The 4Kscore® Test Reduces Prostate Biopsy Rates in Community and Academic Urology Practices
Badrinath Konety, MD,1 Stephen M. Zappala, MD,2 Dipen J. Parekh, MD,3 Danielle Osterhout,4 Jeffrey Schock, DO,4 Randy M. Chudler, MD,4 Gregory M. Oldford, MD,4 Kenneth M. Kernen, MD,4 Jason Hafron, MD4
1Department of Urology, University of Minnesota, Minneapolis, MN; 2Department of Urology, Tufts University School of Medicine, Boston, MA; 3Department of Urology, University of Miami Miller School of Medicine, Miami, FL; 4Michigan Institute of Urology, St. Clair Shores, MI
There is significant concern regarding prostate cancer screening because of the potential for overdiagnosis and overtreatment of men who are discovered to have abnormal prostate specific antigen (PSA) levels and/or digital rectal examination (DRE) results. The 4Kscore® Test (OPKO Diagnostics, LLC) is a blood test that utilizes four kallikrein levels plus clinical information in an algorithm to calculate an individual’s percentage risk (< 1% to > 95%) for aggressive prostate cancer (Gleason score ≥ 7) on prostate biopsy. The 4Kscore Test, as a follow-up test after abnormal PSA and/or DRE test results, has been shown to improve the specificity for predicting the risk of aggressive prostate cancer and reduce unnecessary prostate biopsies. A clinical utility study was conducted to assess the influence of the 4Kscore Test on the decision to perform prostate biopsies in men referred to urologists for abnormal PSA and/or DRE results. The study population included 611 patients seen by 35 academic and community urologists in the United States. Urologists ordered the 4Kscore Test as part of their assessment of men referred for abnormal PSA and/or DRE test results. Results for the patients were stratified into low risk (< 7.5%), intermediate risk (7.5%-19.9%), and high risk (≥ 20%) for aggressive prostate cancer. The 4Kscore Test results influenced biopsy decisions in 88.7% of the men. Performing the 4Kscore Test resulted in a 64.6% reduction in prostate biopsies in patients; the actual percentage of cases not proceeding to biopsy were 94.0%, 52.9%, and 19.0% for men who had low-, intermediate-, and high-risk 4Kscore Test results, respectively. A higher 4Kscore Test was associated with greater likelihood of having a prostate biopsy (P < 0.001). Among the 171 patients who had a biopsy, the 4Kscore risk category is strongly associated with biopsy pathology. The 4Kscore Test, as a follow-up test for an abnormal PSA and/or DRE results, significantly influenced the physician and patient shared decision in clinical practice, which led to a reduction in prostate biopsies while increasing the probability of detecting aggressive cancer.
[Rev Urol. 2015;17(4):231-240 doi: 10.3909/riu0699]
© 2016 MedReviews®, LLC
The 4Kscore® Test Reduces Prostate Biopsy Rates in Community and Academic Urology Practices
Badrinath Konety, MD,1 Stephen M. Zappala, MD,2 Dipen J. Parekh, MD,3 Danielle Osterhout,4 Jeffrey Schock, DO,4 Randy M. Chudler, MD,4 Gregory M. Oldford, MD,4 Kenneth M. Kernen, MD,4 Jason Hafron, MD4
1Department of Urology, University of Minnesota, Minneapolis, MN; 2Department of Urology, Tufts University School of Medicine, Boston, MA; 3Department of Urology, University of Miami Miller School of Medicine, Miami, FL; 4Michigan Institute of Urology, St. Clair Shores, MI
There is significant concern regarding prostate cancer screening because of the potential for overdiagnosis and overtreatment of men who are discovered to have abnormal prostate specific antigen (PSA) levels and/or digital rectal examination (DRE) results. The 4Kscore® Test (OPKO Diagnostics, LLC) is a blood test that utilizes four kallikrein levels plus clinical information in an algorithm to calculate an individual’s percentage risk (< 1% to > 95%) for aggressive prostate cancer (Gleason score ≥ 7) on prostate biopsy. The 4Kscore Test, as a follow-up test after abnormal PSA and/or DRE test results, has been shown to improve the specificity for predicting the risk of aggressive prostate cancer and reduce unnecessary prostate biopsies. A clinical utility study was conducted to assess the influence of the 4Kscore Test on the decision to perform prostate biopsies in men referred to urologists for abnormal PSA and/or DRE results. The study population included 611 patients seen by 35 academic and community urologists in the United States. Urologists ordered the 4Kscore Test as part of their assessment of men referred for abnormal PSA and/or DRE test results. Results for the patients were stratified into low risk (< 7.5%), intermediate risk (7.5%-19.9%), and high risk (≥ 20%) for aggressive prostate cancer. The 4Kscore Test results influenced biopsy decisions in 88.7% of the men. Performing the 4Kscore Test resulted in a 64.6% reduction in prostate biopsies in patients; the actual percentage of cases not proceeding to biopsy were 94.0%, 52.9%, and 19.0% for men who had low-, intermediate-, and high-risk 4Kscore Test results, respectively. A higher 4Kscore Test was associated with greater likelihood of having a prostate biopsy (P < 0.001). Among the 171 patients who had a biopsy, the 4Kscore risk category is strongly associated with biopsy pathology. The 4Kscore Test, as a follow-up test for an abnormal PSA and/or DRE results, significantly influenced the physician and patient shared decision in clinical practice, which led to a reduction in prostate biopsies while increasing the probability of detecting aggressive cancer.
[Rev Urol. 2015;17(4):231-240 doi: 10.3909/riu0699]
© 2016 MedReviews®, LLC
The 4Kscore® Test Reduces Prostate Biopsy Rates in Community and Academic Urology Practices
Badrinath Konety, MD,1 Stephen M. Zappala, MD,2 Dipen J. Parekh, MD,3 Danielle Osterhout,4 Jeffrey Schock, DO,4 Randy M. Chudler, MD,4 Gregory M. Oldford, MD,4 Kenneth M. Kernen, MD,4 Jason Hafron, MD4
1Department of Urology, University of Minnesota, Minneapolis, MN; 2Department of Urology, Tufts University School of Medicine, Boston, MA; 3Department of Urology, University of Miami Miller School of Medicine, Miami, FL; 4Michigan Institute of Urology, St. Clair Shores, MI
There is significant concern regarding prostate cancer screening because of the potential for overdiagnosis and overtreatment of men who are discovered to have abnormal prostate specific antigen (PSA) levels and/or digital rectal examination (DRE) results. The 4Kscore® Test (OPKO Diagnostics, LLC) is a blood test that utilizes four kallikrein levels plus clinical information in an algorithm to calculate an individual’s percentage risk (< 1% to > 95%) for aggressive prostate cancer (Gleason score ≥ 7) on prostate biopsy. The 4Kscore Test, as a follow-up test after abnormal PSA and/or DRE test results, has been shown to improve the specificity for predicting the risk of aggressive prostate cancer and reduce unnecessary prostate biopsies. A clinical utility study was conducted to assess the influence of the 4Kscore Test on the decision to perform prostate biopsies in men referred to urologists for abnormal PSA and/or DRE results. The study population included 611 patients seen by 35 academic and community urologists in the United States. Urologists ordered the 4Kscore Test as part of their assessment of men referred for abnormal PSA and/or DRE test results. Results for the patients were stratified into low risk (< 7.5%), intermediate risk (7.5%-19.9%), and high risk (≥ 20%) for aggressive prostate cancer. The 4Kscore Test results influenced biopsy decisions in 88.7% of the men. Performing the 4Kscore Test resulted in a 64.6% reduction in prostate biopsies in patients; the actual percentage of cases not proceeding to biopsy were 94.0%, 52.9%, and 19.0% for men who had low-, intermediate-, and high-risk 4Kscore Test results, respectively. A higher 4Kscore Test was associated with greater likelihood of having a prostate biopsy (P < 0.001). Among the 171 patients who had a biopsy, the 4Kscore risk category is strongly associated with biopsy pathology. The 4Kscore Test, as a follow-up test for an abnormal PSA and/or DRE results, significantly influenced the physician and patient shared decision in clinical practice, which led to a reduction in prostate biopsies while increasing the probability of detecting aggressive cancer.
[Rev Urol. 2015;17(4):231-240 doi: 10.3909/riu0699]
© 2016 MedReviews®, LLC
Key words
Prostate cancer • Prostate-specific antigen • Digital rectal examination • Biopsy rate • Gleason score • 4Kscore Test • Prostate cancer prognosis
Key words
Prostate cancer • Prostate-specific antigen • Digital rectal examination • Biopsy rate • Gleason score • 4Kscore Test • Prostate cancer prognosis
The elimination of PSA screening means that the 20% to 30% of men who would have presented with an abnormal PSA level and been found to have high-grade prostate cancer may lose an opportunity for a possible cure.
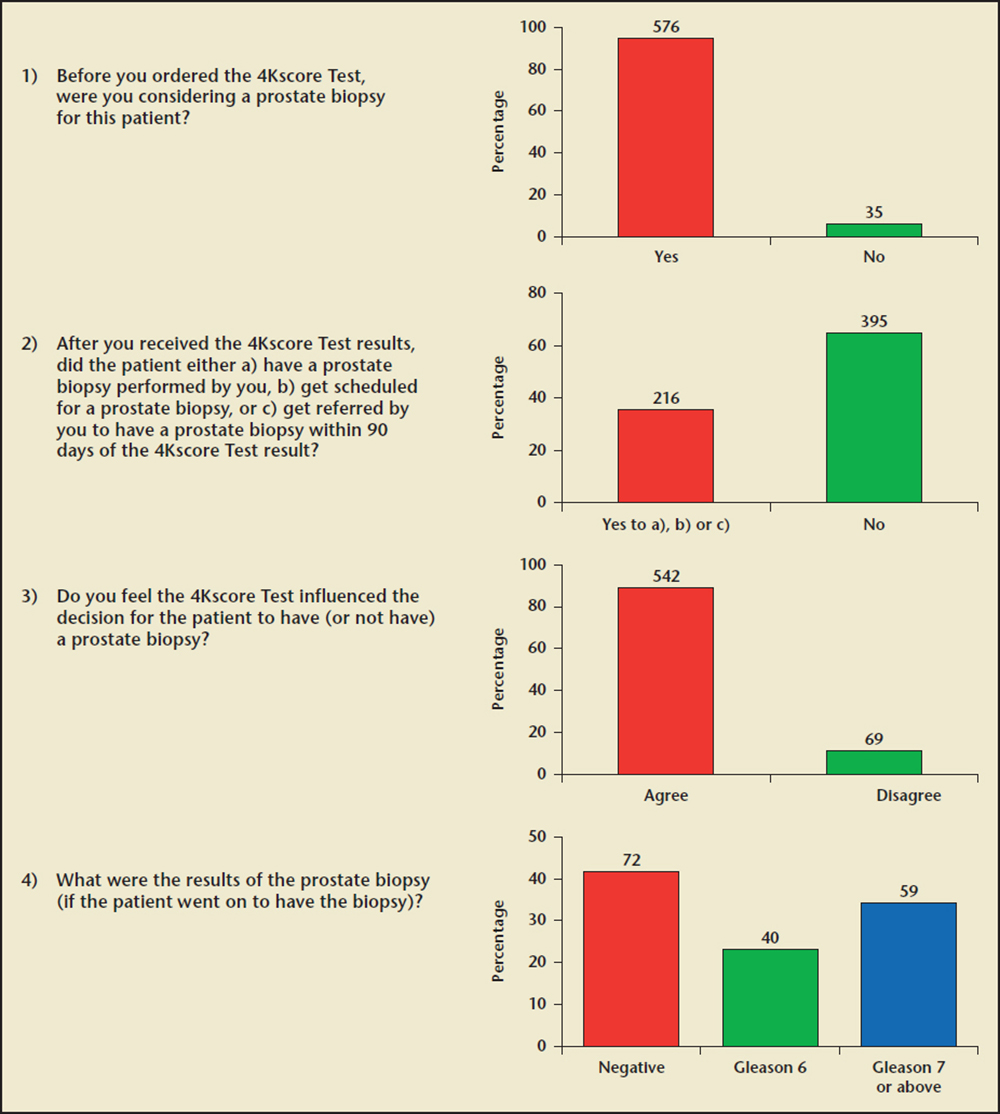
Figure 1. Urologists’ questionnaire for each patient and summary responses.

Figure 1. Urologists’ questionnaire for each patient and summary responses.
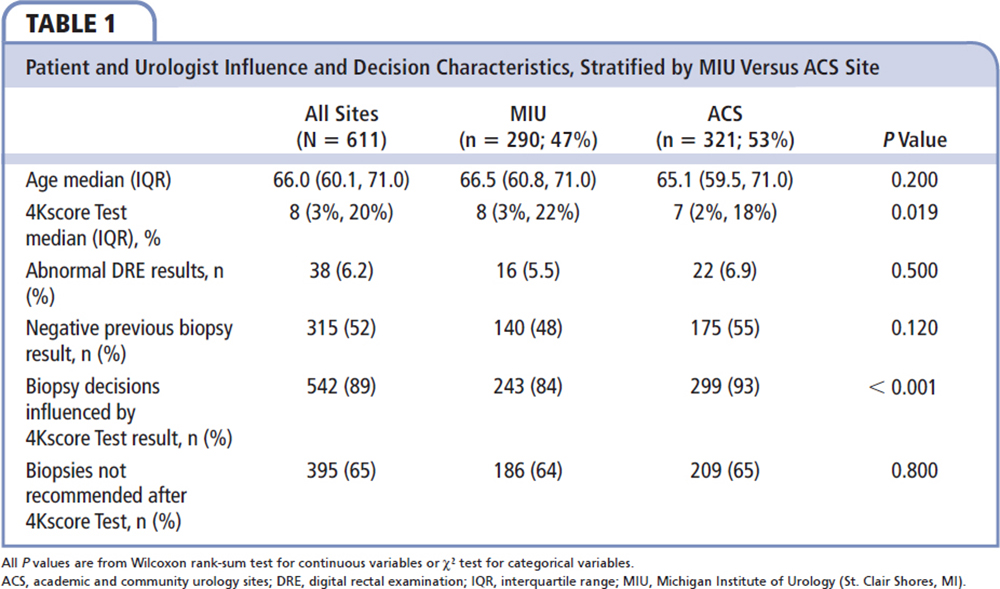

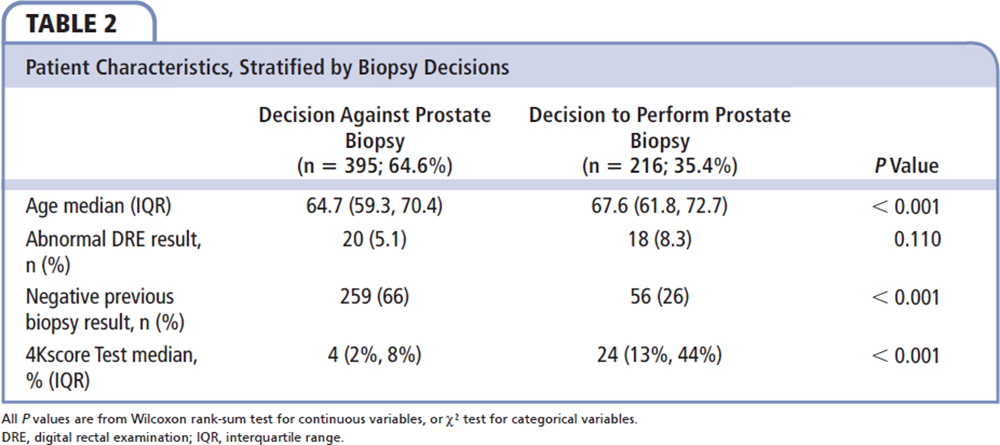

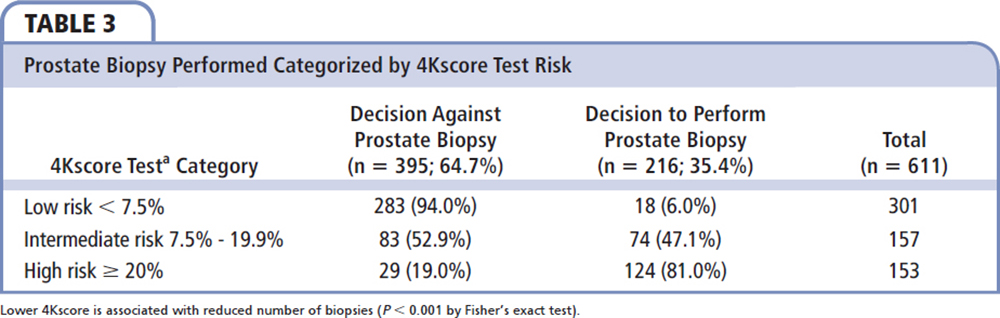

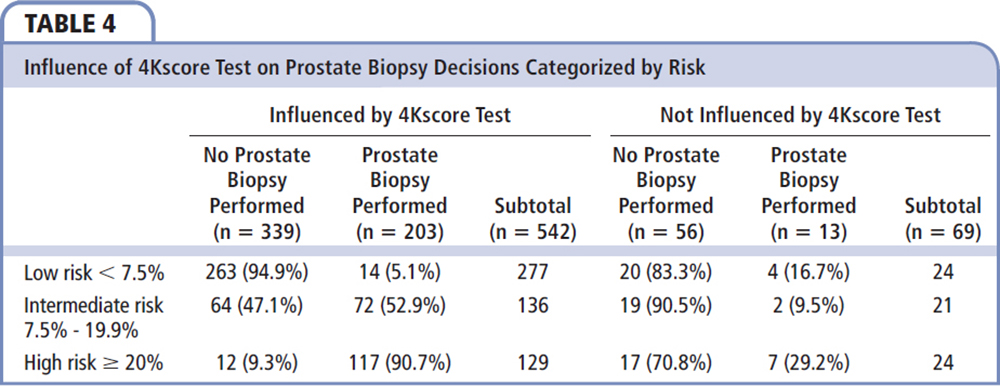

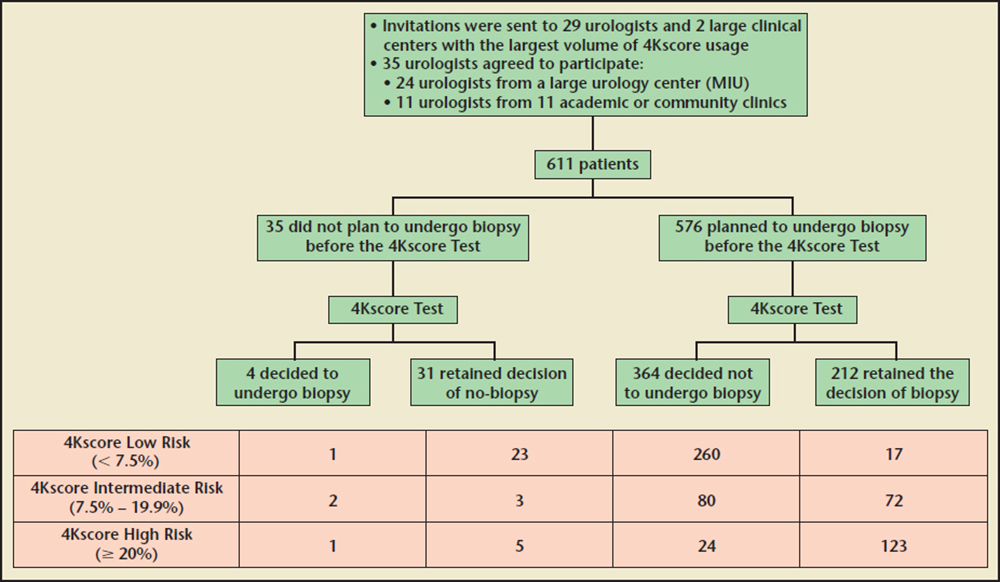
Figure 2. Patients' biopsy decisions before and after the 4Kscore Test was performed. MIU, Michigan Institute of Urology (St. Clair Shores, MI).

Figure2. Patients' biopsy decisions before and after the 4Kscore Test was performed. MIU, Michigan Institute of Urology (St. Clair Shores, MI).

The actual reduction in prostate biopsies in the clinical utility study of 64.6% was greater than the theoretical 30% to 57% reduction reported in retrospective and prospective large studies.
Main Points
• Prostate biopsy is invasive, and has significant costs and complications such as bleeding, urinary retention, and life-threatening infection. There is significant concern regarding prostate cancer screening because of the potential for overdiagnosis and overtreatment of men who are discovered to have abnormal prostate-specific antigen (PSA) levels and/or digital rectal examination (DRE) results.
• The United States Preventative Services Task Force recently advised against routine PSA screening for prostate cancer. The elimination of PSA screening means that the 20% to 30% of men who would have presented with an abnormal PSA level and been found to have high-grade prostate cancer may lose an opportunity for a possible cure.
• The 4Kscore Test incorporates measured blood levels of four kallikrein proteins: total PSA, free PSA, intact PSA, and human kallikrein 2 plus clinical information (age, DRE findings, and a history of prior negative biopsy result) into a proprietary algorithm to calculate an individual man’s percentage risk (, 1% to . 95%) of having Gleason score $ 7 if a prostate biopsy were to be performed.
• Utilization of the 4Kscore Test in men with an abnormal PSA/DRE resulted in an overall 64.6% reduction in prostate biopsies. In the 88.7% of men influenced by the test, 94.9% of men with low-risk scores opted not to proceed with a prostate biopsy and 90.7% of men with high-risk scores did proceed with a prostate biopsy.
• This allows urologists to better risk stratify men for biopsy and ultimately results in more selective treatment of those men with aggressive disease. Conversely, those men not harboring life-threatening disease are able to safely avoid prostate biopsy and overtreatment of indolent disease.
Main Points
• Prostate biopsy is invasive, and has significant costs and complications such as bleeding, urinary retention, and life-threatening infection. There is significant concern regarding prostate cancer screening because of the potential for overdiagnosis and overtreatment of men who are discovered to have abnormal prostate-specific antigen (PSA) levels and/or digital rectal examination (DRE) results.
• The United States Preventative Services Task Force recently advised against routine PSA screening for prostate cancer. The elimination of PSA screening means that the 20% to 30% of men who would have presented with an abnormal PSA level and been found to have high-grade prostate cancer may lose an opportunity for a possible cure.
• The 4Kscore Test incorporates measured blood levels of four kallikrein proteins: total PSA, free PSA, intact PSA, and human kallikrein 2 plus clinical information (age, DRE findings, and a history of prior negative biopsy result) into a proprietary algorithm to calculate an individual man’s percentage risk (, 1% to . 95%) of having Gleason score $ 7 if a prostate biopsy were to be performed.
• Utilization of the 4Kscore Test in men with an abnormal PSA/DRE resulted in an overall 64.6% reduction in prostate biopsies. In the 88.7% of men influenced by the test, 94.9% of men with low-risk scores opted not to proceed with a prostate biopsy and 90.7% of men with high-risk scores did proceed with a prostate biopsy.
• This allows urologists to better risk stratify men for biopsy and ultimately results in more selective treatment of those men with aggressive disease. Conversely, those men not harboring life-threatening disease are able to safely avoid prostate biopsy and overtreatment of indolent disease.
Widespread screening for prostate cancer with serum prostate-specific antigen (PSA) began in 1991, and subsequently a 45% decline in prostate cancer mortality has been observed.1 A recent large European randomized clinical trial also reported a 29% reduction in death from prostate cancer in men undergoing routine screening.2 However, because of a US study that showed no mortality benefits to organized PSA screening,3 and the net physical and psychologic burden of secondary adverse events triggered by PSA testing versus the number of lives saved, the United States Preventative Services Task Force (USPSTF) recently advised against routine PSA screening for prostate cancer.4 The concern of the USPSTF is based on the fact that most men diagnosed with prostate cancer have a tumor that is unlikely to pose a threat to life expectancy. A recent systematic analysis suggested that up to 60% of prostate cancers diagnosed in contemporary studies might be safely observed without a need for immediate intervention.5
One of the primary challenges for urologists is the potential for undergrading of Gleason 6 prostate cancer due to biopsy sampling error; as a result, up to 90% of men with a Gleason 6 prostate cancer still proceed to prostate cancer treatment despite the advent of active surveillance programs. Approximately 66% of patients who are diagnosed with Gleason 6 disease at biopsy will be confirmed to have Gleason 6 cancer after radical prostatectomy.6 Some of these men are considered to have undergone overtreatment, because Gleason 6 cancer is not considered life threatening.7 This subset of men has the potential for developing complications following surgery, including erectile dysfunction, urinary incontinence, and changes in health-related quality of life with disruption of psychologic, sexual, and urinary function.8-12
The prostate biopsy procedure is invasive, and has significant costs and complications such as bleeding, urinary retention, and life-threatening infection. A recent population-based study from Ontario, Canada, revealed a fourfold increase to 4.1% for the rate of hospital admissions after prostate biopsy from 1996 to 2005, with 72% of admissions due to infection.13 These risks, combined with the anxiety involved in undergoing the procedure, present a significant burden to any man considering prostate cancer screening.
The impact of the USPSTF has been a decrease in overall biopsy rates with a subsequent decline in the detection rate of Gleason 7 to 10 high-grade prostate cancers.14 The elimination of PSA screening means that the 20% to 30% of men who would have presented with an abnormal PSA level and been found to have high-grade prostate cancer may lose an opportunity for a possible cure.15 Clearly, there is a need for better risk-stratification tools for men presenting with an abnormal PSA level and/or digital rectal examination (DRE) result in order to both reduce the number of prostate biopsies performed and decrease the rate of Gleason 6 diagnosis and treatment.6
The 4Kscore® Test (OPKO Diagnostics, LLC) incorporates measured blood levels of four kallikrein proteins: total PSA, free PSA, intact PSA, and human kallikrein 2 plus clinical information (age, DRE findings, and a history of prior negative biopsy result) into a proprietary algorithm to calculate an individual man’s percentage risk (< 1% to > 95%) of having Gleason score ≥ 7 if a prostate biopsy were to be performed. The 4Kscore Test has been extensively validated through a total of 12 prospective and retrospective studies published in peer-reviewed journals involving over 22,000 patients from both the United States and Europe.16-23 These studies of men with elevated PSA levels involved cohorts of unscreened and screened men, and those with negative prior prostate biopsy results. Based on analyses published in these studies, the 4Kscore Test would have theoretically resulted in a 45% reduction in prostate biopsies while delaying the diagnosis of aggressive prostate cancer in only a few men (1.3%-4.7%).
The 4Kscore Test is used to accurately determine percentage risk for aggressive prostate cancer (Gleason score ≥ 7) and provide additional information for men being considered for prostate biopsy because of abnormal PSA levels and/or DRE results. This allows urologists to better risk stratify men for biopsy and ultimately results in more selective treatment of those men with aggressive disease. Conversely, those men not harboring life-threatening disease are able to safely avoid prostate biopsy and overtreatment of indolent disease.
With the introduction of any new diagnostic test such as the 4Kscore Test into clinical practice, it is important to assess whether its implementation, in this case as a follow-up test for an abnormal PSA and/or DRE result, influences and changes the physician-patient shared decision-making process and leads to an actual reduction in prostate biopsies. Herein we evaluated the influence of the 4Kscore Test on urologist-patient decisions about proceeding with biopsy in men who have an abnormal PSA and/or DRE result from multiple academic and community urology clinical practices in the United States.
Methods
Study Subject Selection
The 4Kscore Test became commercially available in March 2014, and has been used by over 1600 urologists in both academic and community settings across the United States. In July 2015, we used the database of the 4Kscore Test at OPKO Lab to identify urologists with the largest usage volume of the 4Kscore Test between July 2014 and June 2015. Among this group of urologists, we identified two large centers with multiple users of the 4Kscore Test. Study participation invitations were sent to all of the identified urologists, including those at the two large urology centers. One of the large centers, the Michigan Institute of Urology (MIU; St. Clair Shores, MI), agreed to participate in the study. All MIU urologists who ordered at least one 4Kscore Test (a total of 24 urologists) were invited, and all of them participated. Of the remaining urologists from smaller academic and community practices, 11 of the 29 originally identified urologists agreed to participate. All patients of the participating urologists who had 4Kscore Test results available were included. No additional inclusion criteria were placed on the patients. No restrictions were placed on the urologists in deciding which patients received the 4Kscore Test or in making decisions with the patient about whether to proceed with a prostate biopsy. Urologists received the 4Kscore Test results as a continuum of risk percentage, without the risk categories described here.
An independent institutional review board (IRB) reviewed the study and determined it was exempt from IRB approval because the study was retrospective and the confidentiality of all patient data was assured. Physicians who consented to participate in the study (or their authorized staff) were contacted by study researchers from Lakeside Life Sciences (New Durham, NH) and provided the anonymous patient information used to complete the questionnaire (Figure 1).
In order to ensure complete data sets from subjects enrolled late in the patient accrual period, data were collected until October 16, 2015. Once data sheets were completed, they were returned to Lakeside Life Sciences, where information was entered into a database for statistical analysis. Answers provided by the participating urologists were based upon their review of the patients’ medical records available to them at the time of the study.
Analysis
Patient characteristics, 4Kscore Test results, and biopsy decision data derived from the questionnaire were compared between large and small urology practices. The data were then pooled for analysis, given that there were no major differences in all aspects of the study data between large and small urology practices. Patient characteristics were compared between those who were referred to biopsy after the 4Kscore Test and those who were not, using a Wilcoxon rank-sum test for continuous variables and a χ2 test for categorical variables. The 4Kscore Test results were divided into three categories: < 7.5% (low risk for high-grade cancer)< 7.5% to 19.9% (intermediate risk for high-grade cancer), and ≥ 20% (high risk for high-grade cancer) to evaluate the influence of the 4Kscore Test results on biopsy decisions. The biopsy decisions after the 4Kscore Test were compared across the 4Kscore Test categories using Fisher’s exact test. For those patients whose biopsy data were available, the biopsy outcomes (negative biopsy result, Gleason 6 prostate cancer, or Gleason ≥ 7 aggressive prostate cancer) were compared across the 4Kscore categories using Fisher’s exact test.
Results
There Were No Significant Differences Between the Patients at MIU and the Smaller Academic and Community Sites
A total of 611 patients were enrolled from July 2014 to July 2015, with 290 patients from MIU and 321 patients from smaller academic and community urology sites (ACS) (Table 1). The median age, percentage of patients with an abnormal DRE result, and number of patients with a prior negative biopsy result were not significantly different between MIU and ACS practices. Although statistically significant, the median 4Kscore Test result differed only slightly (8% vs 7%) among the MIU and ACS practices. Clinical decisions were compared for MIU and ACS practices. There was no significant difference in the percentage of patients not recommended for biopsy between the two practice types. Although the percentage of biopsy decisions influenced by the 4Kscore Test was statistically significantly different among MIU and the ACS practices, they were both extremely high (84% for MIU and 93% for ACS). Because of the similarities in patient characteristics and clinical decision making, the two practice types were pooled for subsequent analyses.
The characteristics of the patient and urologist influence and decision characteristics for the pooled patients are also shown in Table 1. All patients in the study had abnormal PSA levels and/or DRE results and received a 4Kscore Test. In addition, 51.6% had a prior negative prostate biopsy result; of these patients, 82.2% did not have a repeat biopsy after the 4Kscore Test.
Table 2 compares the patient characteristics and 4Kscore Test results with the decision to perform prostate biopsy. Men who underwent a prostate biopsy had a higher 4Kscore Test result on average than those who did not have a biopsy (median of 24% vs a median of 4%; P < 0.001). Although all patients either had abnormal PSA levels and/or DRE results, 395 (64.6%) did not undergo biopsies after the 4Kscore Test result was received (Figure 1).
The 4Kscore Test Result Changed Prostate Biopsy Practice
The 4Kscore Test results were categorized as low risk (< 7.5%), intermediate risk (7.5%-19.9%), and high risk (≥ 20%) in 49.3%, 25.7%, and 25.0% of men, respectively. A prostate biopsy was avoided in 94.0% of men at low risk, 52.9% of men at intermediate risk, and 19.0% of men at high risk for aggressive prostate cancer (Table 3). A higher 4Kscore Test result is significantly associated with the decision to proceed with prostate biopsy (P < 0.001).
The Reduction in Actual Prostate Biopsy Rates Was a Result of the 4Kscore Test Influencing the Patient-Physician Decision-making Process
Table 4 categorizes the decisions made based on the 4Kscore Test and whether the patient and urologist were influenced by the 4Kscore Test result. Of the 69 (11.3%) patients for whom the urologists indicated that the 4Kscore Test result did not influence their decisions, the test result did not appear to be associated with a reduction in prostate biopsies performed, with 70% to 90% in each risk category avoiding a prostate biopsy. In contrast, there were 542 (88.7%) patients whose urologists stated that the 4Kscore Test result did influence their decisions about whether to proceed with a prostate biopsy (Figure 1). In these men, the decision to avoid the prostate biopsy correlated to the 4Kscore Test results, with a reduction of 94.9%, 47.1%, and 9.3% in prostate biopsies performed in men with low-risk, intermediate-risk, and high-risk 4Kscore Test results for aggressive prostate cancer, respectively.
The majority of changes in prostate biopsy decisions were primarily because patients and physicians switched from considering a prostate biopsy to not proceeding with a prostate biopsy (Figure 2). Furthermore, the decision to undergo a prostate biopsy was most likely because a high-risk test result further supported the decision to proceed with a prostate biopsy. Interestingly, in physicians and patients who said the 4Kscore Test result influenced their decision (Table 4), there was a 94.9% avoidance of prostate biopsies in the low-risk group and 90.7% of men in the high-risk group underwent a prostate biopsy.
Higher 4Kscore Test Results Were More Likely to Be Associated With High-grade Prostate Cancer Pathology in Men Who Underwent Prostate Biopsy
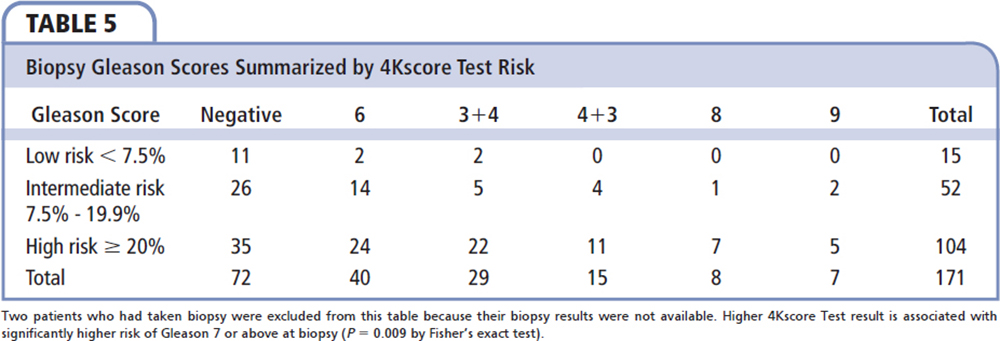
The Gleason scores of 171 patients who had a prostate biopsy during the timeframe of the study are shown in Table 5. The 4Kscore risk category is strongly associated with biopsy-derived Gleason score (P = 0.009). Among the 171 patients who had a biopsy, 45 of the 104 cases (43.3%) with a high-risk 4Kscore (> 20%) had aggressive prostate cancer on prostate biopsy compared with 2 of the 15 (13.3%) with a low-risk 4Kscore (< 7.5%). Both cases with a low-risk 4Kscore were Gleason score 3 + 4 = 7. The patients with 4Kscore ≥ 7.5% (n = 156) had 99 (63.5%) negative or Gleason 6 biopsy results and 57 (36.5%) high-grade cancers diagnosed.
Discussion
Prostate cancer mortality has declined with the advent of PSA screening.24 However, the benefits of PSA screening have to be considered against the harms of performing prostate biopsies in all men with an abnormal PSA level and/or DRE result, and the potential for overtreating men with indolent, low-grade (Gleason 6) prostate cancer.3 Since the USPTF issued a D grade recommendation against PSA screening, several studies have shown reduced levels of PSA screening in the primary care population and an increase in delayed diagnosis of higher-grade cancers.25,26 In response to the PSA dilemma in prostate cancer screening, Penson27 stated, “it is time to accept that prostate cancer screening is not an all-or-none proposition and to accelerate development of personalized screening strategies that are tailored to a man’s individual risk and preferences.” Reducing the financial and quality-of-life cost associated with PSA screening, and increasing the ability to find treatable (aggressive) cancer, is urgently needed. PSA is a sensitive biomarker used to initially identify men at higher risk for aggressive prostate cancer, but it lacks adequate specificity on its own. A blood test that can accurately identify the risk for aggressive, lethal prostate cancer in men with abnormal PSA levels and/or DRE results who are being considered for initial or repeat prostate biopsy, thereby reducing the number of unnecessary prostate biopsies and subsequent treatment of indolent prostate cancers, would be of great value to the public health dilemma associated with PSA screening.
Numerous clinical validity studies of the 4Kscore Test in unscreened, screened, and negative prior prostate biopsy result cohorts have shown its accuracy in correctly determining which patients with abnormal PSA levels and/or DRE results have aggressive prostate cancer. The area under the curve of the 4Kscore Test ranges from 0.80 to 0.90, as compared with 0.51 to 0.77 for total PSA base models to predict high-grade pathology.16-23 Retrospective and prospective large studies have shown that use of the 4Kscore Test could have avoided 30% to 57% of prostate biopsies. The magnitude of the reduction in unnecessary prostate biopsies was similar in populations of men with a very low level of PSA screening,16-18,23 populations of screened men,19-21 and in men who had prior negative prostate biopsy results.22
A decision curve analysis (DCA) allows physicians and patients to determine the net clinical benefit (or harm) the use of a particular test or procedure will have when compared with standard of care at their desired threshold for risk. As applied to the 4Kscore Test, the DCA estimates a net benefit for the 4Kscore Test and other prediction models by summing the benefits (true positives) and subtracting the harms (false positives), in which the latter is weighted by a factor so as to reflect the relative harm of a missed cancer compared with an unnecessary biopsy. A DCA was performed with the 4Kscore Test on multiple cohorts of screened men, unscreened men, and men with a prior negative biopsy result. In each case, the 4Kscore Test gave a favorable net benefit when compared with standard of care or other predictive models built from PSA and clinical data.16-22
Stattin and colleagues28 conducted a nested case-control study measuring 4Kscore Test biomarkers from blood within a population-based cohort in Västerbotten, Sweden. Cohorts of 40-, 50-, and 60-year-old men at the time of initial testing were studied. A total of 12,542 men were followed for > 15 years to determine the risk of distant prostate cancer metastases for PSA and for the 4Kscore Test biomarkers. Results of the 4Kscore Test predicted the risk for distant metastases occurring 5 to 20 years later in men with elevated PSA levels. For example, using a 7.5% threshold for 4Kscore Test, the number of prostate biopsies would have been reduced by 59% in the 50-year-old cohort and 38% in the 60-year-old cohort. As the risk for developing metastatic prostate cancer in men with a 4Kscore Test < 7.5% was < 1% by year 15, there is little risk of missing a lethal cancer even in the absence of frequent follow-up.
The 4Kscore Test has been shown to have the potential to significantly reduce prostate biopsies in men with abnormal PSA levels and/or DRE results, but it is critical that a clinical utility study assess this in clinical practice. The current study was constructed to measure the ability of the 4Kscore Test result to influence the decision of whether or not to proceed with a prostate biopsy in men seen in different types of urology practices who are being considered for an initial or repeat prostate biopsy. Although it is important to evaluate if the 4Kscore Test influenced the shared decision-making process between the urologist and patient of whether to proceed to a prostate biopsy, a less-biased objective measurement is whether the patient actually had a prostate biopsy. This approach assesses whether the information available about the 4Kscore Test, including the inclusion of the 4Kscore Test in the 2015 National Comprehensive Cancer Network guidelines,29 is sufficient to change behavior in a typical urology practice in which the urologist may have inherent incentives to perform a prostate biopsy in any man with an abnormal PSA level and/or DRE result.
Accordingly, a single large urology practice (MIU) was selected to evaluate a center that follows best practices, along with smaller ACS centers at which the reasons that impact the decision to perform a prostate biopsy may be more heterogeneous. This clinical utility study confirms that 4Kscore Test results do change behavior in clinical practice regardless of whether it is in a single large urology practice or smaller ACS centers.
For men being considered for an initial or repeat prostate biopsy for an abnormal PSA and/or DRE result, obtaining the 4Kscore Test as a follow-up resulted in an overall 64.6% reduction in biopsies performed. For those physicians who indicated that the 4Kscore Test influenced their clinical decision making, the risk classification of low risk (4Kscore < 7.5%), intermediate risk (4Kscore 7.5%-19.9%), and high risk (4Kscore ≥ 20%) correlated with 94.9%, 47.1%, and 9.3% reductions in biopsies performed, respectively. The actual reduction in prostate biopsies in the clinical utility study of 64.6% was greater than the theoretical 30% to 57% reduction reported in retrospective and prospective large studies.16-23
The 4Kscore Test influenced the shared decision of whether to proceed to prostate biopsy in 88.7% of the cases studied, with a 94.9% reduction in the prostate biopsy rate in men categorized by the test as low risk and a 90.7% actual biopsy rate in men categorized as high risk for aggressive prostate cancer. In contrast, in the 11.3% of physicians and patients who stated that the 4Kscore did not influence their shared decision, there was no correlation with 4Kscore risk category and the actual performance of a prostate biopsy. This further supports the finding that the 4Kscore Test did influence the decision to conduct a prostate biopsy in the 88.7% of cases for which the urologist indicated that the 4Kscore Test was a consideration in the decision to perform a biopsy. Finally, this clinical utility study showed that results of the 4Kscore Test accurately predicted the actual percentage risk of aggressive prostate cancer for those men whose biopsy data were available in the study.
A limitation of this study is that all of these physicians had already incorporated 4Kscore into their analysis of prostate cancer risk, meaning every patient received a 4Kscore Test. There may also be additional value in testing a cohort with a lower proportion of patients already recommended for or considering a biopsy before the test.
The strengths of the study were that it included physicians from both academic and community-based practices who are in large urology groups or smaller practices. Patients were not excluded for any reason, unless a contraindication for testing was present. Urologists selected patients for the 4Kscore Test using their own criteria, resulting in a study group that reflects real-world practice.
The 4Kscore Test has the ability to identify men who are at risk for aggressive prostate cancer and most likely will benefit from a prostate biopsy, while preventing prostate biopsies in men who are at low risk for aggressive disease. Men determined to be at low risk by the 4Kscore Test (< 7.5%) have a 99% chance to be free of prostate cancer metastases within 15 years of long-term follow-up and may be safely monitored less frequently. This current clinical utility study demonstrates that the 4Kscore Test, as a follow-up test after an abnormal PSA level and/or DRE result, broadly reduced prostate biopsies performed by urologists in actual practice, regardless of type of clinical setting. These findings suggest that PSA screening could be made more specific and personalized, cause less harm, and be more cost effective when coupled with the 4Kscore Test. If physicians provide an individualized risk assessment for aggressive prostate cancer, men who have a low-risk 4Kscore Test result will have a significant reduction of prostate biopsies, whereas men who have a high-risk 4Kscore Test result are more likely to have, and benefit from, a prostate biopsy. ![]()
The authors thank Dr. Mohit Mathur for assisting with manuscript preparation, Dr. Yan Dong for statistical support, and Mike Reeve and Paul Allard for assisting with the organization and monitoring of the clinical trial.
Participating Physicians: Marvin Bondhus, MD, Randy Chudler, MD, Marc Colton, MD, Michael Cotant, MD, Robert Dimitriou, MD, Fadi Eliya, MD, Juan Frontera, MD, Laris Galejs, MD, Marko Gudziak, MD, Jason Hafron, MD, John Harding, MD, Michael Hoff, MD, Mitchell Hollander, MD, William Johnston, MD, Kenneth Kernen, MD, Badrinath Konety, MD, John Lanesky, MD, David Law, MD, James Lugg, MD, Michael Lutz, MD, Martin Madorsky, MD, Gregory McIntosh, MD, John Munoz, MD, Gregory Oldford, MD, Dipen Parekh, MD, Samuel Peretsman, MD, Ashok Reddy, MD, Richard Sarle, MD, Jeffrey Schock, MD, Brian Seifman, MD, Jennifer Sobol, MD, Ned Stein, MD, Kirit Vora, MD, James Yu, MD, Stephen Zappala, MD.
Participating Practices: Urology Center of South Florida (Miami, FL), Garden State Urology (Morris Urology; Denville, NJ), University of Miami Miller School of Medicine (Miami, FL), Cheyenne Urological PC (Cheyenne, WY), Urology Specialist of the Carolinas (Charlotte, NC), Urology Specialty Group (Miami, FL), Marin Urology Center (Greenbrae, CA), Manchester Urology Associates (Manchester, NH), Andover Urology (Andover, MA), Memorial Urology Consultants (Houston, TX), University of Minnesota (Minneapolis and St. Paul, MN), Michigan Institute of Urology (St. Clair Shores, MI).
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9-29.
- Schroder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
- Andriole GL, Crawford ED, Grubb RL 3rd, et al; PLCO Project Team. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125-132.
- Moyer VA, U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120-134.
- Dall’Era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012;62:976-983.
- Eggener SE, Badani K, Barocas DA, et al. Gleason 6 prostate cancer: translating biology into population health. J Urol. 2015;194:626-634.
- Jalloh M, Myers F, Cowan JE, et al. Racial variation in prostate cancer upgrading and upstaging among men with low-risk clinical characteristics. Eur Urol. 2015;67:451-457.
- Barry MJ, Gallagher PM, Skinner JS, Fowler FJ Jr. Adverse effects of robotic-assisted laparoscopic versus open retropubic radical prostatectomy among a nationwide random sample of Medicare-age men. J Clin Oncol. 2012;30:513-518.
- Punnen S, Cowan JE, Chan JM, et al. Long-term health-related quality of life after primary treatment for localized prostate cancer: results from the CaPSURE Registry. Eur Urol. 2014;68:600-608.
- Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368:436-445.
- Resnick MJ, Penson DF. Functional outcomes after treatment for prostate cancer. N Engl J Med. 2013;368:1654.
- Watson E, Shinkins B, Frith E, et al. Symptoms, unmet needs, psychological well-being and health status in survivors of prostate cancer: implications for redesigning follow-up [published online March 27, 2015]. BJU Int. 2015. doi: 10.1111/bju.13122.
- Nam RK, Saskin R, Lee Y, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2013;189(1 suppl):S12-S17.
- Bhindi B, Mamdani M, Kulkarni GS, et al. Impact of the U.S. Preventive Services Task Force recommendations against prostate specific antigen screening on prostate biopsy and cancer detection rates. J Urol. 2015;193:1519-1524.
- Punnen S, Cooperberg MR. The epidemiology of highrisk prostate cancer. Cur Opin Urol. 2013;23:331-336.
- Vickers A, Cronin A, Roobol M, et al. Reducing unnecessary biopsy during prostate cancer screening using a four-kallikrein panel: an independent replication. J Clin Oncol. 2010;28:2493-2498.
- Benchikh A, Savage C, Cronin A, et al. A panel of kallikrein markers can predict outcome of prostate biopsy following clinical work-up: an independent validation study from the European Randomized Study of Prostate Cancer screening, France.BMC Cancer. 2010;10:635.
- Vickers AJ, Cronin AM, Aus G, et al. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: data from the European Randomized Study of Prostate Cancer Screening in Goteborg, Sweden. BMC Med. 2008;6:19.
- Vickers AJ, Cronin AM, Aus G, et al. Impact of recent screening on predicting the outcome of prostate cancer biopsy in men with elevated prostate-specific antigen: data from the European Randomized Study of Prostate Cancer Screening in Gothenburg, Sweden. Cancer. 2010;116:2612-2620.
- Vickers AJ, Cronin AM, Roobol MJ, et al. A four-kallikrein panel predicts prostate cancer in men with recent screening: data from the European Randomized Study of Screening for Prostate Cancer, Rotterdam. Clin Can Res. 2010;16: 3232-3239.
- Parekh DJ, Punnen S, Sjoberg DD, et al. A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer. Eur Urol. 2015;68:464-470.
- Gupta A, Roobol MJ, Savage CJ, et al. A four-kallikrein panel for the prediction of repeat prostate biopsy: data from the European Randomized Study of Prostate Cancer screening in Rotterdam, Netherlands. Br J Cancer. 2010;103:708-714.
- Bryant RJ, Sjoberg DD, Vickers AJ, et al. Predicting high-grade cancer at ten-core prostate biopsy using four kallikrein markers measured in blood in the ProtecT study [published online April 11, 2015]. J Natl Cancer Inst. doi: 10.1093/jnci/djv095.
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control. 2008;19:175-181.
- Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314: 2054-2061.
- Bhindi B, Mamdani M, Kulkarni GS, et al. Impact of the U.S. Preventive Services Task Force recommendations against prostate specific antigen screening on prostate biopsy and cancer detection rates. J Urol. 2015;193:1519-1524.
- Penson DF. The pendulum of prostate cancer screening. JAMA. 2015;314:2031-2033.
- Stattin P, Vickers AJ, Sjoberg DD, et al. Improving the specificity of screening for lethal prostate cancer using prostate-specific antigen and a panel of kallikrein markers: a nested case-control study. Eur Urol. 2015;68:207-213.
- Carroll PR, Parsons JK, Andriole G, et al. Prostate cancer early detection, version 2.2015. J Natl Compr Canc Netw. 2015;13:1534-1561.