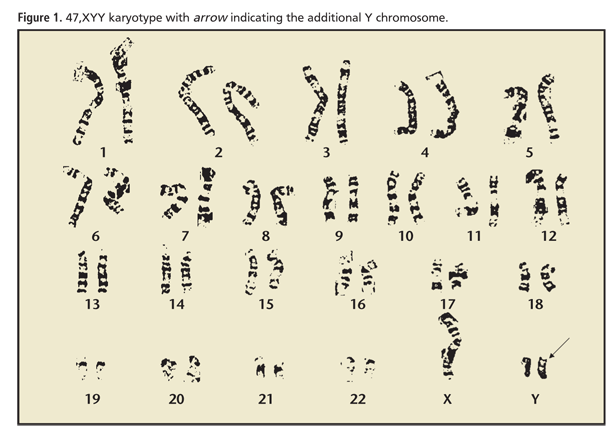
47,XYY Syndrome and Male Infertility
Ina W. Kim, MD, MPH,1 Arjun C. Khadilkar,1 Edmund Y. Ko, MD,2 Edmund S. Sabanegh, Jr, MD1
1Center for Male Fertility, Glickman Urological and Kidney Institute, Cleveland Clinic Foundation, Cleveland, OH; 2Department of Urology, Loma Linda University, Loma Linda, CA
Men with 47,XYY syndrome present with varying physical attributes and degrees of infertility. A retrospective chart review was performed on a male infertility and genetic anomaly database. Three patients with 47,XYY were found. Each presented with > 2 years of infertility. All were tall with elevated body mass indices. Scrotal findings ranged from normal to atrophic testicles. Semen analyses demonstrated oligospermia and varying endocrine profiles. Because of the diverse phenotype and potential lack of symptoms, identification and diagnosis of men with 47,XYY syndrome may be difficult. We recommend careful screening of 47,XYY patients and referral to primary physicians for long-term follow-up for increased incidence of health-related comorbidities.
[ Rev Urol. 2013;15(4):188-196 doi: 10.3909/riu0580]
© 2014 MedReviews®, LLC
Key words
47,XYY syndrome • Infertility syndromes
Men with 47,XXY syndrome have shorter life spans compared with those with normal karyotypes. The median age of survival for men with 47,XXY syndrome is approximately 10.4 years less when compared with a normal control group (77.9 years vs 67.5 years; P < .0001).


Main Points
• Men with 47,XYY syndrome have a diverse spectrum of clinical presentation. Because of the heterogeneous phenotype and potential lack of symptoms, diagnosis may be difficult, especially if fertility is not compromised.
• Patients with low semen parameters may require further assisted reproductive techniques to achieve pregnancy. Genetic evaluation is recommended prior to proceeding.
• Careful screening of these patients and referral to primary physicians is recommended for long-term follow-up given the increased incidence of associated comorbidities.
The 47,XYY sex chromosome variation is the most common sex chromosome anomaly after Klinefelter syndrome (47,XXY),1-3 occurring in approximately 1 out of 1000 live male births.4,5 Parental nondisjunction at meiosis II resulting in an extra Y chromosome produces a 47,XYY karyotype in the affected offspring.6-8 46,XY/47,XYY mosaics from parental nondisjunction during cell division after postzygotic mitosis can result in addition of the extra Y chromosome in early embryonic development.6,8
Most patients with 47,XYY have a delayed diagnosis, with a median age of 17.1 years at diagnosis, as was shown in a Danish cohort study.9 Although most have no phenotypic abnormalities, XYY boys are at greater risk for behavioral problems, mild learning disability, delayed speech and language development, and tall stature.10 Studies have increasingly reported an association between 47,XYY and fertility problems, noting an increased incidence of chromosomally abnormal spermatozoa in the semen of men with 47,XXY syndrome.7,11-15 This greater prevalence of hyperhaploid sperm results in an increased risk of passing the extra Y chromosome to offspring.14 Men with 47,XXY syndrome can have variable sperm counts, ranging from normal to azoospermia.3,8,14,16-18
Here we review pertinent findings on physical examination and laboratory evaluation in three men with 47,XXY syndrome diagnosed during infertility evaluation as well as review the available literature on the subject, with special emphasis on male fertility effects.
Method
A retrospective chart review was performed on an Institutional Review Board–approved database. Electronic charts were reviewed for presenting complaint, past medical history, family history, physical examination, and laboratory/imaging reports, including semen analysis, endocrine studies, and genetic testing.
Case Reports
Patient 1
A 37-year-old man presented with a 3-year history of secondary infertility and miscarriage of his partner’s pregnancies. The patient had a history of chronic low energy and low libido with no previous treatment. He completed puberty in his early teenage years. The patient’s medical history was significant for morbid obesity, Tourette syndrome, facial tics, and excessive blinking. His brother also had fertility issues, although the cause was unknown. The remaining history and review of systems were noncontributory. Physical examination revealed a large obese male with a body mass index (BMI) of 48.69 (height 6 feet 4 inches, weight 400 lbs) and no evidence of gynecomastia. He had a normal phallus and bilaterally descended testes measuring approximately 20 cc. The patient had a left-sided grade 3 and right-sided grade 2 varicocele.
Semen analyses revealed oligoasthenoteratozoospermia, with a concentration of 2.8 to 5.1 M/mL, 45% to 60% motility, and 2% strict normal morphology. Scrotal ultrasonography confirmed bilateral varicoceles. Early morning total serum testosterone was 136 ng/dL (normal range, 220-1000 ng/dL), follicle stimulating hormone (FSH) was 7.1 mU/mL (normal range, 1-10 mU/mL), prolactin was 9.3 ng/mL (normal range, 2-18 ng/mL), and estradiol-17β was 38 pg/mL (normal range, 14-55 pg/mL). Serum growth hormone was normal. A karyotype was obtained that demonstrated 47,XYY. Y-chromosome linked microdeletion test results were negative.
Patient 2
A 27-year-old man presented with a 2-year history of primary infertility. The patient denied any symptoms of hypogonadism. He began puberty at age 13 years. Past medical history was notable for obstructive sleep apnea and asthma. There was no family history of infertility. The remainder of the history was noncontributory.
Physical examination demonstrated a tall, obese man with a BMI of 38.04 (height 6 feet 7 inches, weight 337 lbs) without gynecomastia. The patient was normally virilized with a normal phallus. Bilateral descended testes were atrophic with volumes of 10 cc each. Vas deferens and epididymides were normal. No varicoceles were noted.
Two semen analyses demonstrated severe oligospermia of 2 M/mL on the first specimen, and 21 total sperm on the second specimen. Morning total serum testosterone was 163 ng/dL, FSH 16.1 mU/mL, luteinizing hormone (LH) 4.2 mU/mL (normal range, 1.0-7.0 mU/mL), prolactin 7.2 ng/mL, and estradiol-17β 33 pg/mL. Genetic evaluation demonstrated a 47,XYY karyotype and a normal Y-chromosome linked microdeletion study result.
Patient 3
A 35-year-old man presented with a 5-year history of primary infertility and decreased libido. The patient had a normal childhood and puberty history. The patient’s social history included a 10-year smoking history, as well as extensive drug use including marijuana, ecstasy, ketamine, and mushrooms. He denied any prior anabolic steroid or testosterone abuse. The remainder of his history was noncontributory.
Physical examination demonstrated a tall, well-developed male with a BMI of 37.2 (height 6 feet 5 inches, weight 310 lbs) without gynecomastia. External genitalia were normally developed with bilaterally descended testes. The left testis measured 14 cc and the right measured 16 cc. The patient had a left-sided grade 2 varicocele and a right-sided grade 1 varicocele.
The patient presented with two semen analyses demonstrating severe oligozoospermia with 0.3 M/mL and 4 total motile sperm per 2 μl, respectively. Endocrine evaluation revealed a normal testosterone level of 264 ng/dL, elevated FSH of 17.6 mU/mL, and LH of 11.5 mU/mL. Testicular ultrasonography demonstrated small testicles measuring 2.5 × 1.3 × 2.2 cm on the right and 3.1 × 1.3 × 2.5 cm on the left. Genetic evaluation confirmed 47,XYY karyotype and normal Y-linked microdeletion assay.
Discussion
Fertility Effects
Many men with 47,XYY karyotype are fertile in spite of their sex chromosome abnormalities. Some researchers have suggested that the extra Y chromosome is lost before meiosis,3,6-8 thus conserving fertility in these patients. Studies comparing sperm aneuploidy between fertile and infertile XYY men reveal that most sperm produced by XYY men have a normal karyotype.3,6-8 An arrest point for genetically abnormal germ cells may reside at the primary and secondary spermatocyte or spermatid stages of development leading to a continuous elimination of these cells during spermatogenesis.19 This may cause varying degrees of maturation arrest as well as heterogeneous sperm concentrations seen in men with genetic abnormalities.
Conversely, multiple studies demonstrate XYY men having a significant percentage of sperm mosaicism, aneuploidy, or hyperdiploidy ranging from 0.57% to 77.8%.5,7,13,14,20 The increased rate of disomy YY in men with the 47,XYY karyotype conveys that particular hyperdiploid cells can undergo meiotic division. It has been hypothesized that disomy YY cells emerge because of YY bivalent pairs at meiosis I, and leave the free X univalent within the sex vesicle when eliminated in anaphase.14 Hyperhaploid sperm can undergo meiotic division, thereby increasing the risk of transmission of abnormal genetics to offspring.
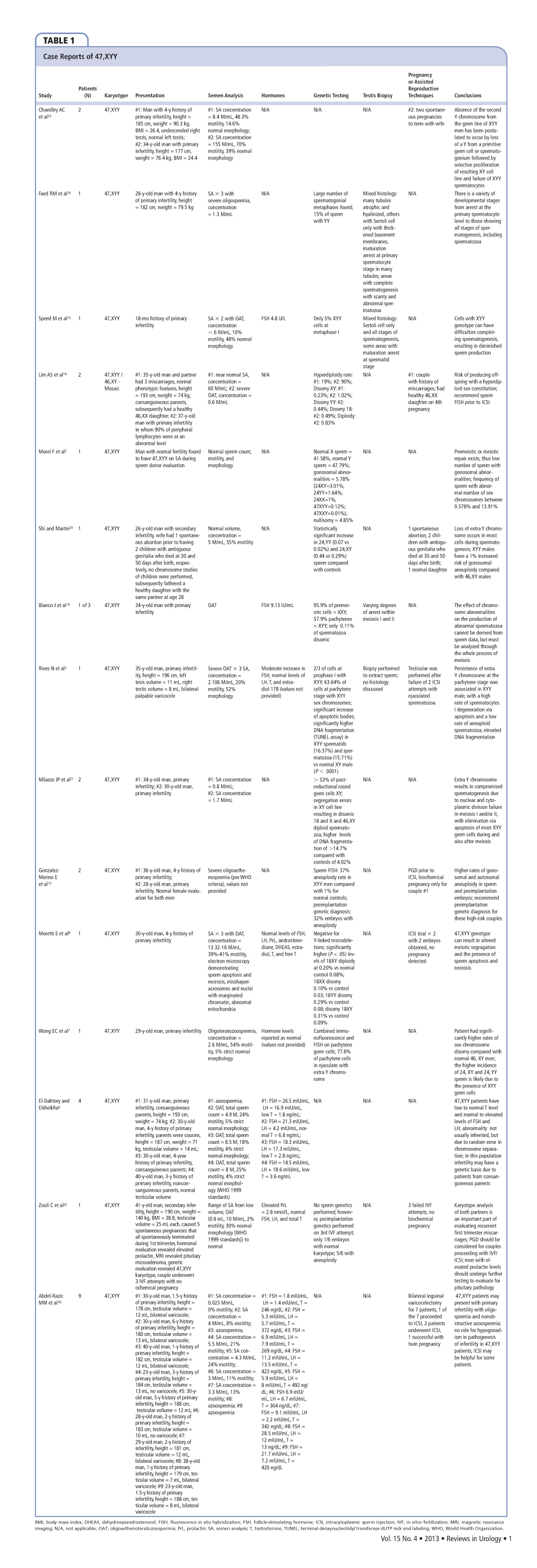
Sperm maturation can be compromised resulting in an increased number of immature sperm.14 Persistence of the extra Y chromosome during meiosis can result in spermatogenesis impairment.21 Sperm counts can range from normal to azoospermia and result in varying fertility in the literature (Table 1).3,8,14,16,17,19 Overall, XYY has a negative effect of sperm count, maturation, and genetics as demonstrated by published case reports and confirmed here.
Fertility Management
Men with 47,XXY syndrome with normal sperm counts can potentially achieve pregnancy spontaneously. However, for those men with 47,XXY syndrome who have difficulty achieving pregnancy, in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) is likely going to be required to achieve pregnancy due to the high prevalence of oligospermia as well as abnormal sperm chromosomal constitution. It has been recommended that oligospermic patients undergoing IVF or ICSI receive medical genetics counseling and potentially preimplantation genetic testing to understand the potential risks to their offspring.13 Men with symptoms of hypogonadism or low total testosterone levels may be started on empiric medical therapy such as clomiphene citrate or anastrozole, if clinically indicated. This can alleviate symptoms of hypogonadism and maximize intratesticular testosterone to optimize spermatogenesis.
Medical Comorbidities
The diagnosis of 47,XYY syndrome often occurs later in life due to the lack of distinguishing phenotypical characteristics compared with men with 46,XY. The symptoms that may lead to chromosomal analysis testing and diagnosis may include behavior problems, increased growth velocity during adolescence, mild learning disability, and delayed speech and language skills.6
Men with 47,XXY syndrome have shorter life spans when compared with those with normal karyotypes. The median age of survival for men with 47,XXY syndrome is approximately 10.4 years less compared with a normal control group (77.9 years vs 67.5 years; P < .0001).9 This shorter lifespan may be due to an increased risk of cancer, pulmonary, neurologic, and unspecified diseases, as well as high-risk behavior and trauma that have been found in men with 47,XXY syndrome.9
Our Experience With 47,XYY Patients
Our three men with 47,XXY syndrome presented with infertility and were found to have varying degrees of oligospermia. Phenotype can be variable. Genetic counseling is recommended for the infertile couple with 47,XYY to understand the potential risks of transmitting this anomaly to offspring as well as health implications for the patient himself. Partners of men with total motile sperm counts > 5 million to 10 million can undergo intrauterine insemination whereas those with severe oligospermia (< 5 million) may require IVF or ICSI to achieve pregnancy. Sperm fluorescence in situ hybridization or preimplantation genetic diagnosis can be considered.13,14,22
Conclusions
Men with 47,XYY syndrome have a diverse spectrum of clinical presentation. Because of the heterogeneous phenotype and potential lack of symptoms, diagnosis may be difficult, especially if fertility is not compromised. However, in our patients and in our review of the literature, it appears that many men with 47,XYY syndrome will likely have decreased fertility potential. These patients may ultimately require assisted reproductive techniques in order to achieve pregnancy. Genetic evaluation is recommended prior to proceeding. We recommend careful screening of these patients and referral to primary physicians for long-term follow-up given the increased incidence of associated comorbidities. ![]()
References
- Gekas J, Thepot F, Turleau C, et al. Chromosomal factors of infertility in candidate couples for ICSI: an equal risk of constitutional aberrations in women and men. Hum Reprod. 2001;16:82-90.
- Hook EB, Hamerton JL. The frequency of chromosome abnormalities detected in consecutive newborn studies- differences between studies- results by sex and by severity of phenotypic involvement. In: Hook EB, Porter IH, eds, Population Cytogenetics. New York: Academic Press; 1977:63-79.
- Rives N, Milazzo JP, North MO, et al. From spermatocytes to spermatozoa in an infertile XYY male. Int J Androl. 2005;28:304-310.
- Jacobs PA, Melville M, Ratcliffe S, et al. A cytogenetic survey of 11,680 newborn infants. Ann Hum Genet. 1974;37:359-376.
- Morel F, Roux C, Bresson JL. Sex chromosome aneuploidies in sperm of 47,XYY men. Arch Androl. 1999;43:27-36.
- El-Dahtory F, Elsheikha HM. Male infertility related to an aberrant karyotype, 47,XYY: four case reports. Cases J. 2009;2:28.
- Wong EC, Ferguson KA, Chow V, Ma S. Sperm aneuploidy and meiotic sex chromosome configurations in an infertile XYY male. Hum Reprod. 2008;23:374-378.
- Moretti E, Anichini C, Sartini B, Collodel G. Sperm ultrastructure and meiotic segregation in an infertile 47,XYY man. Andrologia. 2007;39:229-234.
- Stochholm K, Juul S, Gravholt CH. Diagnosis and mortality in 47,XYY persons: a registry study. Orphanet J Rare Dis. 2010;5:15.
- No authors listed. Children and young adults with sex chromosome aneuploidy— follow-up, clinical and molecular studies. Minaki, Ontario, Canada, June 7-10, 1989. Birth Defects Orig Artic Ser. 1990;26:1-304.
- Blanco J, Rubio C, Simon C, et al. Increased incidence of disomic sperm nuclei in a 47,XYY male assessed by fluorescent in situ hybridization (FISH). Hum Genet. 1997;99:413-416.
- Chevret E, Rousseaux S, Monteil M, et al. Meiotic behaviour of sex chromosomes investigated by three-colour FISH on 35,142 sperm nuclei from two 47,XYY males. Hum Genet. 1997;99:407-412.
- Gonzalez-Merino E, Hans C, Abranowicz M, et al. Aneuploidy study in sperm and preimplantation embryos from nonmosaic 47,XYY men. Fertil Steril 2007;88:600-606.
- Lim AS, Fong Y, Yu SL. Analysis of the sex chromosome constitution of sperm in men with a 47,XYY mosaic karyotype by fluorescence in situ hybridization. Fertil Steril. 1999;72:121-123.
- Speed RM, Faed MJ, Batstone PJ, et al. Persistence of two Y chromosomes through meiotic prophase and metaphase I in an XYY man. Hum Genet. 1991;87:416-420.
- Faed M, Robertson J, MacIntosh WG, Grieve J. Spermatogenesis in an infertile XYY man. Hum Genet. 1976;33:341-347.
- Egozcue S, Blanco J, Vendrell JM, et al. Human male infertility: chromosome anomalies, meiotic disorders, abnormal spermatozoa and recurrent abortion. Hum Reprod Update. 2000;6:93-105.
- Abdel-Razic MM, Abdel-Hamid LA, Elsobky ES. Nonmosaic 47,XYY syndrome presenting with male infertility: case series. Andrologia. 2012;44:200-204.
- Blanco J, Egozcue J, Vidal F. Meiotic behaviour of the sex chromosomes in three patients with sex chromosome anomalies (47,XXY, mosaic 46,XY/47,XXY and 47,XYY) assessed by fluorescence in-situ hybridization. Hum Reprod. 2001;16:887-892.
- Shi Q, Martin RH. Multicolor fluorescence in situ hybridization analysis of meiotic chromosome segregation in a 47,XYY male and a review of the literature. Am J Med Genet. 2000;93:40-46.
- Milazzo JP, Rives N, Mousset-Siméon N, Macé B. Chromosome constitution and apoptosis of immature germ cells present in sperm of two 47,XYY infertile males. Hum Reprod. 2006;21:1749-1758.
- Zouli C, Tsametis C, Papadimas I, Goulis DG. A man with 47,XYY karyotype, prolactinoma and a history of first trimester recurrent miscarriages in his wife. Hormones (Athens). 2011;10:72-75.
- Chandley AC, Fletcher J, Robinson JA. Normal meiosis in two 47,XXY men. Hum Genet. 1976;33:231-240.