Post–Radical Prostatectomy Incontinence: Etiology and Prevention
Kimberley Hoyland, MBBS, Nikhil Vasdev, ChM (Urol), FRCS (Urol), Ahmed Abrof, FRCS (Urol), Gregory Boustead, FRCS (Urol)
Hertfordshire and South Bedfordshire Robotic Urological Cancer Centre, Department of Urology, Lister Hospital, Stevenage, United Kingdom
All patients undergoing a radical prostatectomy (RP) using any surgical approach, be it open, laparoscopic, or robotic, are at risk of developing postprostatectomy urinary incontinence. This side effect of RP has an effect on the patient’s quality of life and can be associated with moderate to severe postoperative morbidity. The authors present a review of the etiology and prevention strategies of postprostatectomy urinary incontinence. Based on the current literature, the authors conclude that there is a paucity of studies that can accurately answer the exact anatomic and physiologic etiologies of postprostatectomy urinary incontinence. The aim of urologic surgeons performing RP should be to reduce the rate of postoperative incontinence rather than attempting to treat it once it has occurred. Further studies aimed at providing a detailed anatomic map of the pelvic anatomy related to continence will help to improve surgical techniques and reduce postoperative urinary incontinence following RP.
[Rev Urol. 2014;16(4):181-188 doi: 10.3909/riu0606]
© 2014 MedReviews®, LLC
Post–Radical Prostatectomy Incontinence: Etiology and Prevention
Kimberley Hoyland, MBBS, Nikhil Vasdev, ChM (Urol), FRCS (Urol), Ahmed Abrof, FRCS (Urol), Gregory Boustead, FRCS (Urol)
Hertfordshire and South Bedfordshire Robotic Urological Cancer Centre, Department of Urology, Lister Hospital, Stevenage, United Kingdom
All patients undergoing a radical prostatectomy (RP) using any surgical approach, be it open, laparoscopic, or robotic, are at risk of developing postprostatectomy urinary incontinence. This side effect of RP has an effect on the patient’s quality of life and can be associated with moderate to severe postoperative morbidity. The authors present a review of the etiology and prevention strategies of postprostatectomy urinary incontinence. Based on the current literature, the authors conclude that there is a paucity of studies that can accurately answer the exact anatomic and physiologic etiologies of postprostatectomy urinary incontinence. The aim of urologic surgeons performing RP should be to reduce the rate of postoperative incontinence rather than attempting to treat it once it has occurred. Further studies aimed at providing a detailed anatomic map of the pelvic anatomy related to continence will help to improve surgical techniques and reduce postoperative urinary incontinence following RP.
[Rev Urol. 2014;16(4):181-188 doi: 10.3909/riu0606]
© 2014 MedReviews®, LLC
Post–Radical Prostatectomy Incontinence: Etiology and Prevention
Kimberley Hoyland, MBBS, Nikhil Vasdev, ChM (Urol), FRCS (Urol), Ahmed Abrof, FRCS (Urol), Gregory Boustead, FRCS (Urol)
Hertfordshire and South Bedfordshire Robotic Urological Cancer Centre, Department of Urology, Lister Hospital, Stevenage, United Kingdom
All patients undergoing a radical prostatectomy (RP) using any surgical approach, be it open, laparoscopic, or robotic, are at risk of developing postprostatectomy urinary incontinence. This side effect of RP has an effect on the patient’s quality of life and can be associated with moderate to severe postoperative morbidity. The authors present a review of the etiology and prevention strategies of postprostatectomy urinary incontinence. Based on the current literature, the authors conclude that there is a paucity of studies that can accurately answer the exact anatomic and physiologic etiologies of postprostatectomy urinary incontinence. The aim of urologic surgeons performing RP should be to reduce the rate of postoperative incontinence rather than attempting to treat it once it has occurred. Further studies aimed at providing a detailed anatomic map of the pelvic anatomy related to continence will help to improve surgical techniques and reduce postoperative urinary incontinence following RP.
[Rev Urol. 2014;16(4):181-188 doi: 10.3909/riu0606]
© 2014 MedReviews®, LLC
Key words
Radical prostatectomy • Urinary incontinence • Urethral length
Key words
Radical prostatectomy • Urinary incontinence • Urethral length
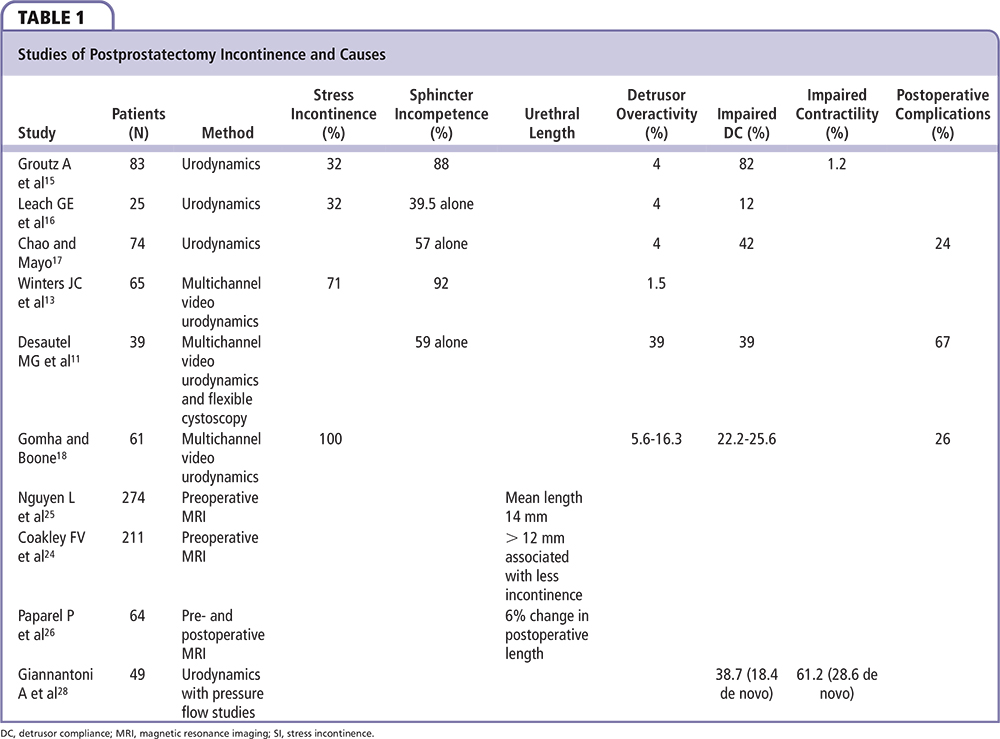


Figure 1. The surgical anatomy of the male urethral sphincter complex prior to radical prostatectomy. B, bladder; DA, detrusor apron; FSS, fascia of the striated sphincter; ML, Mueller’s ligaments; MDR, medical dorsal raphe; NVB, neurovascular bundle; OS, Os ischiadicum; PB, pubis bone; P, prostate; PPL, pubovescicalis ligament; PP, puboperinealis muscle; R, rectum; RU, rectourethralis muscle; SS, striated sphincter.

Figure 1. The surgical anatomy of the male urethral sphincter complex prior to radical prostatectomy. B, bladder; DA, detrusor apron; FSS, fascia of the striated sphincter; ML, Mueller’s ligaments; MDR, medical dorsal raphe; NVB, neurovascular bundle; OS, Os ischiadicum; PB, pubis bone; P, prostate; PPL, pubovescicalis ligament; PP, puboperinealis muscle; R, rectum; RU, rectourethralis muscle; SS, striated sphincter.
Assessment of urethral length by pre- and postoperative endorectal magnetic resonance imaging has demonstrated that longer urethral lengths are associated with more rapid recovery of continence.
Detrusor overactivity arising de novo may be a result of devascularization or denervation of the bladder, or inflammatory changes related to surgery.
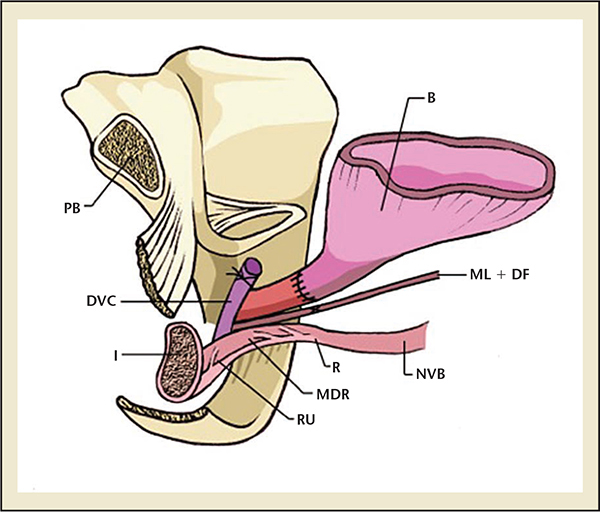
Figure 2. The surgical anatomy of the male urethral complex following a radical prostatectomy. B, bladder; DA, detrusor apron; FSS, fascia of the striated sphincter; ML, Mueller’s ligaments; MDR, medical dorsal raphe; NVB, neurovascular bundle; OS, Os ischiadicum; PB, pubis bone; P, prostate; PPL, pubovescicalis ligament; PP, puboperinealis muscle; R, rectum; RU, rectourethralis muscle; SS, striated sphincter.

Figure 2. The surgical anatomy of the male urethral complex following a radical prostatectomy. B, bladder; DA, detrusor apron; FSS, fascia of the striated sphincter; ML, Mueller’s ligaments; MDR, medical dorsal raphe; NVB, neurovascular bundle; OS, Os ischiadicum; PB, pubis bone; P, prostate; PPL, pubovescicalis ligament; PP, puboperinealis muscle; R, rectum; RU, rectourethralis muscle; SS, striated sphincter.
Evidence to date suggests that the majority of patients with postprostatectomy incontinence have stress incontinence.
Main Points
• All patients undergoing a radical prostatectomy (RP) using any surgical approach (open, laparoscopic, or robotic) are at risk of developing postprostatectomy urinary incontinence.
• Incontinence after RP can broadly be divided into two causes: urethral and detrusor. Urethral causes of incontinence have been found to be a result of either urethral sphincter incompetence, changes in urethral length, or postoperative strictures.
• Nerve-sparing as compared with non–nerve-sparing surgery significantly contributed to increased rates of postoperative continence at 6 months due to preserved innervation of the rhabdosphincter.
• Urethral length also appears to be an important predictor of and factor in postoperative incontinence.
• It is likely that the combination of intraoperative nerve and neurovascular damage has effects on both the detrusor and ureteric function, and this combination results in varying degrees of incontinence, depending on the amount of neurovascular involvement.
Main Points
• All patients undergoing a radical prostatectomy (RP) using any surgical approach (open, laparoscopic, or robotic) are at risk of developing postprostatectomy urinary incontinence.
• Incontinence after RP can broadly be divided into two causes: urethral and detrusor. Urethral causes of incontinence have been found to be a result of either urethral sphincter incompetence, changes in urethral length, or postoperative strictures.
• Nerve-sparing as compared with non–nerve-sparing surgery significantly contributed to increased rates of postoperative continence at 6 months due to preserved innervation of the rhabdosphincter.
• Urethral length also appears to be an important predictor of and factor in postoperative incontinence.
• It is likely that the combination of intraoperative nerve and neurovascular damage has effects on both the detrusor and ureteric function, and this combination results in varying degrees of incontinence, depending on the amount of neurovascular involvement.
Anatomy and Physiology
The anatomy and physiology of continence in men has not been fully elucidated. In men, there is a variety of opinion on neurovascular supply and exact anatomic elements contributing to maintenance of continence. In men, urinary continence is thought to be controlled by five main structures: the detrusor muscle, the internal sphincter, the ureterotrigonal muscles, the levator muscles, and the rhabdo-sphincter.4,5 The male urethral sphincter complex consists of a smooth muscle and skeletal muscle component.5
The smooth muscle component forms the internal or external sphincter, which has been described by Koraitim and colleagues as a separate entity to the bladder musculature, and instead is derived from musculature of the urethra.5 The smooth muscle sphincter surrounds the urethra and lies between the mucosa and the skeletal urethral muscle; along with connective tissue, this makes up the bulk of the urethral wall. There is a distinct layer of longitudinal smooth muscle surrounded by circular smooth muscle, whereby contraction of the circular fibers results in urethral narrowing providing continence, and contraction of the longitudinal fibers widens the urethra for urination. The internal sphincter controls passive continence and holds urine at the level of the vesical orifice; a minimal length is crucial for maintainenance.5
The skeletal muscle sphincter, or rhabdosphincter, surrounds the membranous urethra from the apex of the prostate to the corpus spongiosum, in the shape of an inverted horseshoe.4,5 It then continues proximally over the anterolateral surface of the prostate as the semi-lunar cap.4,5 The caudal part of the muscle is attached to the posterior median raphe, causing movement of the anterior urethral wall toward the posterior wall when contracted.5 The rectourethralis muscle, together with Denonvilliers’ fascia, forms a rigid plate posteriorly and, thus, compression of the anterior urethral wall against this produces a transversely flattened urethral lumen.5 This large surface area results in higher urethral resistance compared with the internal sphincter and hence produces active continence.5 This urethral occlusion occurs in the membranous urethra for rapid and forceful closure, as demonstrated by increased maximum urethral pressure during urethral pressure profilometry and contrast arrest under fluoroscopy.5 The normal preprostatectomy surgical anatomy of the male urethral sphincter complex is demonstrated in Figure 1.
The nervous supply maintaining continence is complex, and not fully determined. The cavernous nerve, which was originally thought to form a bundle structure, has been found to be in this formation in only 30% of patients, whereas 70% have been shown to have plate formation.6 The branches provide innervation to the ipsilateral side of the bladder and urethra, but also have some midline extension to supply the contralateral side.4
The nervous supply of the vesico-urethral smooth muscle is from the hypogastric and pelvic nerves for sympathetic and parasympathetic supply respectively, and the external sphincter receives somatomotor innervation from the pudendal and pelvic nerves.4,7
Post-Radical Prostatectomy Incontinence
Incontinence rates after prostatectomy vary in the current literature, and can be as high as 80%.1,2 There are a number of explanations as to the cause of postprostatectomy incontinence, and it is likely that it is multifactorial in origin (Table 1).
RP removes a number of control mechanisms for urinary continence and potentially damages others. During prostatectomy, the prostate, which has a degree of control of continence as part of the proximal sphincteric unit, is removed.8 In addition, the proximal urethral sphincter is lost; therefore, postoperative continence depends largely on the rhabdosphincter.8,9 Furthermore, the proximity of the neurovascular supply and rhabdosphincter to the prostate puts these structures at high risk of damage intraoperatively. Egawa and colleagues10 demonstrated that the innervation of the urethral sphincter lies only 0.3 to 1.3 cm from the apex of the prostate, making it highly susceptible to injury during apical dissection. In addition to the urethral sphincter, the bladder is also affected by RP, with effects on detrusor innervation and function. The surgical anatomy of the male urethral complex following RP is shown in Figure 2.
Incontinence after RP can broadly be divided into two causes: urethral and detrusor. Urethral causes of incontinence have been found to be a result of urethral sphincter incompetence, changes in urethral length, or postoperative strictures. The evidence for these postoperatively, as well as the effects on continence, is unclear due to lack of definitive evidence from studies.
Multiple studies have demonstrated sphincter incompetence after RP, the majority identifying patients with stress incontinence that is likely a result of sphincteric injury.2,3,9-14 Intrinsic sphincter deficiency on urodynamic testing is present in up to 88% of patients up to 1 year postoperatively.15 Urodynamic testing has shown 39.5% of post-RP patients to have pure sphincteric insufficiency, and only 18.5% of patients demonstrating no sphincteric involvement in their incontinence.16 These results are similar to those of Chao and Mayo,17 who found 37% of incontinent patients to have incontinence solely due to sphincteric weakness, with only 4% showing intact sphincters. Winters and colleagues13 also identified 92% of incontinent patients with sphincteric incompetence. Desautel and associates11 used Valsalva leak point pressures to assess incontinent patients after RP, and found sphincteric injury to be the sole cause of incontinence in 59% of patients and a major contributor in 36%. Gomha and Boone18 also investigated voiding patterns after RP and found all incontinent patients to have stress incontinence, as demonstrated by Valsalva maneuvers and leakage on fluoroscopy.
Urethral sphincter incompetence is generally considered to be the most important contributing factor to post-RP incontinence. Maximal urethral closure pressure (MUCP) appears to be reduced postoperatively, with rates up to 41%.19 Studies have revealed MUCP reductions from 89.6 cm H2O pre-operatively to 65.2 cm H2O postoperatively, as well as higher MUCP in continent compared with incontinent patients.20 Hammerer and Huland20 reported mean MUCP of 68.1 cm H2O in continent patients compared with only 53.1 cm H2O in incontinent patients. Similar results were demonstrated by Dubbelman and associates19 who reported significantly higher pre- and postoperative MUCP in men regaining continence at 6 months postoperatively compared with incontinent patients. They also stated that poor preoperative MUCP is an important prognostic factor for persistent incontinence postoperatively.19
Given that the majority of patients recover continence over a period that is variable, and can range from 6 months to 12 months in most cases, it is less likely that sphincter incompetence is a result of damage to the sphincter itself, and is more likely to be a result of damage to supporting structures and nerves, which recover over time.21 This hypothesis is supported by evidence of autonomic denervation of the membranous urethral mucosa found in 77% to 92% of incontinent men after RP.21 Furthermore, urethral sphincter innervation is closely related to the prostatic apex and preserving the prostatic fascia appears to improve postoperative continence.21 Van der Poel and colleagues21 demonstrated risk reductions of 60% at 6 months postoperatively in patients with preserved prostatic fascia. Hwang and associates22 also demonstrated reduced incontinence rates by preserving the lateral prostatic fascia in robot-assisted laparoscopic prostatectomy (RALP) patients due to reduced nerve damage to branches supplying the urethral sphincter. This is further implied by differences between nerve-sparing and non-nerve-sparing RP incontinence rates. Ko and colleagues23 found prolonged recovery of continence at 3 months in non-nerve-sparing RP compared with nerve-sparing surgery. Additionally, nerve-sparing surgery significantly contributed to increased rates of postoperative continence at 6 months due to preserved innervation of the rhabdosphincter.8 The fact that postoperative continence recovery appears to be maximal at 12 months could be explained by neuropraxic recovery following intraoperative nerve injury.8 Further evidence in favor of this is a study showing recovery of continence in half the time in patients undergoing nerve-sparing operations compared with non-nerve-sparing procedures, with rates of 5.3 months and 10.9 months, respectively.7
Urethral length has also been associated with postoperative continence. Assessment of urethral length by pre- and postoperative endorectal magnetic resonance imaging has demonstrated that longer urethral lengths are associated with more rapid recovery of continence.23,24 The average pre-operative urethral sphincter length has been found to be approximately 14 mm.25,26 Patients with a urethral length > 12 mm postoperatively recovered continence at rates of 89% at 1 year, compared with 77% for patients with urethral lengths < 12 mm.24,25 Paparel and colleagues26found a median change of 6% in maximum urethral lengths postoperatively, and found urethral lengths to be significantly associated with postoperative recovery of continence, with longer pre- and postoperative length associated with greater continence.26 Thus, urethral length appears to be an important predictor of and factor in postoperative incontinence.
Postoperative strictures are reported less in the literature as a cause of post-RP incontinence, but may play a minor role in its etiology. Anastomotic strictures have been reported at rates of approximately 26% postoperatively.18 Studies have found that fibrosis in incontinent patients alters the function of the external urethral sphincter and reduces its elasticity.26 In addition to this, Chao and Mayo17 proposed that anastomotic strictures may extend into the sphincter itself causing impaired urethral closure. This is supported by the findings of Desautel and colleagues11 of urethral scarring and strictures in 67% of patients, all of whom had sphincteric incompetence.
Although sphincteric incompetence is widely reported in the literature, reports of detrusor abnormalities after RP are more contradictory. One of the main reported findings for detrusor changes after RP is reduced detrusor compliance. Impaired compliance has been reported at rates of 8% to 38.9% of patients after RP18,27,28 Reduced compliance has been shown to persist years after RP in some patients, with rates of 9% to 11% reported in the literature.18 Furthermore, measurements of bladder capacity have been shown to reduce postoperatively; Gomha and colleagues reported compliance of 37 mL/cm H2O preoperatively reducing to 23 mL/cm H2O at 6 months after surgery.18 Patients with reduced bladder capacities or preoperative compliance have been found to have less improvement in postoperative voiding symptoms.29 The finding of reduced compliance after RP could be explained by reduced bladder perfusion induced by long-term increased intravesical pressure from bladder outflow obstruction, which would explain its pre- and postoperative presence.27 However, this does not explain those patients with de novo reduced compliance. The wide dissection during RP can result in partial decentralization of the bladder, which could explain those patients with impaired compliance arising de novo postoperatively.27
In addition to reduced compliance, both pre- and postoperative detrusor hypocontractility are prevalent, with rates of up to 42.8% preoperatively, rising to 61.2% postoperatively.28 Giannantoni and associates28 found de novo hypo-contractility in 28.6% of patients after RP. Matsukawa and colleagues30 reported much lower rates of hypocontractility, with only 9.1% arising de novo, but persisting long term. Detrusor hypocontractility is likely to be preexisting in many due to long-term bladder outflow obstruction.18 Those arising de novo cases could be the result of bladder denervation due to intra-operative nerve disruption.18,28 Giannantoni and associates28 found persisting detrusor hypocontractility in only 10% of patients at 8 months postoperatively, which would fit the model of a neuropraxic injury. Impaired contractility due to relief of obstruction would be expected to coincide with high postvoid residuals; however, these patients tend to have residual volumes of < 100 mL, making this less likely, and further favoring the explanation of a denervation injury as a root cause.27 The association of hypocontractility with sphincter incompetence could suggest that hypocontractility is a result of lack of urethral resistance, such that poor detrusor pressures are sufficient for normal flow rate, and indicating that the finding of hypocontractility may be less significant in causing incontinence.27
Detrusor overactivity has been reported in varying rates following RP, ranging from 13% to 67.3%.15,18,28 However, despite high postoperative rates, preoperative rates also appear to be high. Giannantoni and associates28 found 67.3% of patients to have postoperative detrusor overactivity, but 55.1% also had it preoperatively. Similarly, Song and colleagues29 found preoperative rates of 38% and rates of 51.4% at 3 years postoperatively. Detrusor overactivity has been reported as the sole cause of incontinence in only 4% of patients, and it is highly associated with sphincter dysfunction; 23% to 42% of cases are associated with sphincter insufficiency.12,13,16,17,27 Patients with incontinence have higher rates of bladder instability than continent patients after RP, with rates of 57% and 38%, respectively.20 Detrusor overactivity arising de novo may be a result of devascularization or denervation of the bladder, or inflammatory changes related to surgery.29 The strong association between detrusor overactivity and sphincter incompetence may be due to activation of the vesicourethral reflex due to sphincter incompetence.27
Discussion
Evidence to date suggests that the majority of patients with post-prostatectomy incontinence have stress incontinence.18 Given the anatomic findings so far, the most likely explanation for this stress incontinence is sphincteric incompetence, as evidenced from urodynamic studies, as well as comparisons between nerve-sparing and non-nerve-sparing prostatectomy incontinence rates. This is under standable given the anatomic layout of the male pelvis and the intimate relationship of the prostate to the elements necessary for urinary continence. RP is, in effect, causing continence to solely rely on the integrity and function of the distal sphincteric unit.29 Given the proximity of this to the prostate, apical dissection is key to the maintenance of continence. There is a delicate balance between cancer control and continence with apical dissection, and interindividual variation, as well as the need for clear surgical margins, can impact on the integrity of the rhabdosphincter and neurovascular bundle.6 Nerve-sparing prostatectomy offers greater rates of continence due to neurovascular bundle preservation; however, this does not eradicate postoperative incontinence, as anatomic studies show a plate-like formation of nerves rather than the traditional bundle concept, making dissection more difficult. Rates of incontinence are lower and recovery quicker with robotic prostatectomy due to greater magnification and maneuverability, allowing more precise dissection around the apex. Continence rates following RALP have been demonstrated as high as 88% compared with 71% following laparoscopic prostatectomy.31-34 Furthermore, average time to postoperative continence has been shown to be 44 days with RALP compared with 160 days for retropubic radical prostatectomy,35 which could be a result of increased nerve preservation and reduced damage to the ureteric sphincter.
Although an element of bladder dysfunction is likely to exist in these patients, it is strongly associated with sphincter incompetence, and it is difficult to distinguish whether detrusor dysfunction is a cause or consequence of this. Detrusor overactivity and hypocontractility may be the result of intraoperative neuropraxic injuries, or may be the result of ureteric dysfunction impeding detrusor function. The high prevalence of preoperative detrusor abnormalities suggests that these problems are preexisting in a large number of patients and, therefore, RP may only unmask rather than cause these issues. The low number of de novo cases of detrusor abnormalities could suggest that these have less impact on postoperative clinical incontinence, but this is difficult to speculate. It is likely that the combination of intraoperative nerve and neurovascular damage has effects on both the detrusor and ureteric function, and this combination results in varying degrees of incontinence, depending on the amount of neurovascular involvement.
Different surgical techniques are being improvised, such as posterior musculofascial reconstruction after RP.36 In a systematic analysis the authors reviewed outcomes reported in comparative studies analyzing the influence of reconstruction of the posterior aspect of the rhabdosphincter after RP using the Rocco suture. The authors found that the cumulative analysis of comparative studies showed that reconstruction of the posterior musculofascial plate improves early return of continence within the first 30 d after RP (P = .004), whereas continence rates 90 days after surgery were not affected by use of the reconstruction technique. The benefit of posterior bladder neck reconstruction is also not proven to improve urinary incontinence in patients following RP.37 In the context of patients undergoing a robotic prostatectomy, a systemic analysis by Ficarra and colleagues38 found that urinary incontinence after robot-assisted radical prostatectomy is influenced by preoperative patient characteristics, surgeon experience, surgical technique, and methods used to collect and report data. Posterior musculofascial reconstruction seems to offer a slight advantage with regard to 1-month urinary continence recovery.38
In summary, current evidence would suggest the following principles to surgeons performing RP to facilitate early and long-term urinary continence include
- Avoiding overdissection of the apical musculature and skeletonizing the urethra
- Maintaining maximal urethral length without compromising cancer control (this also avoids foreshortening or bunching of a long urethra by sutures placed too deeply, akin to a closed concertina effect)
- Nerve-sparing approach where oncologically safe
- Bladder neck sparing where feasible or bladder neck reconstruction to prevent funneling of a patulous bladder neck
Conclusions
In the current literature, there is a paucity of studies that can accurately answer the exact anatomic and physiologic etiologies of post- prostatectomy urinary incontinence. The aim of urologic surgeons performing an RP should be to reduce the rate of postoperative incontinence rather than an attempt to treat it once it has occurred. Further studies aimed at providing a detailed anatomic map of the pelvic anatomy related to continence will help improve surgical techniques and reduce postoperative incontinence, which continues to be a significant cause of postoperative morbidity in patients undergoing an RP. ![]()
The authors report no real or apparent conflict of interest.
References
- Goluboff ET, Saidi JA, Mazer S, et al. Urinary continence after radical prostatectomy: the Columbia experience. J Urol. 1998;159:1276-1280.
- Wilson LC, Gilling PJ. Post-prostatectomy urinary incontinence: a review of surgical treatment options. BJU Int. 2011;107(suppl 3):7-10.
- Catarin MV, Manzano GM, Nóbrega JA, et al. The role of membranous urethral afferent autonomic innervation in the continence mechanism after nerve sparing radical prostatectomy: a clinical and prospective study. J Urol. 2008;180: 2527-2531.
- Golomb J, Chertin B, Mor Y. Anatomy of urinary continence and neurogenic incontinence. Therapy. 2009;6:151-155.
- Koraitim MM. The male urethral sphincter complex revisited: an anatomical concept and its physiological correlate. J Urol. 2008;179:1683-1689.
- Hinata N, Sejima T, Takenaka A. Progress in pelvic anatomy from the viewpoint of radical prostatectomy. Int J Urol. 2013;20:260-270.
- Loughlin KR, Prasad MM. Post-prostatectomy urinary incontinence: a confluence of 3 factors. J Urol. 2010;183:871-877.
- Song C, Doo CK, Hong JH, et al. Relationship between the integrity of the pelvic floor muscles and early recovery of continence after radical prostatectomy. J Urol. 2007;178:208-211.
- Atiemo HO, Vasavada S, Rackley R, Moy L. Evaluating and managing urinary incontinence after prostatectomy: beyond pads and diapers. Cleve Clin J Med. 2007;74:57-63.
- Egawa S, Minei S, Iwamura M, et al. Urinary continence following radical prostatectomy. Jpn J Clin Oncol. 1997;27:71-75.
- Desautel MG, Kapoor R, Badlani GH. Sphincteric incontinence: the primary cause of post-prostatectomy incontinence in patients with prostate cancer. Neurourol Urodyn. 1997;16:153-160.
- Ficazzola MA, Nitti VW. The etiology of postradical prostatectomy incontinence and correlation of symptoms with urodynamic findings. J Urol. 1998;160:1317-1320.
- Winters JC, Appell RA, Rackley RR. Urodynamic findings in postprostatectomy incontinence. Neurourol Urodyn. 1998;17:493-498.
- Takenaka A, Tewari AK. Anatomical basis for carrying out a state-of-the-art radical prostatectomy. Int J Urol. 2012;19:7-19.
- Groutz A, Blaivas JG, Chaikin DC, et al. The pathophysiology of post-radical prostatectomy incontinence: a clinical and video urodynamic study. J Urol. 2000;163:1767-1770.
- Leach GE, Trockman B, Wong A, et al. Post-prostatectomy incontinence: urodynamic findings and treatment outcomes. J Urol. 1996;155:1256-1259.
- Chao R, Mayo ME. Incontinence after radical prostatectomy: detrusor or sphincter causes. J Urol. 1995; 154:16-18.
- Gomha MA, Boone TB. Voiding patterns in patients with post-prostatectomy incontinence: urodynamic and demographic analysis. J Urol. 2003;169:1766-1769.
- Dubbelman YD, Groen J, Wildhagen MF, et al. Urodynamic quantification of decrease in sphincter function after radical prostatectomy: relation to postoperative continence status and the effect of intensive pelvic floor muscle exercises. Neurourol Urodyn. 2012;31:646-651.
- Hammerer P, Huland H. Urodynamic evaluation of changes in urinary control after radical retropubic prostatectomy. J Urol. 1997;157:233-236.
- Van der Poel HG, de Blok W, Joshi N, van Muilekom E. Preservation of lateral prostatic fascia is associated with urine continence after robotic-assisted prostatectomy. Eur Urol. 2009;55:892-900.
- Hwang JJ, Kim BY, Uchiol EM. Improving urinary continence after radical prostatectomy: review of surgical modifications. Korean J Urol. 2009;50:935-941.
- Ko YH, Coelho RF, Chauhan S, et al. Factors affecting return of continence 3 months after robotic-assisted radical prostatectomy: analysis from a large prospective data by a single surgeon. J Urol. 2012;187: 190-194.
- Coakley FV, Eberhardt S, Kattan MW, et al. Urinary continence after radical retropubic prostatectomy: relationship with membranous urethral length on preoperative endorectal magnetic resonance imaging. J Urol. 2002;168:1032-1035.
- Nguyen L, Jhaveri J, Tewari A. Surgical technique to overcome anatomical shortcoming: balancing post-prostatectomy continence outcomes of urethral sphincter lengths on preoperative magnetic resonance imaging. J Urol. 2008;179:1907-1911.
- Paparel P, Akin O, Sandhu JS, et al. Recovery of urinary continence after radical prostatectomy: association with urethral length and urethral fibrosis measured by preoperative and postoperative endorectal magnetic resonance imaging. Eur Urol. 2009;55:629-637.
- Porena M, Mearini E, Mearini L, et al. Voiding dysfunction after radical retropubic prostatectomy: more than external urethral sphincter deficiency. Eur Urol. 2007;52:38-45.
- Giannantoni A, Mearini E, Di Stasi SM, et al. Assessment of bladder and urethral sphincter function before and after radical retropubic prostatectomy. J Urol. 2004;171:1563-1566.
- Song C, Lee J, Hong JH, et al. Urodynamic interpretation of changing bladder function and voiding pattern after radical prostatectomy: a long-term follow-up. BJU Int. 2010;106:681-686.
- Matsukawa Y, Hattori R, Komatsu T, et al. De novo detrusor underactivity after laparoscopic radical prostatectomy. Int J Urol. 2010;17:643-648.
- Leach GE, Yip CM, Donovan BJ. Post-prostatectomy incontinence: the influence of bladder dysfunction. J Urol. 1987;138:574-578.
- Tewari A, Srivasatava A, Menon M; Members of the VIP Team. A prospective comparison of radical retropubic and robot-assisted prostatectomy: experience in one institution. BJU Int. 2003;92:205-210.
- Rocco B, Matei DV, Melegari S, et al. Robotic vs open prostatectomy in a laparoscopically naïve centre: a matched-pair analysis. BJU Int. 2009;104:991-995.
- Montanari E, Del Nero A, Bernardini P, et al. Epidemiology and physiopathology of urinary incontinence after radical prostatectomy [article in Italian]. Arch Ital Urol Androl. 2001;73:121-126.
- Mottrie A, Gallina A, De Wil P, et al. Balancing continence function and oncological outcomes during robot-assisted radical prostatectomy (RARP). BJU Int. 2011;108(6 Pt 2):999-1006.
- Rocco B, Cozzi G, Spinelli MG, et al. Posterior musculofascial reconstruction after radical prostatectomy: a systematic review of the literature. Eur Urol. 2012;62:779-790.
- Sutherland DE, Linder B, Guzman AM, et al. Posterior rhabdosphincter reconstruction during robotic assisted radical prostatectomy: results from a phase II randomized clinical trial. J Urol. 2011;185:1262-1267.
- Ficarra V, Novara G, Rosen RC, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robotic assisted radical prostatectomy. Eur Urol. 2012;62:405-417.