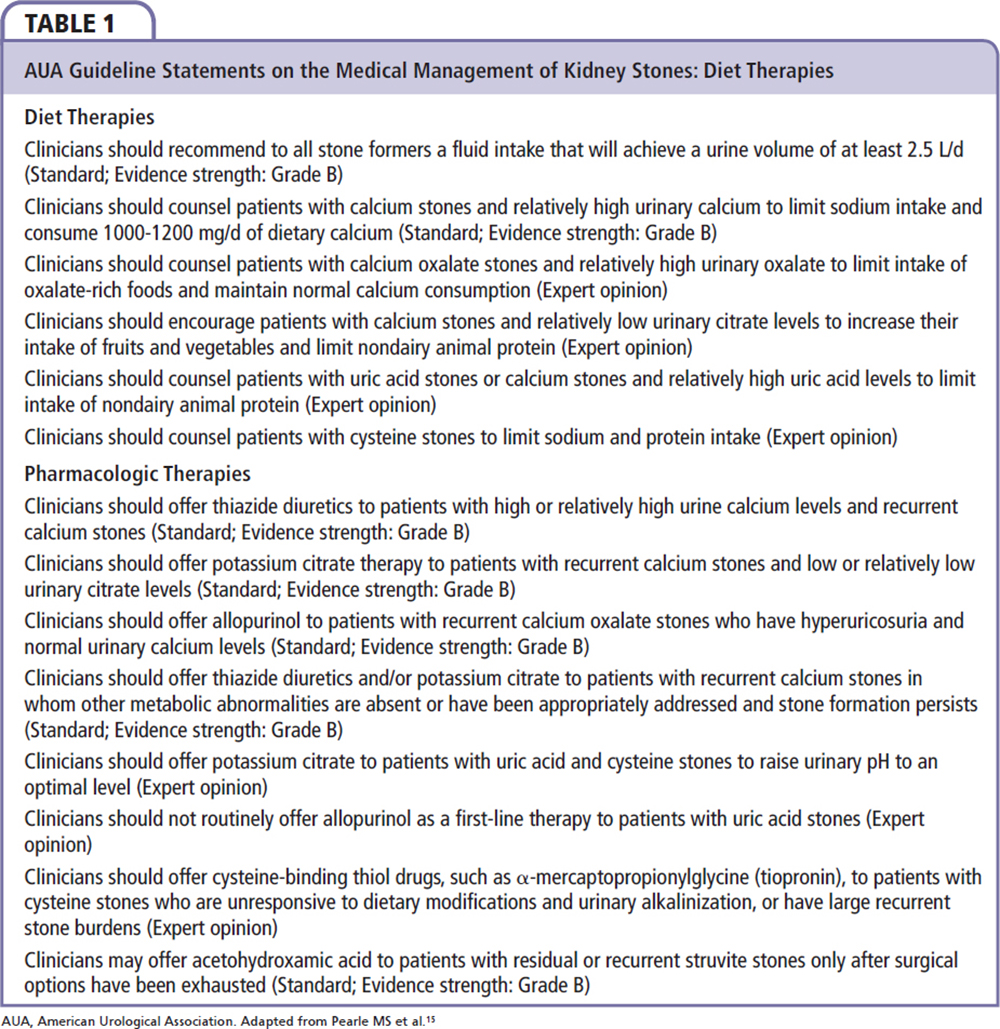
Urologic management of nephrolithiasis promotes general healthy lifestyle changes to reduce the risk of recurrent episodes. The speedy recovery from clinical episodes, and the identification of any systemic conditions that may have predisposed the patient to the disease process, are also important goals. The foundation for the AUA guidelines comes from the Agency for Healthcare Research and Quality’s systematic review entitled Recurrent Nephrolithiasis in Adults: Comparative Effectiveness of Preventative Medical Strategies.15,50 Unfortunately, the relative infrequency of acute nephrolithic events has made the study of preventative and therapeutic measures difficult, and there is limited available quality evidence upon which to base definitive guidelines.15 Diet and lifestyle changes are recognized as effective tools for reducing the formation of nephrocalculi, although the magnitude of benefit has not been directly compared with pharmacologic therapy.15 In view of this, recommendations remain inclusive of both diet therapy and pharmacotherapy in most clinical settings (Table 1).
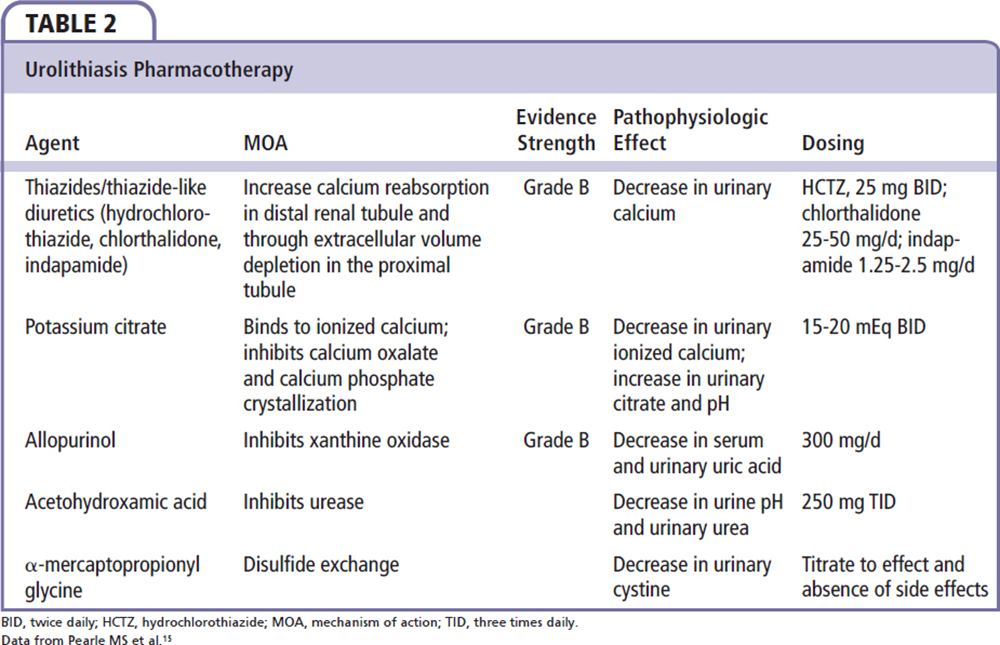
With regard to uric acid nephrolithiasis specifically, medical treatment has been directed at reducing and/or reversing processes pivotal in creating the conditions that lead to urolithiasis (low urinary pH, hyperuricosuria, and low urinary volume).51 Therapeutic measures include increasing urine flow and urine pH to between 6.0 and 6.5 with potassium citrate (30-60 mEq daily in divided doses), as well as the possible addition of allopurinol or febuxostat in patients with urine pH > 6.0 and increased cell turnover or recalcitrant stone formation.15,50,52 This may also be of benefit to patients with calcium oxalate nephrolithiasis, as uric acid crystals can serve as the catalyst in heterogeneous nucleation with calcium oxalate crystals.53 Additional pharmacologic recommendations are summarized in Table 2. Although the AUA guidelines make dietary recommendations dependent on stone type and metabolic derangements, they do not include weight loss or exercise as a possible preventative and treatment strategy for recurrent nephrolithiasis.15
The profound escalation in the incidence and prevalence of obesity, hypertension, diabetes, and MetS, all insulin-resistant states, appears to be associated with an accompanying increase in the incidence of nephrolithiasis. The provocation of hyperuricemia, hyperuricosuria, and lowered urine pH, driven in part by insulin resistance and higher intakes of organic acids in the form of animal proteins and purine-containing compounds, seems to be facilitating these unfortunate trends.
The cost estimates of treating nephrolithiasis extend from $3.8 billion to beyond $10 billion per year.26,54 It is estimated that this annual cost may increase to greater than $15 billion by 2030.26,55 This anticipated increase in the cost of nephrolithiasis is exacerbated, in part, by the progressive increases in rates of obesity and diabetes, and economic factors including inflation and the overall cost of healthcare delivery.26 Turney and Reynard55 offer cogent arguments about these trends, and discuss the importance of a continued focus on prevention of nephrolithiasis as opposed to a monolithic treatment approach.
The management of nephrolithiasis associated with MetS and diabetes has been similar to the general management of nephrolithiasis.6 Both weight loss and exercise are associated with reductions in insulin resistance. Whether recommendations for an aggressive approach to lifestyle changes, to effect meaningful weight loss through calorie restriction, and increased calorie expenditure through exercise, might reduce the frequency of nephrolithiasis and promote more effective treatment of established cases has not been adequately tested. These recommendations, however, are associated with reductions in cardiometabolic complications of MetS and diabetes, and may very well be associated with favorable effects on the processes promoting nephrolithiasis. Recently, it has been proposed that diet and exercise should be encouraged for patients with MetS in order to prevent nephrolithiasis.56 It seems appropriate, perhaps even compelling, for the urologist to emphasize these therapeutic lifestyle changes, simultaneous with the current treatments for nephrolithiasis, even before specific effects regarding the complications of nephrolithiasis are known. Albeit a consensus position, we concur with the multidisciplinary team approach, including the urologist, nephrologist, primary care provider, and dietitian, as suggested by Monga and colleagues,56 when treating a patient with MetS and nephrolithiasis. We agree with the suggestion of Daudon and associates32 to investigate for type 2 diabetes and components of MetS whenever presented with a patient with uric acid nephrolithiasis, even if this approach lacks strong direction from randomized, controlled, definitive studies.19
Conclusions
Patients with MetS have an increased risk of nephrolithiasis, which includes a heightened prevalence of uric acid nephrolithiasis, promoted by low urinary pH, hyperuricosuria, and low urinary volume. Weight loss and exercise, the main lifestyle treatments of MetS, counter abdominal obesity and the resulting insulin resistance, which appears to play an important role in the pathogenesis of low urine pH and uric acid precipitation. These measures also reduce cardiovascular events and the development of diabetes. Recommendations to improve lifestyle, through weight loss and exercise, will likely offer a beneficial adjunctive treatment option for nephrolithiasis complicated by MetS. Although definitive therapeutic recommendations must await further study, it seems both reasonable and justifiable for the urologist, working within a multidisciplinary team, to recommend these important lifestyle changes to the patient with the dual diagnoses of nephrolithiasis and MetS, along with currently accepted empirical treatments of nephrolithiasis. ![]()
References
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356-359.
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415-1428.
- Stone NJ, Bilek S, Rosenbaum S. Recent National Cholesterol Education Program Adult Treatment Panel III update: adjustments and options. Am J Cardiol. 2005;96:53E-59E.
- Grundy SM, Cleeman JI, Daniels SR, et al; American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735-2752.
- Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev. 2008;29:777-822.
- Rendina D, De Filippo G, D’Elia L, Strazzullo P. Metabolic syndrome and nephrolithiasis: a systematic review and meta-analysis of the scientific evidence. J Nephrol. 2014;27:371-376.
- Akman T, Binbay M, Erbin A, et al. The impact of metabolic syndrome on long-term outcomes of percutaneous nephrolithotomy (PCNL). BJU Int. 2012;110(11 Pt C):E1079-E1083.
- Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455-462.
- West B, Luke A, Durazo-Arvizu RA, et al. Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988-1994. Am J Kidney Dis. 2008;51:741-747.
- Kohjimoto Y, Sasaki Y, Iguchi M, et al. Association of metabolic syndrome traits and severity of kidney stones: results from a nationwide survey on urolithiasis in Japan. Am J Kidney Dis. 2013;61: 923-929.
- Reiner AP, Kahn A, Eisner BH, et al. Kidney stones and subclinical atherosclerosis in young adults: the CARDIA study. J Urol. 2011;185:920-925.
- Alexander RT, Hemmelgarn BR, Wiebe N, et al; Alberta Kidney Disease Network. Kidney stones and cardiovascular events: a cohort study. Clin J Am Soc Nephrol. 2014;9:506-512.
- Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004;15:3225-3232.
- Pearle MS, Goldfarb DS, Assimos DG, et al; American Urological Assocation. Medical management of kidney stones: AUA guideline. J Urol. 2014;192:316-324.
- Scales CD Jr, Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
- Borghi L, Meschi T, Guerra A, et al. Essential arterial hypertension and stone disease. Kidney Int. 1999;55:2397-2406.
- Strazzullo P, Barba G, Vuotto P, et al. Past history of nephrolithiasis and incidence of hypertension in men: a reappraisal based on the results of the Olivetti Prospective Heart Study. Nephrol Dial Transplant. 2001;16:2232-2235.
- Lieske JC, de la Vega LS, Gettman MT, et al. Diabetes mellitus and the risk of urinary tract stones: a population-based case-control study. Am J Kidney Dis. 2006, 48:897-904.
- Burns CM, Wortmann RL. Disorders of purine and pyrimidine metabolism. In: Longo DL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012:3181-3187.
- Lange JN, Mufarrij PW, Wood KD, et al. The association of cardiovascular disease and metabolic syndrome with nephrolithiasis. Curr Opin Urol. 2012;22:154-159.
- Cho ST, Jung SI, Myung SC, Kim TH. Correlation of metabolic syndrome with urinary stone composition. Int J Urol. 2013;20:208-213.
- Scales CD Jr, Curtis LH, Norris RD, et al. Changing gender prevalence of stone disease. J Urol. 2007;177:979-982.
- Inci M, Demirtas A, Sarli B, et al. Association between body mass index, lipid profiles, and types of urinary stones. Ren Fail. 2012;34:1140-1143.
- Nowfar S, Palazzi-Churas K, Chang DC, Sur RL. The relationship of obesity and gender prevalence changes in United States inpatient nephrolithiasis. Urology. 2011;78:1029-1033.
- Antonelli JA, Maalouf NM, Pearle MS, Lotan Y. Use of the National Health and Nutrition Examination Survey to calculate the impact of obesity and diabetes on cost and prevalence of urolithiasis in 2030. Eur Urol. 2014;66:724-729.
- Fujimura M, Chin K, Sekita N, et al. Visceral fat accumulation as a risk factor for urinary stone formation. J Urol. 2009;181(suppl):518-519.
- Zhou T, Watts K, Agalliu I, et al. Effects of visceral fat area and other metabolic parameters on stone composition in patients undergoing percutaneous nephrolithotomy. J Urol. 2013;190: 1416-1420.
- Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68:1230-1235.
- Weinberg AE, Patel CJ, Chertow GM, Leppert JT. Diabetic severity and risk of kidney stone disease. Eur Urol. 2014;65:242-247.
- Meydan N, Barutca S, Caliskan S, Camsari T. Urinary stone disease in diabetes mellitus. Scand J Urol Nephrol. 2003;37:64-70.
- Daudon M, Traxer O, Conort P, et al. Type 2 diabetes increases the risk for uric acid stones. J Am Soc Nephrol. 2006;17:2026-2033.
- Cappuccio FP, Siani A, Barba G, et al. A prospective study of hypertension and the incidence of kidney stones in men. J Hypertens. 1999;17:1017-1022.
- Tang J, Mettler P, McFann K, Chonchol M. The association of prevalent kidney stone disease with mortality in US adults: the National Health and Nutrition Examination Survey III, 1988-1994. Am J Nephrol. 2013;37:501-506.
- Obligado SH, Goldfarb DS. The association of nephrolithiasis with hypertension and obesity: a review. Am J Hypertens. 2008;21:257-264.
- Torricelli FC, De SK, Gebreselassie S, et al. Dyslipidemia and kidney stone risk. J Urol. 2014;191:667-672.
- Taylor EN, Curhan GC. Body size and 24-hour urine composition. Am J Kidney Dis. 2006;48:905-915.
- Desai A, Ahmad M, Tawfik, et al. Association of body mass index, stone type and 24 hour-urine chemistry—a retrospective evaluation from a stone center. J Urol. 2009;181(suppl):522.
- Eisner BH, Porten SP, Bechis SK, Stoller ML. Diabetic kidney stone formers excrete more oxalate and have lower uirne pH than nondiabetic stone formers. J Urol. 2010;183:2244-2248.
- Cameron MA, Maalouf NM, Adams-Huet B, et al. Urine composition in type 2 diabetes: predisposition to uric acid nephrolithiasis. J Am Soc Nephrol. 2006;17:1422-1428.
- Maalouf NM, Cameron MA, Moe OW, et al. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2:883-888.
- Shekarriz B, Stoller ML. Uric acid nephrolithiasis: current concepts and controversies. J Urol. 2002; 168(4 Pt 1):1307-1314.
- Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest. 2005;115:2598-2608.
- Tracy CR, Best S, Bagrodia A, et al. Animal protein and the risk of kidney stones: a comparative metabolic study of animal protein sources. J Urol. 2014;192:137-141.
- Reddy ST, Wang CY, Sakhaee K, et al. Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis. 2002;40:265-274.
- Sasaki Y, Kohjimoto Y, Iba A, et al. Weight loss intervention reduces the risk of kidney stone formation in a rat model of metabolic syndrome. Int J Urol. 2015;22:404-409.
- Vujovic A, Keoghane S. Management of renal stone disease in obese patients. Nat Clin Pract Urol. 2007;4:671-676.
- Tarplin S, Ganesan V, Monga M. Stone formation and management after bariatric surgery. Nat Rev Urol. 2015;12:263-270.
- Chen T, Godebu E, Horgan S, et al. The effect of restrictive bariatric surgery on urolithiasis. J Endourol. 2013;27:242-244.
- Xu H, Zisman AL, Coe FL, Worcester EM. Kidney stones: an update on current pharmacological management and future directions. Expert Opin Pharmacother. 2013;14:435-447.
- Sakhaee K. Recent advances in the pathophysiology of nephrolithiasis. Kidney Int. 2009;75:585-595.
- Becker MA, Kisicki J, Khosravan R, et al. Febuxostat (TMX-67), a novel, non-purine, selective inhibitor of xanthine oxidase, is safe and decreases serum urate in healthy volunteers. Nucleosides Nucleotides Nucleic Acids. 2004;23:1111-1116.
- Pearle MS, Lotan Y. Urinary lithiasis: etiology, epidemiology, and pathogenesis. In: Wein AJ, Kavoussi LR, Novick AC, eds. Campbell-Walsh Urology. 10th ed. Philadelphia, PA: Elsevier Saunders; 2012: 1257-1286.
- Scales CD Jr, Saigal CS, Hanley JM, et al; NIDDK Urologic Diseases in America Project. The impact of unplanned postprocedure visits in the management of patients with urinary stones. Surgery. 2014;155: 769-775.
- Turney BW, Reynard JM. The cost of stone surgery. Eur Urol. 2014;66:730-731.
- Monga M, Calle JC, Gonzalez CM. How to prevent stone formation in patients with metabolic syndrome. Urology Times. May 7, 2014. http://urologytimes.modernmedicine.com/
urology-times/content/tags/christopher-m-gonzalez/how-prevent-stone-formation-patients-metabolic-syn?page=full. Accessed August 9, 2015. - Eisner BH, Porten SP, Bechis SK, Stoller ML. Diabetic kidney stone formers excrete more oxalate and have lower urine pH than nondiabetic stone formers. J Urol. 2010;183:2244-2248.
- Torricelli FC, De S, Li I, et al. Can obese stone formers follow dietary recommendations? J Endourol. 2014;28:248-251.